Retinoids in Fungal Infections: From Bench to Bedside
Abstract
:1. Introduction
2. Methods and Study Design
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
3.1. Effectiveness of Retinoids against Opportunistic Fungi That Colonize the Skin and Mucosae in Humans
3.1.1. Candida
3.1.2. Rhodotorula Mucilaginosa
3.1.3. Malassezia
3.2. Effectiveness of Retinoids against Environmental Human Pathogenic Filamentous Fungi
3.2.1. Aspergillus spp.
3.2.2. Fonsecaea spp.
3.2.3. Dermatophytes
3.3. Efficacy of Retinoids against Pneumocystis
Pneumocystis
| Fungi | Spp. | Clinical/Experimental Model | Pathological Model | Retinoid | Combination | Results | Reference |
|---|---|---|---|---|---|---|---|
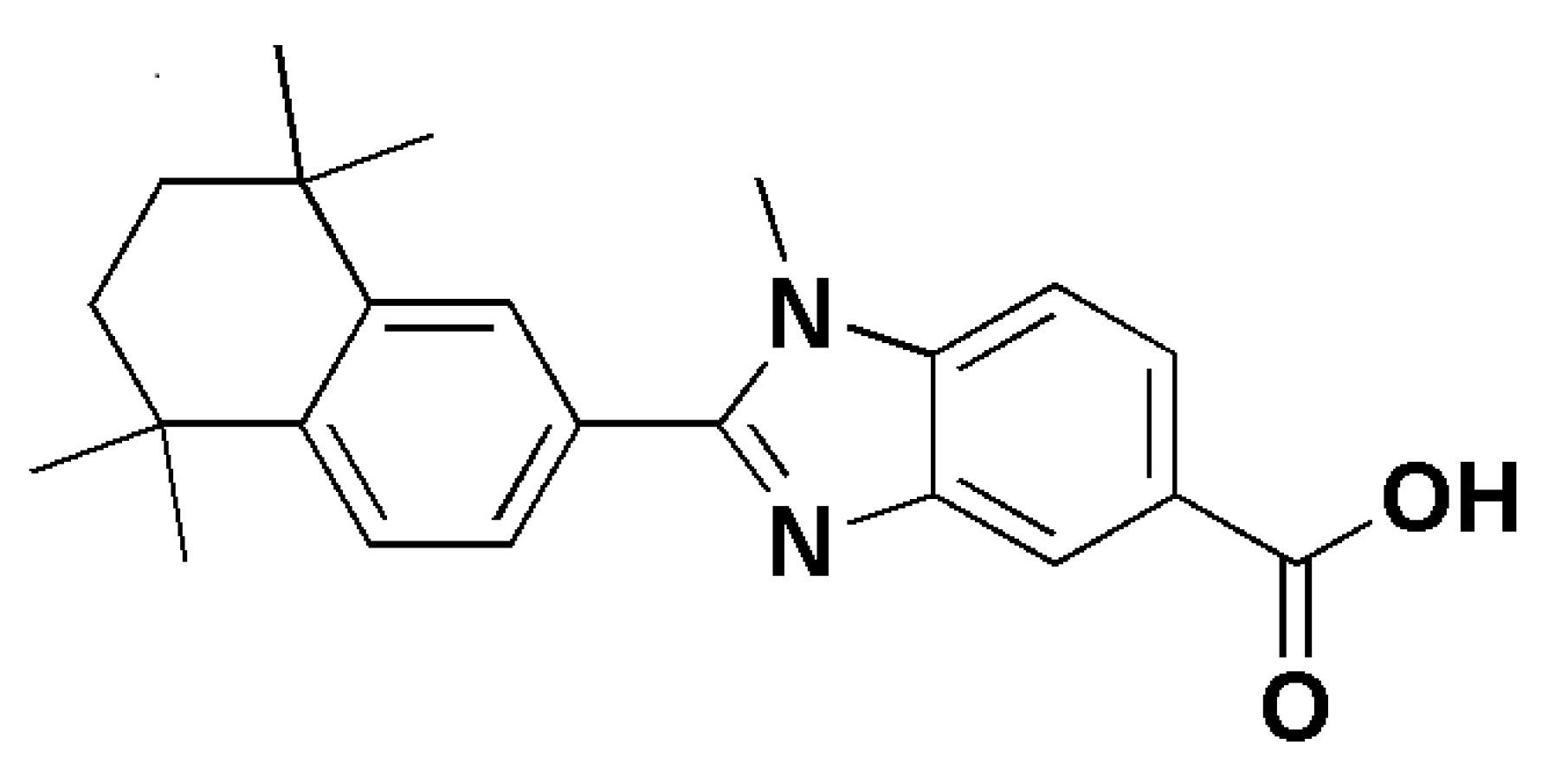
| Candida | albicans | In vitro culture | Retinoid derivatives containing a benzimidazole moiety | Antimicrobial activity MIC 1.56μg/mL | [16] | ||
| albicans | In vitro culture | 1 g of Tazarotene 0.1% gel dissolved in 3mL of physiological solution | Fungistatic activity | [12] | |||
| glabrata | In vitro culture | 1 g of Tazarotene 0.1% gel dissolved in 3mL of physiological solution | Fungistatic activity | [12] | |||
| krusei | In vitro culture | Retinoid derivatives containing a benzimidazole moiety | Antimicrobial activity MIC 1.56μg/mL | [16] | |||
| Not specified | Human | Chronic hyperplastic candidiasis nystatin-resistant | 0.18% isotretinoin applied twice a day for one month | Clinical resolution after one month | [17] | ||
| Malassezia | furfur | Human | Pityriasis versicolor | Retinoic acid 0.05% cream vs. retinoic acid 0.05% lotion twice daily for 3 weeks | 50 patients totally. 33 patients (73.33%) and 3 weeks in 45 patients (97.83%) with a mean of 2.27 weeks | [25] | |
| Not specified | Human | Pityriasis versicolor | Adapalene gel vs. ketoconazole 2% | Clinical resolution after 4 weeks and 30/40 (75%) presented mycological negative test vs. Ketoconazole cream 2% 28/40 (70%) and 28/40 (70%) | [26] | ||
| Not specified | Human | Pityriasis versicolor | Oral isotretinoin 20 mg/day (0,4 mg/kg/day) for 6 weeks | Clinical resolution after 6 weeks | [28] | ||
| Aspergillus | fumigatus | structural bioinformatic analysis | ATRA | Competitive inhbitor of the Hsp90 ATP-binding site | [13] | ||
| fumigatus | In vitro colture | ATRA | Fungistatic activity (0.5 and 1 mM) down-regulation of HSP90 mRNa and protein expression; enhances the phagocytosis of macrophages (5 or 10mM) | [13] | |||
| fumigatus | Rat | Invasive pulmonary aspergillosis (IPA) | ATRA, 2 mg/kg i.p. for 6 days | Alone; versus posaconazole; versus vehicol | Reduction in mortality of IPA | [13] | |
| niger | Human | Onychomycosis | Tazarotene 0.1% gel twice daily for three months | Alone or plus tioconazole (28% nail paint) | Clinical resolution of OM after three months | [32] | |
| flavus | Human | Onychomycosis | Tazarotene 0.1% gel twice daily for three months | Alone or plus tioconazole (28% nail paint) | Clinical resolution of OM after three months | [32] | |
| fumigatus | Murine model | Keratitis | Fenretinide 100 μM subconjunctival injection | Inhibition of neutrophil recruitment and IL-1β production | [34] | ||
| Dermatophyte (Trichophyton) | rubrum | Human | Onychomycosis | Tazarotene 0.1% gel once daily for 12 weeks | Clinical resolution | [12] | |
| mentagrophytes | Human | Onychomycosis | Tazarotene 0.1% gel once daily for 12 weeks | Clinical resolution | [12] | ||
| verrucosum | In vitro culture | 1 g of Tazarotene 0.1% gel dissolved in 3 mL of physiological solution | Fungistatic activity | [12] | |||
| tonsurans | In vitro culture | 1 g of Tazarotene 0.1% gel dissolved in 3 mL of physiological solution | Fungistatic activity | [12] | |||
| Dermatophyte (Epidermophyton) | floccosum | Human | Onychomycosis | Tazarotene 0.1% gel once daily for 12 weeks | Fungistatic activity | [12] | |
| Pneumocystis | jiroveci | Mice and rats | pneumonia | ATRA 5 mg/kg/day in 8% DMSO | Primaquine 2 mg/kg/day in water | Engaged myeloid-derived suppressor cells | [48] |
| Fonsecaea | Not specified | Human | Chromoblastomycosis | Acitretin 50 mg/day | Itraconazole and 200 mg/day topical imiquimod for 5 weeks | Clinical resolution | [37] |
| Not specified | Human | Chromoblastomycosis | Acitretin 20 mg/day for 5 weeks | Itraconazole and 200 mg/day topical imiquimod for 5 weeks | Clinical resolution | [37] | |
| monophora | Human | Chromoblastomycosis | Acitretin 20 mg/Kg for 1 month | Itraconazole 200 mg/day for 1 month | Clinical resolution | [38] | |
| Rhodotorula | mucilaginosa | Human | Onychomycosis and psoriasis | Oral acitretin 10 mg/day for 8 weeks | Topical calcipotriol/betamethasone for 8 weeks | Clinical improvement within 8 weeks | [21] |
4. Discussion
Unmet Needs in Fungal Infections
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPA | Allergic Bronchial Pulmonary Aspergillosis |
| AIDS | Acquired Immunodeficiency Syndrome |
| AML | Acute Myeloid Leukaemia |
| APL | Acute Promyelocytic Leukaemia |
| ATRA | All-Trans Retinoic Acid |
| CPA | Chronic Pulmonary Aspergillosis |
| DDSs | Drug Delivery Systems |
| DMSO | Dimethyl sulfoxide |
| EPSs | Extracellular Polymeric Substances |
| IBA | Invasive Bronchial Aspergillosis |
| IL-1β | Interleukin 1 beta |
| IFN-γ | Interferon gamma |
| IPA | Invasive Pulmonary Aspergillosis |
| HIV | Human Immunodeficiency Virus |
| HSP90 | Heat Shock Protein 90 |
| JNK | c-Jun N-terminal Kinase |
| LOX-1 | Lectin-type Oxidized LDL receptor 1 |
| MDR | Multidrug resistance |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MPO | Myeloperoxidase |
| NEs | Nanoemulsions |
| NLCs | Nanostructured Lipid Carriers |
| OM | Onychomycosis |
| OSI | Onychomycosis Severity Index |
| PcP | Pneumocystis Pneumonia |
| qRT-PCR | Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction |
| QS | Quorum Sensing |
| RARs | Retinoic Acid Receptors |
| SAFS | Severe Asthma with Fungal Sensitization |
| SLNs | Solid Lipid Nanoparticles |
| SMX | sulfamethoxazole |
| TMP | trimethoprim |
| TPGS | D-α-tocopheryl-polyethylene-glycol-succinate |
References
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [CrossRef]
- Mayer, F.L.; Kronstad, J.W. Cryptococcus neoformans. Trends Microbiol. 2020, 28, 163–164. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.-A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., III. Mycoses Study Group Education and Research Consortium (MSG-ERC); European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection–Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Ibáñez-Martínez, E.; Ruiz-Gaitán, A.; Pemán-García, J. Update on the diagnosis of invasive fungal infection. Rev. Espanola Quimioter. 2017, 30 (Suppl. S1), 16–21. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host–commensal–pathogen interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Dika, E.; Bianchi, L. Clinical efficacy and reflectance confocal microscopy monitoring in moderate-severe skin aging treated with a polyvinyl gel containing retinoic and glycolic acid: An assessor-blinded 1-month study proof-of-concept trial. J. Cosmet. Dermatol. 2021, 20, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Di Prete, M.; Gaziano, R.; Lanna, C.; Orlandi, A.; Di Francesco, P.; Bianchi, L.; Campione, E. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines 2021, 9, 237. [Google Scholar] [CrossRef]
- Girmenia, C.; Coco, F.L.; Breccia, M.; Latagliata, R.; Spadea, A.; D’Andrea, M.; Gentile, G.; Micozzi, A.; Alimena, G.; Martino, P.; et al. Infectious complications in patients with acute promyelocytic leukaemia treated with the AIDA regimen. Leukemia 2003, 17, 925–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campione, E.; Paterno, E.J.; Diluvio, L.; Costanza, G.; Bianchi, L.; Carboni, I.; Chimenti, S.; Orlandi, A.; Marino, D.; Favalli, C. Tazarotene as alternative topical treatment for onychomycosis. Drug Des. Dev. Ther. 2015, 9, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal Effect of All- trans Retinoic Acid against Aspergillus fumigatus In vitro and in a Pulmonary Aspergillosis In vivo Model. Antimicrob. Agents Chemother. 2021, 65, e01874-20. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216 (Suppl. S3), S445–S451. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.L.; De Almeida Júnior, J.N.; Guinea, J. Emerging multidrug-resistant Candida species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Alagoz, Z.A.; Yildiz, S.; Buyukbingol, E. Antimicrobial Activities of Some Tetrahydronaphthalene-Benzimidazole Derivatives. Chemotherapy 2007, 53, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Scardina, G.A.; Ruggieri, A.; Messina, P. Chronic hyperplastic candidosis: A pilot study of the efficacy of 0.18% isotretinoin. J. Oral Sci. 2009, 51, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, P.; Vamvoukaki, R.; Samonis, G. Rhodotorula species infections in humans: A systematic review. Mycoses 2019, 62, 90–100. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, S.C.; Kong, F.; Fan, X.; Cheng, J.-W.; Hou, X.; Zhou, M.-L.; Wang, H.; Xu, Y.-C. Five-year China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study of invasive fungal infections caused by noncandidal yeasts: Species distribution and azole susceptibility. Infect. Drug Resist. 2018, 11, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Martini, K.; Müller, H.; Huemer, H.P.; Höpfl, R. Nail psoriasis masqueraded by secondary infection withRhodotorula mucilaginosa. Mycoses 2013, 56, 690–692. [Google Scholar] [CrossRef]
- Jarros, I.C.; Veiga, F.F.; Corrêa, J.L.; Barros, I.L.E.; Gadelha, M.C.; Voidaleski, M.F.; Pieralisi, N.; Pedroso, R.B.; Vicente, V.A.; Negri, M.; et al. Microbiological and virulence aspects of Rhodotorula mucilaginosa. EXCLI J. 2020, 19, 687–704. [Google Scholar] [PubMed]
- Ge, G.; Li, D.; Mei, H.; Lu, G.; Zheng, H.; Liu, W.; Shi, D. Different toenail onychomycosis due to Rhodotorula mucilaginosa and Candida parapsilosis in an immunocompetent young adult. Med. Mycol. Case Rep. 2019, 24, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.F.B.; Huang, G.; Jia, Q.; Yuan, L.; Li, Y.; Tu, Z. Mixed Infection of Toe Nail Caused by Trichosporon asahii and Rhodotorula mucilaginosa. Mycopathologia 2020, 185, 373–376. [Google Scholar] [CrossRef]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56 (Suppl. S1), S10–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handojo, I.; Subagjo, B.; Hadi, S. The effect of topical retinoic acid (Airol) in the treatment of tinea versicolor. Southeast Asian J. Trop. Med. Public Health 1977, 8, 93–98. [Google Scholar]
- Shi, T.-W.; Ren, X.-K.; Yu, H.-X.; Tang, Y.-B. Roles of Adapalene in the Treatment of Pityriasis Versicolor. Dermatology 2012, 224, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Tanase, C.; Pascu, G.-A.; Todoran, N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals 2020, 13, 217. [Google Scholar] [CrossRef]
- Yazici, S.; Baskan, E.B.; Saricaoglu, H. Long-term remission of recurren pityriasis versicolor with short-term systemic isotretinoin therapy. J. Dermatol. Cosmetol. 2018, 2, 1. [Google Scholar] [CrossRef]
- Quindós, G. Epidemiología de las micosis invasoras: Un paisaje en continuo cambio [Epidemiology of invasive mycoses: A landscape in continuous change]. Rev. Iberoam. Micol. 2018, 35, 171–178. [Google Scholar] [CrossRef]
- Gago, S.; Overton, N.L.D.; Ben-Ghazzi, N.; Novak-Frazer, L.; Read, N.D.; Denning, D.W.; Bowyer, P. Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat. Commun. 2018, 9, 3835. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, S.S.A.; Omar, G.A.; Mahmoud, M.T.; Said, M. Comparative study between the effect of topical tazarotene 0.1 gel alone vs. its combination with tioconazole nail paint in treatment of onychomycosis. Dermatol. Ther. 2020, 33, e14333. [Google Scholar] [CrossRef] [PubMed]
- Carratù, M.R.; Marasco, C.; Mangialardi, G.; Vacca, A. Retinoids: Novel immunomodulators and tumour-suppressive agents? Br. J. Pharmacol. 2012, 167, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Che, C.; Liu, K.; Zhang, J.; Jiang, N.; Yuan, K.; Zhao, G. Fenretinide Inhibits Neutrophil Recruitment and IL-1β Production in Aspergillus fumigatus Keratitis. Cornea 2018, 37, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, P.M.; Pindycka-Piaszczyńska, M.; Piaszczyński, M. Chromoblastomycosis. Adv. Dermatol. Allergol. 2014, 31, 310–321. [Google Scholar] [CrossRef]
- De Brito, A.C.; Bittencourt, M.D.J.S. Chromoblastomycosis: An etiological, epidemiological, clinical, diagnostic, and treatment update. An. Bras. Dermatol. 2018, 93, 495–506. [Google Scholar] [CrossRef]
- Belda, W.; Criado, P.R.; Passero, L.F.D. Case Report: Treatment of Chromoblastomycosis with Combinations including Acitretin: A Report of Two Cases. Am. J. Trop. Med. Hyg. 2020, 103, 1852–1854. [Google Scholar] [CrossRef]
- Bao, F.; Wang, Q.; Yu, C.; Shang, P.; Sun, L.; Zhou, G.; Wu, M.; Zhang, F. Case Report: Successful Treatment of Chromoblastomycosis Caused by Fonsecaea monophora in a Patient with Psoriasis Using Itraconazole and Acitretin. Am. J. Trop. Med. Hyg. 2018, 99, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Bitew, A. Dermatophytosis: Prevalence of Dermatophytes and Non-Dermatophyte Fungi from Patients Attending Arsho Advanced Medical Laboratory, Addis Ababa, Ethiopia. Dermatol. Res. Pract. 2018, 2018, 8164757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méhul, B.; De Coi, N.; Grundt, P.; Genette, A.; Voegel, J.J.; Monod, M. Detection ofTrichophyton rubrumandTrichophyton interdigitale in onychomycosis using monoclonal antibodies against Sub6 (Tri r 2). Mycoses 2019, 62, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Kassir, M.; Karagaiah, P.; Sonthalia, S.; Katsambas, A.; Galadari, H.; Gupta, M.; Lotti, T.; Wollina, U.; Abdelmaksoud, A.; Grabbe, S.; et al. Selective RAR agonists for acne vulgaris: A narrative review. J. Cosmet. Dermatol. 2020, 19, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.E. Potential anti-inflammatory effects of topical retinoids and retinoid analogues. Adv. Ther. 2002, 19, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, R.; Campione, E.; Iacovelli, F.; Marino, D.; Pica, F.; Di Francesco, P.; Aquaro, S.; Menichini, F.; Falconi, M.; Bianchi, L. Antifungal activity of Cardiospermum halicacabum L. (Sapindaceae) against Trichophyton rubrum occurs through molecular interaction with fungal Hsp90. Drug Des. Dev. Ther. 2018, 12, 2185–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, J.A. Pneumocystis jiroveci. Semin. Respir. Crit. Care Med. 2020, 41, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, A.; Norris, K.; Mousa, J. Pneumocystis pneumonia: Immunity, Vaccines and Treatments. Pathogens 2021, 10, 236. [Google Scholar] [CrossRef]
- Kato, H.; Samukawa, S.; Takahashi, H.; Nakajima, H. Diagnosis and treatment of Pneumocystis jirovecii pneumonia in HIV-infected or non-HIV-infected patients—difficulties in diagnosis and adverse effects of trimethoprim-sulfamethoxazole. J. Infect. Chemother. 2019, 25, 920–924. [Google Scholar] [CrossRef]
- Pereira-Díaz, E.; Moreno-Verdejo, F.; De La Horra, C.; Guerrero, J.A.; Calderón, E.J.; Medrano, F.J. Changing Trends in the Epidemiology and Risk Factors of Pneumocystis Pneumonia in Spain. Front. Public Health 2019, 7, 275. [Google Scholar] [CrossRef]
- Lei, G.-S.; Zhang, C.; Shao, S.; Jung, H.-W.; Durant, P.J.; Lee, C.-H. All-Trans Retinoic Acid in Combination with Primaquine Clears Pneumocystis Infection. PLoS ONE 2013, 8, e53479. [Google Scholar] [CrossRef]
- Lee, J.-M.; Seo, J.-H.; Kim, Y.-J.; Kim, Y.-S.; Ko, H.-J.; Kang, C.-Y. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int. J. Cancer 2012, 131, 741–751. [Google Scholar] [CrossRef]
- Mirza, N.; Fishman, M.; Fricke, I.; Dunn, M.; Neuger, A.M.; Frost, T.J.; Lush, R.M.; Antonia, S.; Gabrilovich, D.I. All-trans-Retinoic Acid Improves Differentiation of Myeloid Cells and Immune Response in Cancer Patients. Cancer Res. 2006, 66, 9299–9307. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Waterer, G. Advances in anti-fungal therapies. Mycopathologia 2021, 1–8. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 4265. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, R.; Singh, C.; Kaur, S.; Goyal, A.K.; Singh, K.K.; Singh, B. Inhalational Drug Delivery in Pulmonary Aspergillosis. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 183–217. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A new bioavailable fenretinide formulation with antiproliferative, antimetabolic, and cytotoxic effects on solid tumors. Cell Death Dis. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orienti, I.; Nguyen, F.; Guan, P.; Kolla, V.; Calonghi, N.; Farruggia, G.; Chorny, M.; Brodeur, G.M. A Novel Nanomicellar Combination of Fenretinide and Lenalidomide Shows Marked Antitumor Activity in a Neuroblastoma Xenograft Model. Drug Des. Dev. Ther. 2019, 13, 4305–4319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paolo, D.; Pastorino, F.; Zuccari, G.; Caffa, I.; Loi, M.; Marimpietri, D.; Brignole, C.; Perri, P.; Cilli, M.; Nico, B.; et al. Enhanced anti-tumor and anti-angiogenic efficacy of a novel liposomal fenretinide on human neuroblastoma. J. Control. Release 2013, 170, 445–451. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Charoenputtakhun, P.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. All-trans retinoic acid-loaded lipid nanoparticles as a transdermal drug delivery carrier. Pharm. Dev. Technol. 2013, 19, 164–172. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Nacher, A.; Carbone, C.; Valenti, D.; Maccioni, A.M.; Sinico, C.; Fadda, A.M. Development of novel diolein–niosomes for cutaneous delivery of tretinoin: Influence of formulation and In vitro assessment. Int. J. Pharm. 2014, 477, 176–186. [Google Scholar] [CrossRef]
- AlGahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Íñigo, M.; Del Pozo, J.L. Fungal biofilms: From bench to bedside. Rev. Espanola Quimioter. 2019, 31 (Suppl. S1), 35–38. [Google Scholar]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; De Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R. Treatment of Invasive Candidiasis: A Narrative Review. J. Fungi 2018, 4, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, C.H.; Morelli, K.A.; Schultz, D.; Nadell, C.D.; Cramer, R.A. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 22473–22483. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; She, P.; Zhou, L.; Liu, Y.; Chen, L.; Luo, Z.; Wu, Y. Bactericidal and Anti-biofilm Activity of the Retinoid Compound CD437 Against Enterococcus faecalis. Front. Microbiol. 2019, 10, 2301. [Google Scholar] [CrossRef] [PubMed]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef] [PubMed]
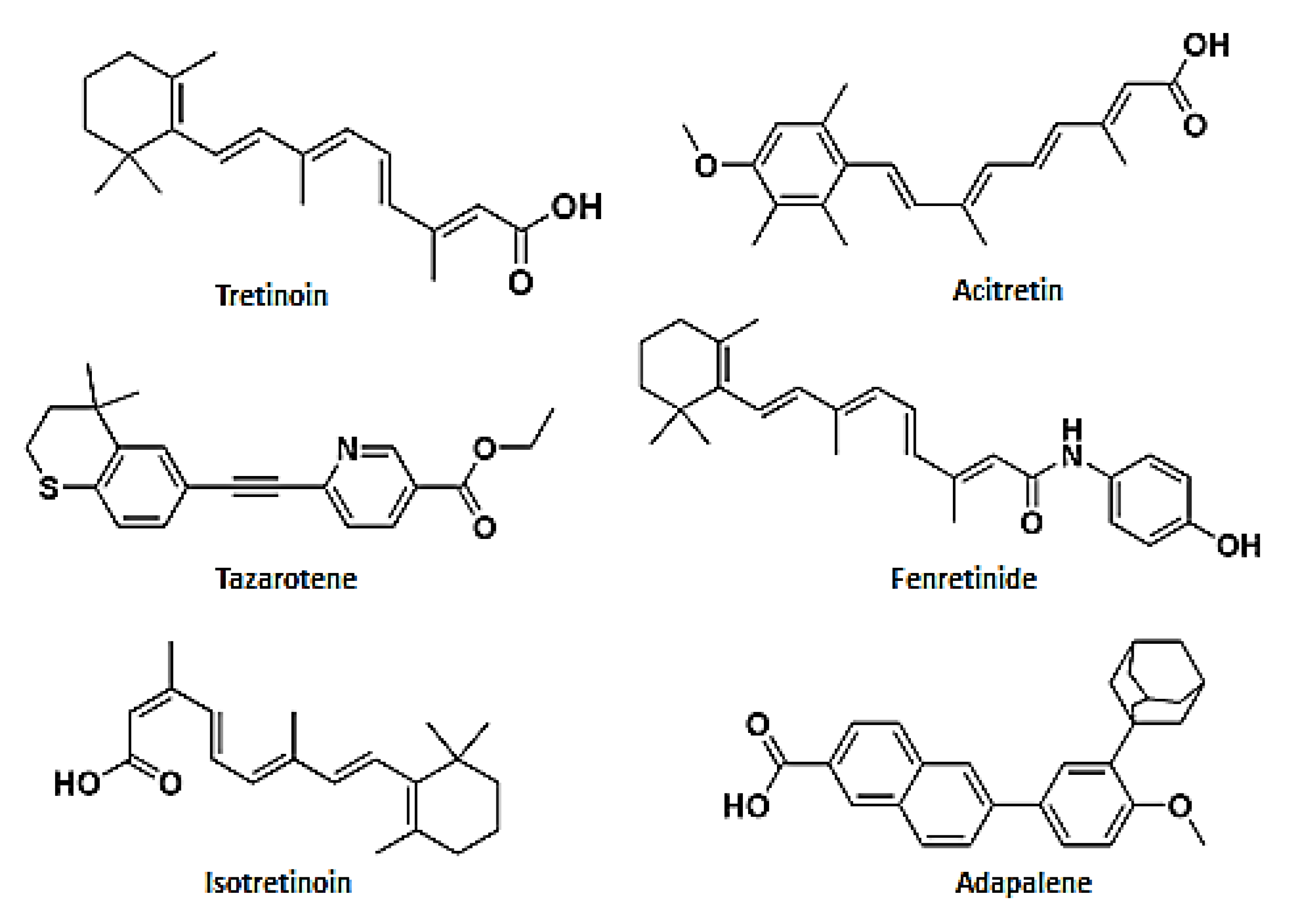
| Tretinoin | Tazarotene | Isotretinoin | Acitretin | Fenretinide | Adapalene | |
|---|---|---|---|---|---|---|
| In vitro studiies | A. fumigatus | C. glabrata | A. fumigatus | |||
| C. albicans | C. albicans | A. niger | ||||
| Microsporum spp. | T. verrucosum | C. albicans | ||||
| Trichophyton spp. | ||||||
| Epidermophyton spp. | ||||||
| In vivo studies | M. furfur | A. niger | M. furfur | F. monophora | A. fumigatus | M. furfur |
| A. fumigatus | T. rubrum | P. jiroveci | R. mucilaginosa | |||
| T. tonsurans | ||||||
| T. mentagrophytes | ||||||
| E. floccosum | ||||||
| C. albicans | ||||||
| A. flavus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. https://doi.org/10.3390/ph14100962
Cosio T, Gaziano R, Zuccari G, Costanza G, Grelli S, Di Francesco P, Bianchi L, Campione E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals. 2021; 14(10):962. https://doi.org/10.3390/ph14100962
Chicago/Turabian StyleCosio, Terenzio, Roberta Gaziano, Guendalina Zuccari, Gaetana Costanza, Sandro Grelli, Paolo Di Francesco, Luca Bianchi, and Elena Campione. 2021. "Retinoids in Fungal Infections: From Bench to Bedside" Pharmaceuticals 14, no. 10: 962. https://doi.org/10.3390/ph14100962
APA StyleCosio, T., Gaziano, R., Zuccari, G., Costanza, G., Grelli, S., Di Francesco, P., Bianchi, L., & Campione, E. (2021). Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals, 14(10), 962. https://doi.org/10.3390/ph14100962










