Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy
Abstract
:1. Introduction
2. Transmembrane Mucin Structure
2.1. Core Structural Characteristics
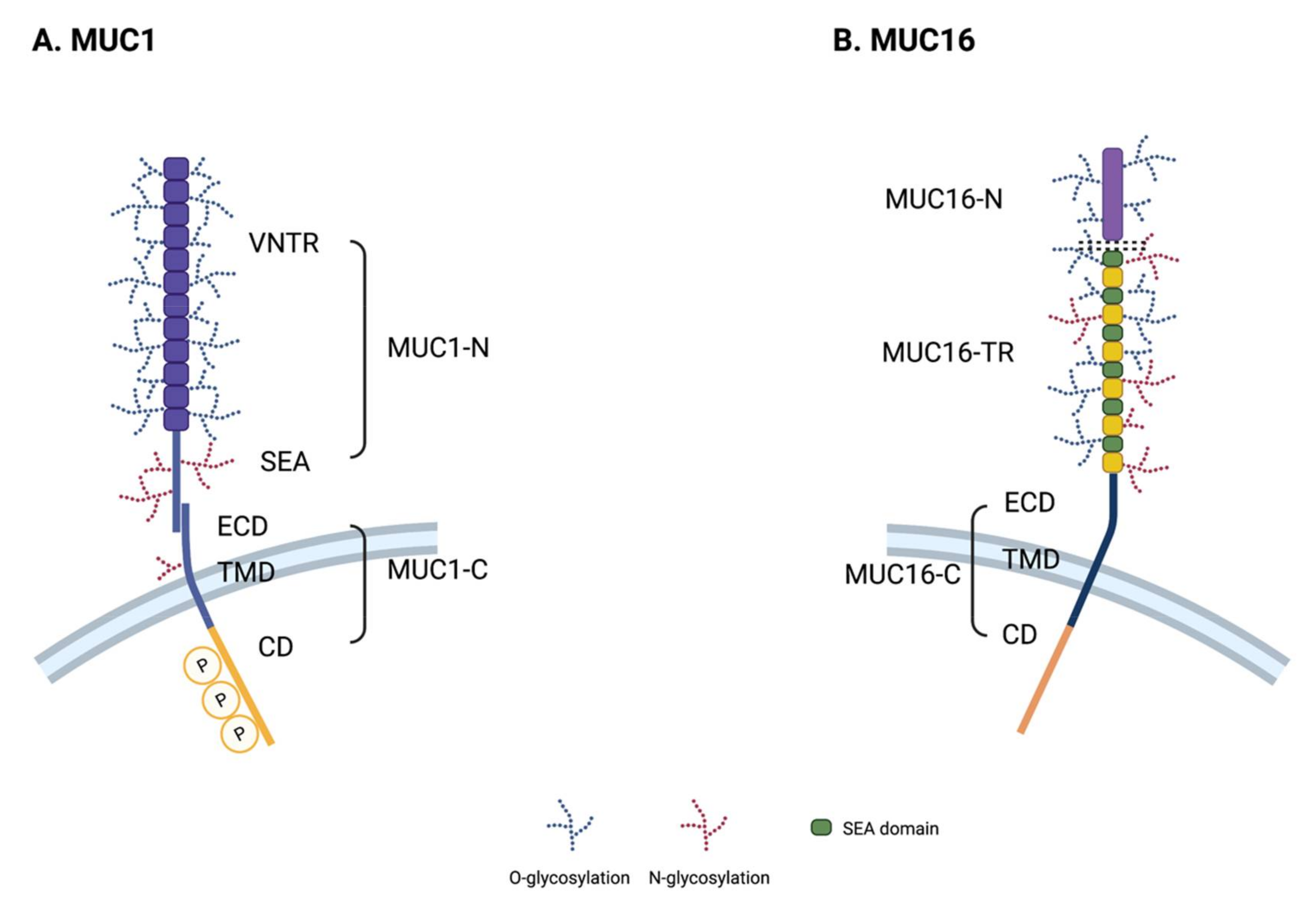
2.2. Structure of MUC1
2.3. Structure of MUC16
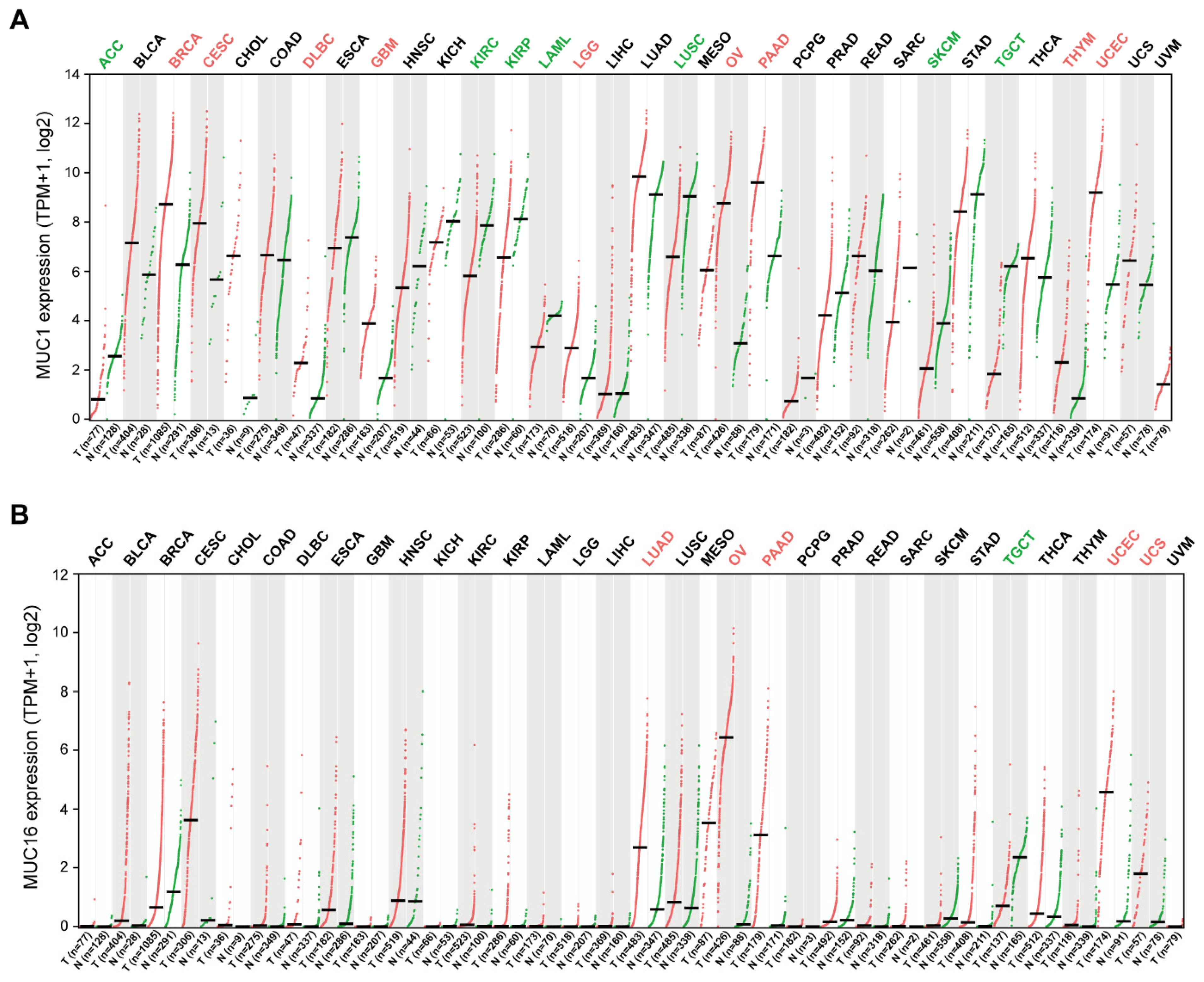
3. The Role of Transmembrane Mucins in Tumorigenesis
3.1. Uncontrolled Proliferation
3.2. Evading Cell Death and Resistance to Stress
3.3. Reprogramming Energy Metabolism
3.4. EMT and Metastasis
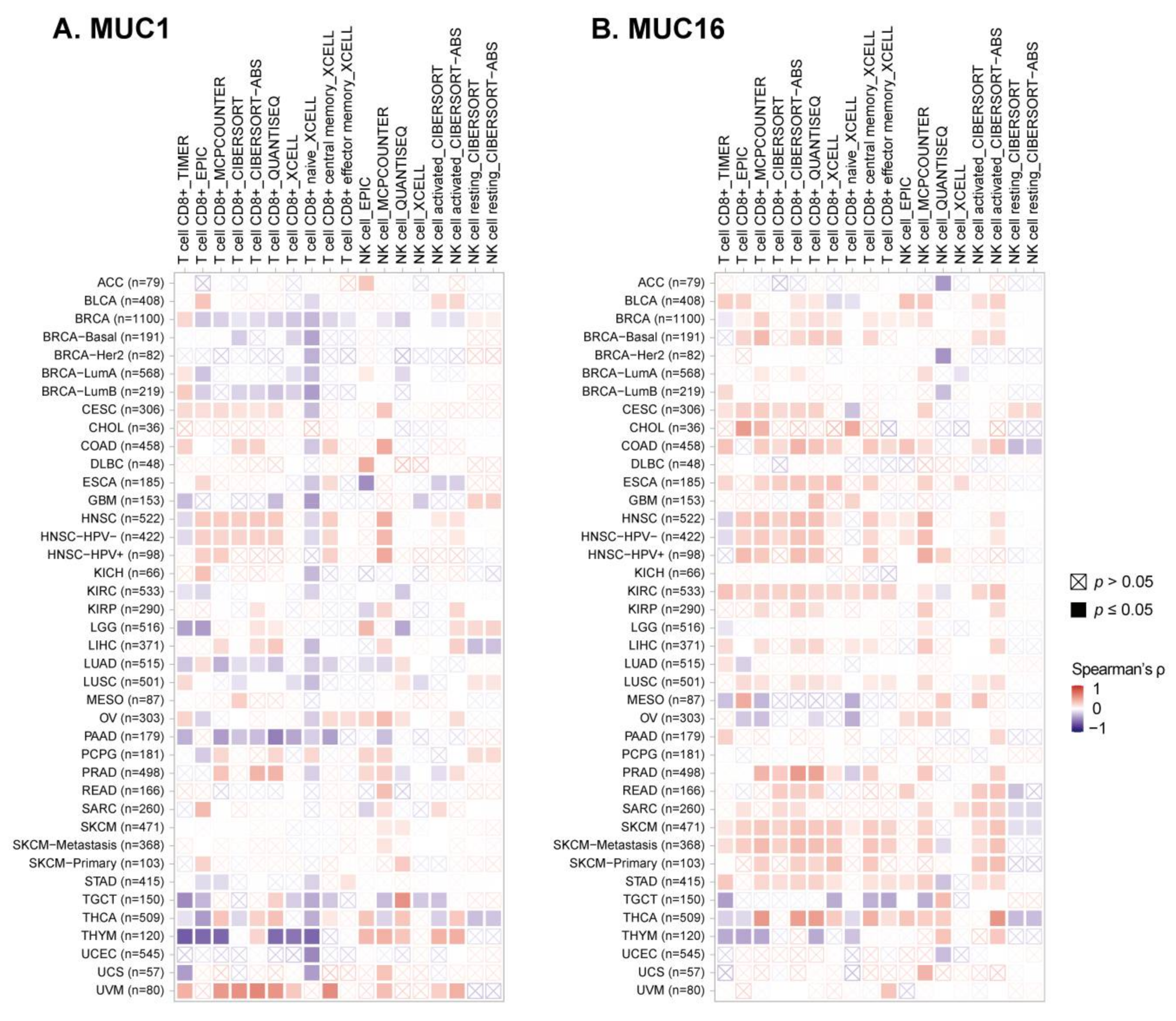
3.5. Avoiding Immune Surveillance
4. Targeting Transmembrane Mucins for Cancer Treatment
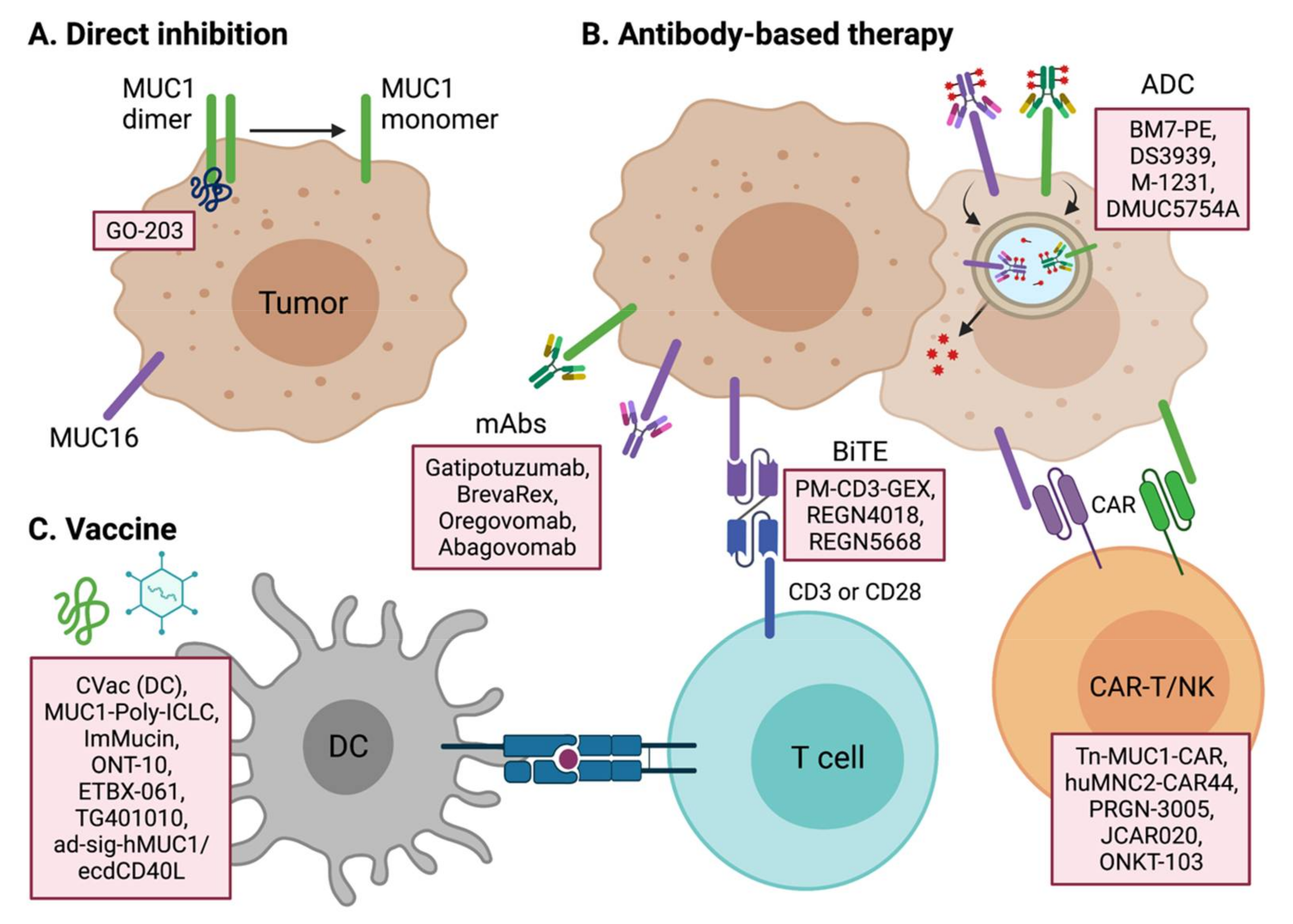
4.1. Therapeutic Targeting of MUC1
4.2. Therapeutic Targeting of MUC16 and Other Mucins
4.3. Tumor Vaccines
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, J.; Rossen, J.W.; Buller, H.A.; Einerhand, A.W. The MUC family: An obituary. Trends Biochem. Sci. 2002, 27, 126–131. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Gendler, S.J.; Lancaster, C.A.; Taylor-Papadimitriou, J.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Lalani, E.N.; Wilson, D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990, 265, 15286–15293. [Google Scholar] [CrossRef]
- Batra, S.K.; Hollingsworth, M.A. Expression of the human mucin gene, Muc 1, in normal tissues and metastatic pancreatic tumors. Int. J. Pancreatol. 1991, 10, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.S.; Batra, S.K.; Qi, W.N.; Metzgar, R.S.; Hollingsworth, M.A. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem. 1990, 265, 15294–15299. [Google Scholar] [CrossRef]
- Patton, S.; Gendler, S.J.; Spicer, A.P. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim. Biophys. Acta 1995, 1241, 407–423. [Google Scholar] [CrossRef]
- Gendler, S.J. MUC1, the renaissance molecule. J. Mammary Gland Biol. Neoplasia 2001, 6, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, D.; Chen, D.; Kharbanda, S.; Kufe, D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene 2003, 22, 6107–6110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cozzi, P.J. MUC1 is a promising therapeutic target for prostate cancer therapy. Curr. Cancer Drug. Targets 2007, 7, 259–271. [Google Scholar] [CrossRef]
- Joshi, M.D.; Ahmad, R.; Yin, L.; Raina, D.; Rajabi, H.; Bubley, G.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol. Cancer Ther. 2009, 8, 3056–3065. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.F.; Yang, E.; Li, J.; Xing, P.X. MUC1 cytoplasmic tail: A potential therapeutic target for ovarian carcinoma. Exp. Rev. Anticancer Ther. 2006, 6, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Zeimet, A.G.; Offner, F.A.; Muller-Holzner, E.; Widschwendter, M.; Abendstein, B.; Fuith, L.C.; Daxenbichler, G.; Marth, C. Peritoneum and tissues of the female reproductive tract as physiological sources of CA-125. Tumour Biol. 1998, 19, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Argueso, P.; Spurr-Michaud, S.; Russo, C.L.; Tisdale, A.; Gipson, I.K. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2487–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, Y.; Endo, K.; Kawamura, Y.; Yoshida, T.; Saga, T.; Watanabe, Y.; Koizumi, M.; Nakashima, T.; Konishi, J.; Yamaguchi, N.; et al. Normal bronchial mucus contains high levels of cancer-associated antigens, CA125, CA19-9, and carcinoembryonic antigen. Cancer 1990, 65, 506–510. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Xu, F.J.; Yu, Y.H.; Barnhill, S.; Zhang, Z.; Mills, G.B. CA 125: The past and the future. Int. J. Biol. Markers 1998, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Capstick, V.; Maclean, G.D.; Suresh, M.R.; Bodnar, D.; Lloyd, S.; Shepert, L.; Longenecker, B.M.; Krantz, M. Clinical evaluation of a new two-site assay for CA125 antigen. Int. J. Biol. Markers 1991, 6, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Samant, U.; Hyland, S.; Chaudhari, P.R.; Wels, W.S.; Bandyopadhyay, D. Target-specific cytotoxic activity of recombinant immunotoxin scFv(MUC1)-ETA on breast carcinoma cells and primary breast tumors. Mol. Cancer Ther. 2007, 6, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Bandyopadhyay, D. MUC1: A target molecule for cancer therapy. Cancer Biol. Ther. 2007, 6, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.G. O-glycosylation of the mucin type. Biol. Chem. 2001, 382, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L., 3rd; Rossi, E.A.; Komatsu, M.; Price-Schiavi, S.A.; Huang, D.; Guy, P.M.; Carvajal, M.E.; Fregien, N.; Carraway, C.A.; Carraway, K.L. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J. Biol. Chem. 1999, 274, 5263–5266. [Google Scholar] [CrossRef] [Green Version]
- Al Masri, A.; Gendler, S.J. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene 2005, 24, 5799–5808. [Google Scholar] [CrossRef] [Green Version]
- Kinlough, C.L.; Poland, P.A.; Bruns, J.B.; Harkleroad, K.L.; Hughey, R.P. MUC1 membrane trafficking is modulated by multiple interactions. J. Biol. Chem. 2004, 279, 53071–53077. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bharti, A.; Chen, D.; Gong, J.; Kufe, D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol. Cell Biol. 1998, 18, 7216–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.; Kharbanda, S.; Kufe, D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995, 55, 4000–4003. [Google Scholar]
- Wei, X.; Xu, H.; Kufe, D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol. Cell 2006, 21, 295–305. [Google Scholar] [CrossRef]
- Yamamoto, M.; Bharti, A.; Li, Y.; Kufe, D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 1997, 272, 12492–12494. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Xu, H.; Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 2005, 7, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Oosterkamp, H.M.; Scheiner, L.; Stefanova, M.C.; Lloyd, K.O.; Finstad, C.L. Comparison of MUC-1 mucin expression in epithelial and non-epithelial cancer cell lines and demonstration of a new short variant form (MUC-1/Z). Int. J. Cancer 1997, 72, 87–94. [Google Scholar] [CrossRef]
- Zrihan-Licht, S.; Vos, H.L.; Baruch, A.; Elroy-Stein, O.; Sagiv, D.; Keydar, I.; Hilkens, J.; Wreschner, D.H. Characterization and molecular cloning of a novel MUC1 protein, devoid of tandem repeats, expressed in human breast cancer tissue. Eur. J. Biochem. 1994, 224, 787–795. [Google Scholar] [CrossRef]
- Zhang, L.; Vlad, A.; Milcarek, C.; Finn, O.J. Human mucin MUC1 RNA undergoes different types of alternative splicing resulting in multiple isoforms. Cancer Immunol. Immunother. 2013, 62, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Ramasamy, S.; Kharbanda, S.; Kufe, D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16. Gene 2006, 373, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Blalock, T.D.; Spurr-Michaud, S.J.; Tisdale, A.S.; Heimer, S.R.; Gilmore, M.S.; Ramesh, V.; Gipson, I.K. Functions of MUC16 in corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4509–4518. [Google Scholar] [CrossRef] [Green Version]
- Akita, K.; Tanaka, M.; Tanida, S.; Mori, Y.; Toda, M.; Nakada, H. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial cancer cells reduces cell-cell adhesion. Eur. J. Cell Biol. 2013, 92, 257–263. [Google Scholar] [CrossRef]
- Tsutsumida, H.; Goto, M.; Kitajima, S.; Kubota, I.; Hirotsu, Y.; Wakimoto, J.; Batra, S.K.; Imai, K.; Yonezawa, S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer 2007, 55, 195–203. [Google Scholar] [CrossRef]
- Hanaoka, J.; Kontani, K.; Sawai, S.; Ichinose, M.; Tezuka, N.; Inoue, S.; Fujino, S.; Ohkubo, I. Analysis of MUC4 mucin expression in lung carcinoma cells and its immunogenicity. Cancer 2001, 92, 2148–2157. [Google Scholar] [CrossRef]
- Guddo, F.; Giatromanolaki, A.; Koukourakis, M.I.; Reina, C.; Vignola, A.M.; Chlouverakis, G.; Hilkens, J.; Gatter, K.C.; Harris, A.L.; Bonsignore, G. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J. Clin. Pathol. 1998, 51, 667–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakha, E.A.; Boyce, R.W.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.; Ellis, I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Sekine, H.; Ohno, T.; Abe, M.; Keefe, K.; Kufe, D.W. Use of a murine monoclonal antibody for detection of circulating plasma DF3 antigen levels in breast cancer patients. J. Clin. Investig. 1985, 75, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolopoulos, V.; McKenzie, I.F. Cellular mucins: Targets for immunotherapy. Crit. Rev. Immunol. 1994, 14, 293–309. [Google Scholar] [CrossRef]
- Choudhury, A.; Moniaux, N.; Winpenny, J.P.; Hollingsworth, M.A.; Aubert, J.P.; Batra, S.K. Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J. Biochem. 2000, 128, 233–243. [Google Scholar] [CrossRef]
- Balague, C.; Gambus, G.; Carrato, C.; Porchet, N.; Aubert, J.P.; Kim, Y.S.; Real, F.X. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology 1994, 106, 1054–1061. [Google Scholar] [CrossRef]
- Andrianifahanana, M.; Moniaux, N.; Schmied, B.M.; Ringel, J.; Friess, H.; Hollingsworth, M.A.; Buchler, M.W.; Aubert, J.P.; Batra, S.K. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: A potential role of MUC4 as a tumor marker of diagnostic significance. Clin. Cancer Res. 2001, 7, 4033–4040. [Google Scholar]
- Wang, R.Q.; Fang, D.C. Alterations of MUC1 and MUC3 expression in gastric carcinoma: Relevance to patient clinicopathological features. J. Clin. Pathol. 2003, 56, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Utsunomiya, T.; Yonezawa, S.; Sakamoto, H.; Kitamura, H.; Hokita, S.; Aiko, T.; Tanaka, S.; Irimura, T.; Kim, Y.S.; Sato, E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: Its relationship with the prognosis of the patients. Clin. Cancer Res. 1998, 4, 2605–2614. [Google Scholar]
- Walsh, M.D.; Young, J.P.; Leggett, B.A.; Williams, S.H.; Jass, J.R.; McGuckin, M.A. The MUC13 cell surface mucin is highly expressed by human colorectal carcinomas. Hum. Pathol. 2007, 38, 883–892. [Google Scholar] [CrossRef]
- Duncan, T.J.; Watson, N.F.; Al-Attar, A.H.; Scholefield, J.H.; Durrant, L.G. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J. Surg. Oncol. 2007, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, H.A.; Bast, R.C. CA 125 in ovarian cancer: Advances and controversy. Clin. Chem. 1998, 44, 1379–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, S.C.; Singh, A.P.; Ruiz, F.; Johansson, S.L.; Jain, M.; Smith, L.M.; Moniaux, N.; Batra, S.K. Aberrant expression of MUC4 in ovarian carcinoma: Diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod. Pathol. 2006, 19, 1386–1394. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regad, T. Targeting RTK Signaling Pathways in Cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Yu, W.; Li, Q.; Kuwahara, H.; Yin, L.; Carraway, K.L., 3rd; Kufe, D. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J. Biol. Chem. 2001, 276, 35239–35242. [Google Scholar] [CrossRef] [Green Version]
- Pochampalli, M.R.; el Bejjani, R.M.; Schroeder, J.A. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene 2007, 26, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.A.; Thompson, M.C.; Gardner, M.M.; Gendler, S.J. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 2001, 276, 13057–13064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Raina, D.; Chen, W.; Li, G.; Huang, L.; Kufe, D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol. Cancer Res. 2006, 4, 873–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Liao, X.; Lv, Y.; Pang, Z.; Wang, Y.; Li, Q.; Liao, Y.; Ye, Q.; Chen, G.; Zhao, K.; et al. MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death Dis. 2017, 8, e2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, S.; Daneshvar, K.; Roy, L.D.; Grover, P.; Kidiyoor, A.; Mosley, L.; Sahraei, M.; Mukherjee, P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013, 2, e51. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Agata, N.; Chen, D.; Li, Y.; Yu, W.H.; Huang, L.; Raina, D.; Chen, W.; Kharbanda, S.; Kufe, D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004, 5, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Raina, D.; Kharbanda, S.; Kufe, D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J. Biol. Chem. 2004, 279, 20607–20612. [Google Scholar] [CrossRef] [Green Version]
- Kharbanda, S.; Ren, R.; Pandey, P.; Shafman, T.D.; Feller, S.M.; Weichselbaum, R.R.; Kufe, D.W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 1995, 376, 785–788. [Google Scholar] [CrossRef]
- Raina, D.; Ahmad, R.; Kumar, S.; Ren, J.; Yoshida, K.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J. 2006, 25, 3774–3783. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Raina, D.; Trivedi, V.; Ren, J.; Rajabi, H.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat. Cell Biol. 2007, 9, 1419–1427. [Google Scholar] [CrossRef]
- Yin, L.; Huang, L.; Kufe, D. MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J. Biol. Chem. 2004, 279, 45721–45727. [Google Scholar] [CrossRef] [Green Version]
- Agata, N.; Ahmad, R.; Kawano, T.; Raina, D.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008, 68, 6136–6144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boivin, M.; Lane, D.; Piche, A.; Rancourt, C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol. 2009, 115, 407–413. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Salfity, S.; Seshacharyulu, P.; Rachagani, S.; Thomas, A.; Das, S.; Majhi, P.D.; Nimmakayala, R.K.; Vengoji, R.; Lele, S.M.; et al. MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53. Clin. Cancer Res. 2017, 23, 3906–3917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaika, N.V.; Gebregiworgis, T.; Lewallen, M.E.; Purohit, V.; Radhakrishnan, P.; Liu, X.; Zhang, B.; Mehla, K.; Brown, R.B.; Caffrey, T.; et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 13787–13792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.K.; Gunda, V.; Abrego, J.; Haridas, D.; Mishra, A.; Souchek, J.; Chaika, N.V.; Yu, F.; Sasson, A.R.; Lazenby, A.J.; et al. MUC16-mediated activation of mTOR and c-Myc reprograms pancreatic cancer metabolism. Oncotarget 2015, 6, 19118–19131. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, M.; Ahmad, R.; Alam, M.; Uchida, Y.; Kufe, D. MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS ONE 2011, 6, e28234. [Google Scholar] [CrossRef]
- Turgeon, M.O.; Perry, N.J.S.; Poulogiannis, G. DNA Damage, Repair, and Cancer Metabolism. Front. Oncol. 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Gunda, V.; Souchek, J.; Abrego, J.; Shukla, S.K.; Goode, G.D.; Vernucci, E.; Dasgupta, A.; Chaika, N.V.; King, R.J.; Li, S.; et al. MUC1-Mediated Metabolic Alterations Regulate Response to Radiotherapy in Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 5881–5891. [Google Scholar] [CrossRef] [Green Version]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Pitroda, S.P.; Khodarev, N.N.; Beckett, M.A.; Kufe, D.W.; Weichselbaum, R.R. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 5837–5841. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, P.; Nath, S.; Nye, M.D.; Zhou, R.; Ahmad, M.; Mukherjee, P. SMAD4-independent activation of TGF-beta signaling by MUC1 in a human pancreatic cancer cell line. Oncotarget 2018, 9, 6897–6910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, H.; Alam, M.; Takahashi, H.; Kharbanda, A.; Guha, M.; Ahmad, R.; Kufe, D. MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 2014, 33, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Rajabi, H.; Yamamoto, M.; Jin, C.; Ahmad, R.; Zhang, Y.; Kui, L.; Li, W.; Yasumizu, Y.; Hong, D.; et al. Targeting MUC1-C Inhibits TWIST1 Signaling in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2019, 18, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Muniyan, S.; Haridas, D.; Chugh, S.; Rachagani, S.; Lakshmanan, I.; Gupta, S.; Seshacharyulu, P.; Smith, L.M.; Ponnusamy, M.P.; Batra, S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016, 7, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Wesseling, J.; van der Valk, S.W.; Hilkens, J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol. Biol. Cell 1996, 7, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Baeckstrom, D.; Brevinge, H.; Hansson, G.C. Secreted MUC1 mucins lacking their cytoplasmic part and carrying sialyl-Lewis a and x epitopes from a tumor cell line and sera of colon carcinoma patients can inhibit HL-60 leukocyte adhesion to E-selectin-expressing endothelial cells. J. Cell Biochem. 1996, 60, 538–549. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [Green Version]
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. eLife 2017, 6, e26476. [Google Scholar] [CrossRef] [PubMed]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautes-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Finotello, F.; Mayer, C.; Plattner, C.; Laschober, G.; Rieder, D.; Hackl, H.; Krogsdam, A.; Loncova, Z.; Posch, W.; Wilflingseder, D.; et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Yee, L.; Carraway, K.L. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999, 59, 2229–2236. [Google Scholar]
- Van de Wiel-van Kemenade, E.; Ligtenberg, M.J.; de Boer, A.J.; Buijs, F.; Vos, H.L.; Melief, C.J.; Hilkens, J.; Figdor, C.G. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J. Immunol. 1993, 151, 767–776. [Google Scholar]
- Kim, Y.J.; Borsig, L.; Han, H.L.; Varki, N.M.; Varki, A. Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am. J. Pathol. 1999, 155, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.; Hartnell, A.; Happerfield, L.; Miles, D.W.; Burchell, J.; Taylor-Papadimitriou, J.; Crocker, P.R. Macrophage-tumour cell interactions: Identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 1999, 98, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Brinkman-Van der Linden, E.C.; Varki, A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J. Biol. Chem. 2000, 275, 8625–8632. [Google Scholar] [CrossRef] [Green Version]
- Regimbald, L.H.; Pilarski, L.M.; Longenecker, B.M.; Reddish, M.A.; Zimmermann, G.; Hugh, J.C. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996, 56, 4244–4249. [Google Scholar]
- Agrawal, B.; Krantz, M.J.; Reddish, M.A.; Longenecker, B.M. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat. Med. 1998, 4, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rughetti, A.; Pellicciotta, I.; Biffoni, M.; Backstrom, M.; Link, T.; Bennet, E.P.; Clausen, H.; Noll, T.; Hansson, G.C.; Burchell, J.M.; et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J. Immunol. 2005, 174, 7764–7772. [Google Scholar] [CrossRef] [Green Version]
- Monti, P.; Leone, B.E.; Zerbi, A.; Balzano, G.; Cainarca, S.; Sordi, V.; Pontillo, M.; Mercalli, A.; Di Carlo, V.; Allavena, P.; et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J. Immunol. 2004, 172, 7341–7349. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.A.; Bauer, S.; Lu, W.; Guo, J.; Walter, S.; Bushnell, T.P.; Lillehoj, E.P.; Georas, S.N. Deletion of the mucin-like molecule muc1 enhances dendritic cell activation in response to toll-like receptor ligands. J. Innate Immunol. 2010, 2, 123–143. [Google Scholar] [CrossRef] [Green Version]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seelenmeyer, C.; Wegehingel, S.; Lechner, J.; Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci. 2003, 116, 1305–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belisle, J.A.; Gubbels, J.A.; Raphael, C.A.; Migneault, M.; Rancourt, C.; Connor, J.P.; Patankar, M.S. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007, 122, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Jing, Y.; Morrison, J.C.; Belisle, J.A.; Lattanzio, F.A.; Deng, Y.; Wong, N.K.; Morris, H.R.; Dell, A.; Clark, G.F. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol. Oncol. 2005, 99, 704–713. [Google Scholar] [CrossRef]
- Bouillez, A.; Rajabi, H.; Jin, C.; Samur, M.; Tagde, A.; Alam, M.; Hiraki, M.; Maeda, T.; Hu, X.; Adeegbe, D.; et al. MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene 2017, 36, 4037–4046. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Hiraki, M.; Jin, C.; Rajabi, H.; Tagde, A.; Alam, M.; Bouillez, A.; Hu, X.; Suzuki, Y.; Miyo, M.; et al. MUC1-C Induces PD-L1 and Immune Evasion in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Gaemers, I.C.; Vos, H.L.; Volders, H.H.; van der Valk, S.W.; Hilkens, J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 2001, 276, 6191–6199. [Google Scholar] [CrossRef] [Green Version]
- Morgado, M.; Sutton, M.N.; Simmons, M.; Warren, C.R.; Lu, Z.; Constantinou, P.E.; Liu, J.; Francis, L.L.; Conlan, R.S.; Bast, R.C., Jr.; et al. Tumor necrosis factor-alpha and interferon-gamma stimulate MUC16 (CA125) expression in breast, endometrial and ovarian cancers through NFkappaB. Oncotarget 2016, 7, 14871–14884. [Google Scholar] [CrossRef] [Green Version]
- Cascio, S.; Zhang, L.; Finn, O.J. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-kappaB p65 and binding to cytokine promoters: Importance of extracellular domain. J. Biol. Chem. 2011, 286, 42248–42256. [Google Scholar] [CrossRef] [Green Version]
- Graham, R.A.; Burchell, J.M.; Taylor-Papadimitriou, J. The polymorphic epithelial mucin: Potential as an immunogen for a cancer vaccine. Cancer Immunol. Immunother. 1996, 42, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.; Agarwal, P.; Lee, J.; Bharti, A.; McKnight, C.J.; Sharma, P.; Kharbanda, S.; Kufe, D. Characterization of the MUC1-C Cytoplasmic Domain as a Cancer Target. PLoS ONE 2015, 10, e0135156. [Google Scholar] [CrossRef]
- Ahmad, R.; Alam, M.; Hasegawa, M.; Uchida, Y.; Al-Obaid, O.; Kharbanda, S.; Kufe, D. Targeting MUC1-C inhibits the AKT-S6K1-elF4A pathway regulating TIGAR translation in colorectal cancer. Mol. Cancer 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GongSun, X.; Zhao, Y.; Jiang, B.; Xin, Z.; Shi, M.; Song, L.; Qin, Q.; Wang, Q.; Liu, X. Inhibition of MUC1-C regulates metabolism by AKT pathway in esophageal squamous cell carcinoma. J. Cell Physiol. 2019, 234, 12019–12028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigeta, K.; Hasegawa, M.; Kikuchi, E.; Yasumizu, Y.; Kosaka, T.; Mizuno, R.; Mikami, S.; Miyajima, A.; Kufe, D.; Oya, M. Role of the MUC1-C oncoprotein in the acquisition of cisplatin resistance by urothelial carcinoma. Cancer Sci. 2020, 111, 3639–3652. [Google Scholar] [CrossRef]
- Raina, D.; Uchida, Y.; Kharbanda, A.; Rajabi, H.; Panchamoorthy, G.; Jin, C.; Kharbanda, S.; Scaltriti, M.; Baselga, J.; Kufe, D. Targeting the MUC1-C oncoprotein downregulates HER2 activation and abrogates trastuzumab resistance in breast cancer cells. Oncogene 2014, 33, 3422–3431. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Jin, C.; Hata, T.; Yasumizu, Y.; Zhang, Y.; Hong, D.; Maeda, T.; Miyo, M.; Hiraki, M.; Suzuki, Y.; et al. MUC1-C Integrates Chromatin Remodeling and PARP1 Activity in the DNA Damage Response of Triple-Negative Breast Cancer Cells. Cancer Res. 2019, 79, 2031–2041. [Google Scholar] [CrossRef] [Green Version]
- Bouillez, A.; Rajabi, H.; Pitroda, S.; Jin, C.; Alam, M.; Kharbanda, A.; Tagde, A.; Wong, K.K.; Kufe, D. Inhibition of MUC1-C Suppresses MYC Expression and Attenuates Malignant Growth in KRAS Mutant Lung Adenocarcinomas. Cancer Res. 2016, 76, 1538–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Kufe, T.; Avigan, D.; Kufe, D. Targeting MUC1-C is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated myeloma cell death. Blood 2014, 123, 2997–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Tagde, A.; Gali, R.; Tai, Y.T.; Hideshima, T.; Anderson, K.; Avigan, D.; Kufe, D. MUC1-C is a target in lenalidomide resistant multiple myeloma. Br. J. Haematol. 2017, 178, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Washington, A.; Leaf, R.K.; Bhargava, P.; Clark, R.A.; Kupper, T.S.; Stroopinsky, D.; Pyzer, A.; Cole, L.; Nahas, M.; et al. Decitabine Priming Enhances Mucin 1 Inhibition Mediated Disruption of Redox Homeostasis in Cutaneous T-Cell Lymphoma. Mol. Cancer Ther. 2017, 16, 2304–2314. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yin, L.; Stroopinsky, D.; Rajabi, H.; Puissant, A.; Stegmaier, K.; Avigan, D.; Kharbanda, S.; Kufe, D.; Stone, R. MUC1-C oncoprotein promotes FLT3 receptor activation in acute myeloid leukemia cells. Blood 2014, 123, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Bouillez, A.; Adeegbe, D.; Jin, C.; Hu, X.; Tagde, A.; Alam, M.; Rajabi, H.; Wong, K.K.; Kufe, D. MUC1-C promotes the suppressive immune microenvironment in non-small cell lung cancer. Oncoimmunology 2017, 6, e1338998. [Google Scholar] [CrossRef] [Green Version]
- Perepelyuk, M.; Sacko, K.; Thangavel, K.; Shoyele, S.A. Evaluation of MUC1-Aptamer Functionalized Hybrid Nanoparticles for Targeted Delivery of miRNA-29b to Nonsmall Cell Lung Cancer. Mol. Pharm. 2018, 15, 985–993. [Google Scholar] [CrossRef]
- Sacko, K.; Thangavel, K.; Shoyele, S.A. Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates. Nanomaterials 2019, 9, 1052. [Google Scholar] [CrossRef] [Green Version]
- Engebraaten, O.; Sivam, G.; Juell, S.; Fodstad, O. Systemic immunotoxin treatment inhibits formation of human breast cancer metastasis and tumor growth in nude rats. Int. J. Cancer 2000, 88, 970–976. [Google Scholar] [CrossRef]
- Wu, G.; Kim, D.; Kim, J.N.; Park, S.; Maharjan, S.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.J. A Mucin1 C-terminal Subunit-directed Monoclonal Antibody Targets Overexpressed Mucin1 in Breast Cancer. Theranostics 2018, 8, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Maharjan, S.; Kim, D.; Kim, J.N.; Park, B.K.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.J. A Novel Monoclonal Antibody Targets Mucin1 and Attenuates Growth in Pancreatic Cancer Model. Int. J. Mol. Sci. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielczyk, A.; Stahn, R.; Faulstich, D.; Loffler, A.; Marten, A.; Karsten, U.; Goletz, S. PankoMab: A potent new generation anti-tumour MUC1 antibody. Cancer Immunol. Immunother. 2006, 55, 1337–1347. [Google Scholar] [CrossRef]
- Posey, A.D., Jr.; Schwab, R.D.; Boesteanu, A.C.; Steentoft, C.; Mandel, U.; Engels, B.; Stone, J.D.; Madsen, T.D.; Schreiber, K.; Haines, K.M.; et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016, 44, 1444–1454. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, P.; Scatena, R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Schultes, B.C.; Baum, R.P.; Niesen, A.; Noujaim, A.A.; Madiyalakan, R. Anti-idiotype induction therapy: Anti-CA125 antibodies (Ab3) mediated tumor killing in patients treated with Ovarex mAb B43.13 (Ab1). Cancer Immunol. Immunother. 1998, 46, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Pietragalla, A.; Duranti, S.; Daniele, G.; Nero, C.; Ciccarone, F.; Lorusso, D.; Fagotti, A.; Scambia, G. Oregovomab: An investigational agent for the treatment of advanced ovarian cancer. Expert Opin. Investig. Drugs 2021, 30, 103–110. [Google Scholar] [CrossRef]
- Brewer, M.; Angioli, R.; Scambia, G.; Lorusso, D.; Terranova, C.; Panici, P.B.; Raspagliesi, F.; Scollo, P.; Plotti, F.; Ferrandina, G.; et al. Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: A randomized phase II study. Gynecol. Oncol. 2020, 156, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.; Buzzonetti, A.; Fossati, M.; Scambia, G.; Fattorossi, A.; Madiyalakan, M.R.; Mahnke, Y.D.; Nicodemus, C. Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients. Cancer Immunol. Immunother. 2020, 69, 383–397. [Google Scholar] [CrossRef]
- Berek, J.S.; Taylor, P.T.; Gordon, A.; Cunningham, M.J.; Finkler, N.; Orr, J., Jr.; Rivkin, S.; Schultes, B.C.; Whiteside, T.L.; Nicodemus, C.F. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J. Clin. Oncol. 2004, 22, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.; Taylor, P.; McGuire, W.; Smith, L.M.; Schultes, B.; Nicodemus, C.F. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J. Clin. Oncol. 2009, 27, 418–425. [Google Scholar] [CrossRef]
- Reinartz, S.; Kohler, S.; Schlebusch, H.; Krista, K.; Giffels, P.; Renke, K.; Huober, J.; Mobus, V.; Kreienberg, R.; DuBois, A.; et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: Immunological response and survival (phase Ib/II). Clin. Cancer Res. 2004, 10, 1580–1587. [Google Scholar] [CrossRef] [Green Version]
- Sabbatini, P.; Harter, P.; Scambia, G.; Sehouli, J.; Meier, W.; Wimberger, P.; Baumann, K.H.; Kurzeder, C.; Schmalfeldt, B.; Cibula, D.; et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: A phase III trial of the AGO OVAR, COGI, GINECO, and GEICO--the MIMOSA study. J. Clin. Oncol. 2013, 31, 1554–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzonetti, A.; Fossati, M.; Catzola, V.; Scambia, G.; Fattorossi, A.; Battaglia, A. Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: Relationship with survival-a substudy of the MIMOSA trial. Cancer Immunol. Immunother. 2014, 63, 1037–1045. [Google Scholar] [CrossRef]
- Battaglia, A.; Fossati, M.; Buzzonetti, A.; Scambia, G.; Fattorossi, A. A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients. Immunol. Lett. 2017, 191, 35–39. [Google Scholar] [CrossRef]
- Liu, J.F.; Moore, K.N.; Birrer, M.J.; Berlin, S.; Matulonis, U.A.; Infante, J.R.; Wolpin, B.; Poon, K.A.; Firestein, R.; Xu, J.; et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 2016, 27, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Haber, L.; Kelly, M.P.; Vazzana, K.; Canova, L.; Ram, P.; Pawashe, A.; Finney, J.; Jalal, S.; Chiu, D.; et al. A Mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci. Transl. Med. 2019, 11, 7534. [Google Scholar] [CrossRef]
- Khan, S.; Zafar, N.; Khan, S.S.; Setua, S.; Behrman, S.W.; Stiles, Z.E.; Yallapu, M.M.; Sahay, P.; Ghimire, H.; Ise, T.; et al. Clinical significance of MUC13 in pancreatic ductal adenocarcinoma. HPB 2018, 20, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Nishii, Y.; Yamaguchi, M.; Kimura, Y.; Hasegawa, T.; Aburatani, H.; Uchida, H.; Hirata, K.; Sakuma, Y. A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells. Int. J. Oncol. 2015, 46, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.C.; Ebeling, M.C.; Maher, D.M.; Koch, M.D.; Watanabe, A.; Aburatani, H.; Lio, Y.; Jaggi, M. MUC13 mucin augments pancreatic tumorigenesis. Mol. Cancer Ther. 2012, 11, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.L.; Quinn, M.A.; Grant, P.T.; Allen, D.G.; Jobling, T.W.; White, S.C.; Zhao, A.; Karanikas, V.; Vaughan, H.; Pietersz, G.; et al. A phase 2, single-arm study of an autologous dendritic cell treatment against mucin 1 in patients with advanced epithelial ovarian cancer. J. Immunother. Cancer 2014, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.J.; Benigno, B.; Berek, J.; Chang, J.; Mason, J.; Mileshkin, L.; Mitchell, P.; Moradi, M.; Recio, F.O.; Michener, C.M.; et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J. Immunother. Cancer 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Kovjazin, R.; Volovitz, I.; Kundel, Y.; Rosenbaum, E.; Medalia, G.; Horn, G.; Smorodinsky, N.I.; Brenner, B.; Carmon, L. ImMucin: A novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine 2011, 29, 4676–4686. [Google Scholar] [CrossRef]
- Carmon, L.; Avivi, I.; Kovjazin, R.; Zuckerman, T.; Dray, L.; Gatt, M.E.; Or, R.; Shapira, M.Y. Phase I/II study exploring ImMucin, a pan-major histocompatibility complex, anti-MUC1 signal peptide vaccine, in multiple myeloma patients. Br. J. Haematol. 2015, 169, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, R.K.; Lee, K.M.; McKolanis, J.; Hitbold, E.; Schraut, W.; Moser, A.J.; Warnick, E.; Whiteside, T.; Osborne, J.; Kim, H.; et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol. Immunother. 2005, 54, 254–264. [Google Scholar] [CrossRef]
- Lepisto, A.J.; Moser, A.J.; Zeh, H.; Lee, K.; Bartlett, D.; McKolanis, J.R.; Geller, B.A.; Schmotzer, A.; Potter, D.P.; Whiteside, T.; et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008, 6, 955–964. [Google Scholar] [PubMed]
- Pestano, L.A.; Christian, B.; Koppenol, S.; Millard, J.; Christianson, G.; Klucher, K.; Rosler, R.; Peterson, S.R. Abstract 762: ONT-10, a liposomal vaccine targeting hypoglycosylated MUC1, induces a potent cellular and humoral response and suppresses the growth of MUC1 expressing tumors. Cancer Res. 2011, 71, 762. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Bedell, C.; Klucher, K.; Vo, A.; Whiting, S. Phase 1 dose escalation of ONT-10, a therapeutic MUC1 vaccine, in patients with advanced cancer. J. Immunother. Cancer 2013, 1, P240. [Google Scholar] [CrossRef] [Green Version]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.E.; Bosquee, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Gatti-Mays, M.E.; Redman, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Bilusic, M.; Sater, H.A.; Marte, J.L.; Cordes, L.M.; et al. A Phase I Trial Using a Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury)-Based Immunotherapy Vaccine Regimen in Patients with Advanced Cancer. Oncologist 2020, 25, 479.e899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quoix, E.; Lena, H.; Losonczy, G.; Forget, F.; Chouaid, C.; Papai, Z.; Gervais, R.; Ottensmeier, C.; Szczesna, A.; Kazarnowicz, A.; et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): Results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016, 17, 212–223. [Google Scholar] [CrossRef]
- Deisseroth, A.; Tang, Y.; Zhang, L.; Akbulut, H.; Habib, N. TAA/ecdCD40L adenoviral prime-protein boost vaccine for cancer and infectious diseases. Cancer Gene Ther. 2013, 20, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.J.Y.; Chia, J.W.K.; Chong, H.-S.; Li, X.; Tan, S.H.; Hopkins, R.; Wang, W.-W.; Toh, H.C. First-in-man study of Ad-sig-hMUC1/ecdCD40L vaccine for immunotherapy of MUC1 overexpressing epithelial cancers. J. Clin. Oncol. 2018, 36, 3098. [Google Scholar] [CrossRef]
| Drug Name | Company | Drug Type | Indication | Development Status | Identifiers or References | Recruitment Status | Start Date |
|---|---|---|---|---|---|---|---|
| Anti-mucin-1 antibodies | University of California Davis | scFv antibody | Cancer | ||||
| Mucin-1 inhibitor | Quest PharmaTech | IgE monoclonal antibody | Cancer | Preclinical | |||
| MUC1 inhibitors | Minerva Biotechnologies Corp. | Humanized single chain antibody | Cancer | Discovery | |||
| Yttrium civatuzumab tetraxetan | Garden State Cancer Center | Radio-labeled antibody | Cancer | ||||
| Pab-001 | Peptron | ADC | Cancer | Preclinical | |||
| DS-3939 | Daiichi Sankyo | ADC | Cancer | Preclinical | |||
| SPmAb | Vaxil BioTherapeutics | Monoclonal antibody | Cancer | Preclinical | |||
| BrevaRex | Quest PharmaTech | Anti-idiotype mAb | Multiple Myeloma, Cancer | Phase 2 | |||
| BM7-PE | Oslo University Hospital | ADC | Metastatic colorectal cancer | Phase 2 | NCT04550897 | Recruiting | 31 August 2020 |
| Gatipotuzumab (PankoMab-GEX) | Glycotope GmbH | IgG1 antibody | Advanced ovarian cancer | Phase 2 | NCT01899599 | Completed | September 2013 |
| PM-CD3-GEX | Glycotope GmbH | BiTE | NSCLC, Ovary tumor | Preclinical | |||
| PM-IL15-GEX | Glycotope GmbH | Antibody fused to IL-15 | Solid tumor | Preclinical | |||
| PM-PDL-GEX | Glycotope GmbH | Trifunctional antibody (MUC1, PD-L1, and FcγR) | Ovary tumor | Preclinical | |||
| HuMab-Tn | MabVax Therapeutics Holdings | Monoclonal antibody | Cancer | Discovery | |||
| M-1231 | EMD Serono; Sutro Biopharma | ADC | Metastatic cancer | Phase 1 | NCT04695847 | Recruiting | 13 January 2021 |
| Anti-CD3/anti-MUC1 BiTE CIK cell therapy | Benhealth Biopharmaceutical (Shenzhen) | BiTE; Cell therapy | Metastatic cancer | Phase 2 | NCT03146637 | Recruiting | 1 May 2017 |
| GO-203-2C | Genus Oncology LLC | Peptide | R/R AML | Phase 2 | NCT02204085 | Active, not recruiting | September 2014 |
| GO-203-2C | Genus Oncology LLC | Peptide | Solid tumor | Phase 1 | NCT01279603 | Completed | 19 January 2011 |
| Mucin1-aptamer | Thomas Jefferson University | Aptamer | NSCLC | Preclinical | |||
| rHSP-MUC1 fusion protein | Chengdu Xinlibang Bio-pharmaceutical | Fusion protein | Breast tumor | Phase 1 | |||
| P-MUC1C-ALLO1 | Poseida Therapeutics | CAR-T | Solid tumor | Preclinical | |||
| P-MUC1C-101 | Poseida Therapeutics | CAR-T | Solid tumor | Preclinical | |||
| ICTCAR-052 | Innovative Cellular Therapeutics | CAR-T | Breast tumor | Phase 1 | |||
| ICTCAR-046 | Innovative Cellular Therapeutics | CAR-T | Pancreatic tumor | Phase 1 | |||
| ICTCAR-053 | Innovative Cellular Therapeutics | CAR-T | Pancreatic tumor | Phase 1 | |||
| ICTCAR-043 | Innovative Cellular Therapeutics | CAR-T | Breast tumor | Phase 1 | |||
| Tn MUC-1 CAR-T | Tmunity Therapeutics | CAR-T | Cancer | Phase 1 | NCT04025216 | Recruiting | 10 October 2019 |
| Anti-MUC1 CAR-T cell therapy (w or w/o) PD-1 knockout T cell | Guangzhou Anjie Biomedical Technology | CAR-T | Metastatic cancer | Phase 2/3 | NCT03525782 | Recruiting | 1 February 2018 |
| MUC-1 pCAR | Leucid Bio | CAR-T | Cancer | Preclinical | |||
| huMNC2-CAR44 T cells | Minerva Biotechnologies Corp. | CAR-T | Cancer | Phase 1 | NCT04020575 | Recruiting | 15 January 2020 |
| Anti-MUC1 CAR-pNK | PersonGen Biomedicine (Suzhou) | CAR-NK | MUC1-positive R/R solid tumor | Phase 2 | NCT02839954 | Unknown | 21 July 2016 |
| Anti-MUC1 CAR-T cell therapy | PersonGen Biomedicine (Suzhou) | CAR-T | MUC1-positive advanced refractory solid tumor | Phase 2 | NCT02587689 | Unknown | 27 October 2015 |
| TAB-28z | OncoTab | CAR-T | Breast tumor | Preclinical | |||
| Anti-MUC-1 CAR-T cell therapy | Baylor College of Medicine | CAR-T | Breast tumor | Preclinical | |||
| ONKT-103 | ONK Therapeutics | CAR-NK | Solid tumor | Preclinical | |||
| Anti-MUC1 CAR-T cell therapy + anti-CTLA4 + anti-PD-1 antibodies | Shanghai Cell Therapy Research Institute | CAR-T | MUC1-positive advanced solid tumor | Phase 2 | NCT03179007 | Unknown | 7 June 2017 |
| Drug Name | Company | Drug Type | Indication | Development Status | Identifiers or References | Recruitment Status | Start Date |
|---|---|---|---|---|---|---|---|
| Mab-AR-9.6 | Quest PharmaTech | Monoclonal antibody | Pancreas tumor | Preclinical | |||
| Oregovomab (OvaRex) | Quest PharmaTech | Monoclonal antibody; Anti-idiotype induction therapy | Cancer | Phase 3 | NCT04498117 | Recruiting | 25 August 2020 |
| RG-7458 (DMUC-5754A) | Genentech | ADC | Ovarian cancer, Pancreatic cancer | Discontinued | NCT01335958 | Completed | Day, April 2011 |
| RG-7882 (DMUC-4064A) | Genentech | ADC | Ovarian cancer, Pancreatic cancer | Discontinued | NCT02146313 | Completed | 22 June 2014 |
| EDO-772P | Mundipharma EDO GmbH | ADC | Ovarian cancer | ||||
| Abagovomab | Menarini; CellControl Biomedical | Anti-idiotype mAb | Ovarian cancer | Phase 2/3 | NCT00418574 | Terminated (Failed) | Day, December 2006 |
| NAV-005 | Navrogen | IgG1 Fc fusion protein | Cancer | Discovery | |||
| REGN-4018 | Regeneron Pharmaceuticals | BiTE (MUC16/CD3) | Ovarian cancer | Phase 2 | NCT03564340 | Recruiting | 21 May 2018 |
| REGN-5668 | Regeneron Pharmaceuticals | BiTE (MUC16/CD28) | Ovarian cancer | Phase 2 | NCT04590326 | Recruiting | 9 December 2020 |
| PRGN-3005 | Precigen | CAR-T | Ovarian cancer | Phase 1 | NCT03907527 | Recruiting | 30 April 2019 |
| TC-220 | TCR2 Therapeutics | CAR-T | Ovarian cancer | Preclinical | |||
| TC-410 | TCR2 Therapeutics | CAR-T | Ovarian cancer | Discovery | |||
| Anti-MUC16 CAR-T/PD-1 scFv | Eureka Therapeutics | CAR-T | Solid tumor | Preclinical | |||
| JCAR-020 | Bristol-Myers Squibb | CAR-T | Cancer | Phase 1 | NCT02498912 | Active, not recruiting | Day, August 2015 |
| Targeting other mucins | |||||||
| MUC13 antibody | University of Tennessee | Monoclonal antibody | Cancer | Discovery | |||
| AMG-199 | Amgen | BiTE (MUC17/CD3) | Stomach cancer, Esophagus cancer | Phase 1 | NCT04117958 | Recruiting | 20 January 2020 |
| Drug Name | Company | Drug Type | Indication | Development Status | Identifiers or References | Recruitment Status | Start Date |
|---|---|---|---|---|---|---|---|
| Mucin-1 vaccines | |||||||
| Cvac | Sydys/Prima BioMed | Autologous DC vaccine | Ovarian cancer, Colorectal cancer, Triple-negative breast cancer | Phase 2 | NCT01068509 | Completed | July 2010 |
| MUC1-Poly-ICLC | University of Pittsburgh | Peptide vaccine | Colon cancer, NSCLC | Phase 2 | NCT00773097 | Completed | October 2008 |
| MUC1-DC-CTL | Beijing Doing Biomedical Technology | DC vaccine; T-cell stimulator | Stomach tumor | Preclinical | |||
| ETBX-061 | Etubics Corp | Adenovirus vaccine | Colon cancer | Phase 1/2 | NCT03563157 | Active, not recruiting | 25 May 2018 |
| Advanced cancer | Phase 1 | NCT03384316 | Completed | 31 January 2018 | |||
| Hormone refractory prostate cancer | Phase 1 | NCT03481816 | Completed | 24 July 2018 | |||
| ImMucin | Vaxil BioTherapeutics | Peptide vaccine | Breast cancer, Multiple myeloma, Ovary tumor | Phase 2 | NCT01232712 | Completed | September 2010 |
| Emepepimut-S (Tecemotide/Stimuvax/L-BLP25) | Merck Serono | Liposome-encapsulated peptide vaccine | Cancer | Phase 3 Discontinued | NCT00409188 | ||
| MTI | ViaMune/GeoVax | Peptide vaccine | Cancer | Preclinical | |||
| GEO-CM01 | GeoVax | Modified-vaccina Ankara virus-like particles (MVA-VLP) | Cancer | Preclinical | |||
| ONT-10 | Cascadian Therapeutics | Glycopeptide vaccine | Cancer | Phase 1b | NCT02270372 | Completed | November 2014 |
| TG4010 | Transgene SA | MVA | NSCLC | Phase 2/3 (Suspended) | NCT01383148 | Terminated | April 2012 |
| TG4010 Ad-sig-hMUC-1/ecdCD40L vaccine | Transgene SA MicroVAX LLC | MVA Adenovirus vaccine | NSCLC Cancer | Phase 2 | NCT02823990 | Active, not recruiting | 14 December 2016 |
| Phase 1 | NCT02140996 | Unknown | September 2014 | ||||
| GI-6108 | NantCell/Celgene/GlobeImmune | Tarmogen vaccine | Cancer | Preclinical | |||
| Mucin-16 vaccine | |||||||
| OvcaVax | Theralink Technologies | CA-125/IL-2/GM-CSF vaccine | Ovary tumor | Preclinical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Choi, S.; Park, Y.; Jin, H.-s. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals 2021, 14, 1053. https://doi.org/10.3390/ph14101053
Lee D-H, Choi S, Park Y, Jin H-s. Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals. 2021; 14(10):1053. https://doi.org/10.3390/ph14101053
Chicago/Turabian StyleLee, Dong-Hee, Seunghyun Choi, Yoon Park, and Hyung-seung Jin. 2021. "Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy" Pharmaceuticals 14, no. 10: 1053. https://doi.org/10.3390/ph14101053
APA StyleLee, D.-H., Choi, S., Park, Y., & Jin, H.-s. (2021). Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals, 14(10), 1053. https://doi.org/10.3390/ph14101053






