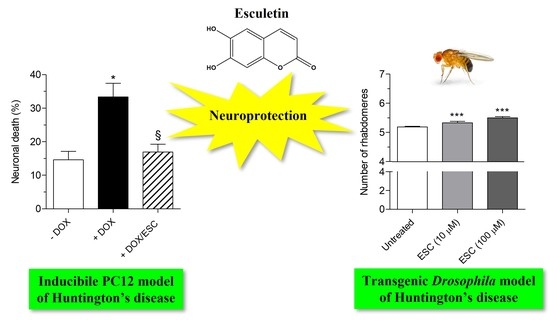
Esculetin Provides Neuroprotection against Mutant Huntingtin-Induced Toxicity in Huntington’s Disease Models
Abstract
1. Introduction
2. Results and Discussion
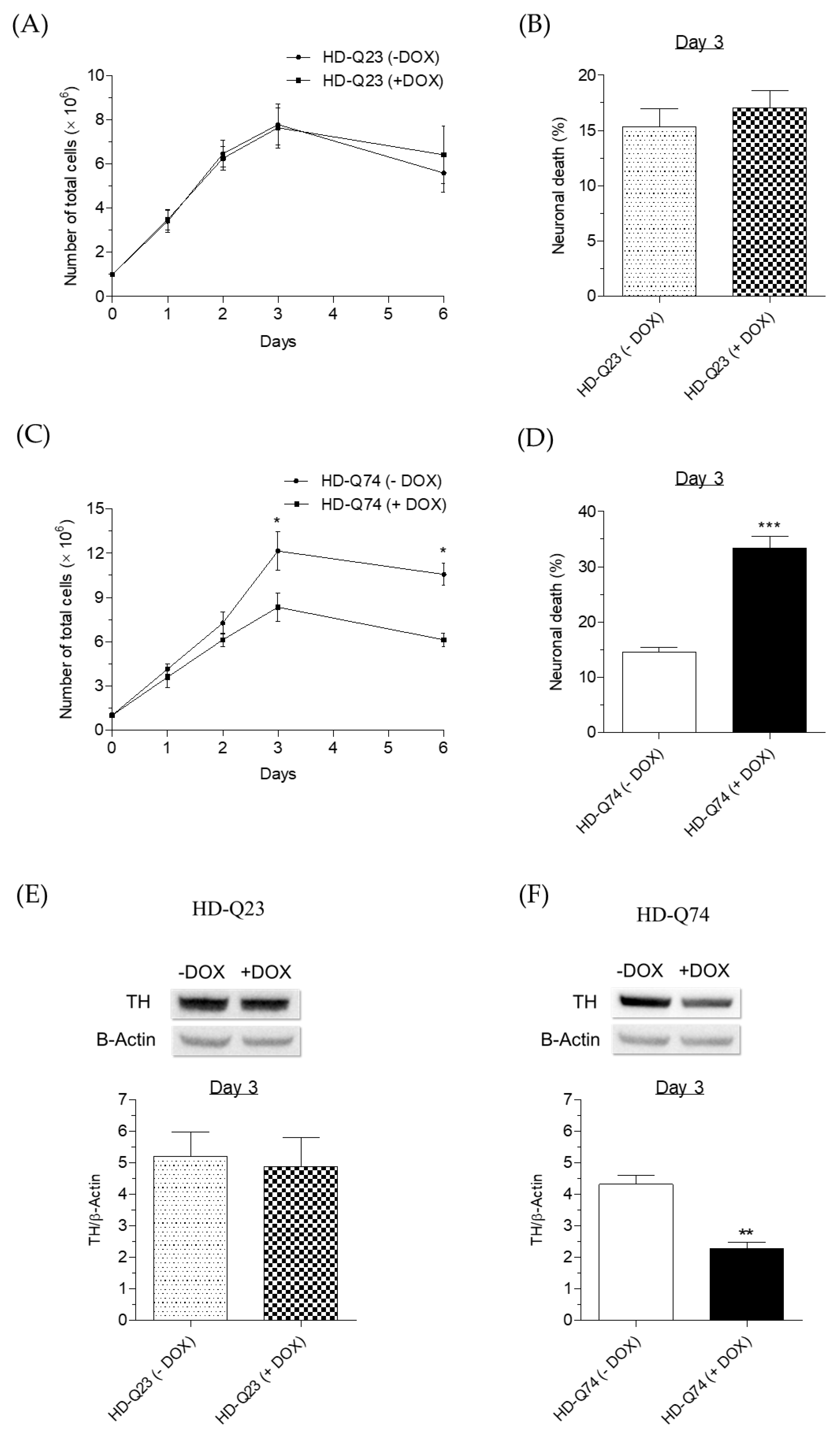
2.1. Neurotoxicity Induced by mHTT in HD-Q74 Cells
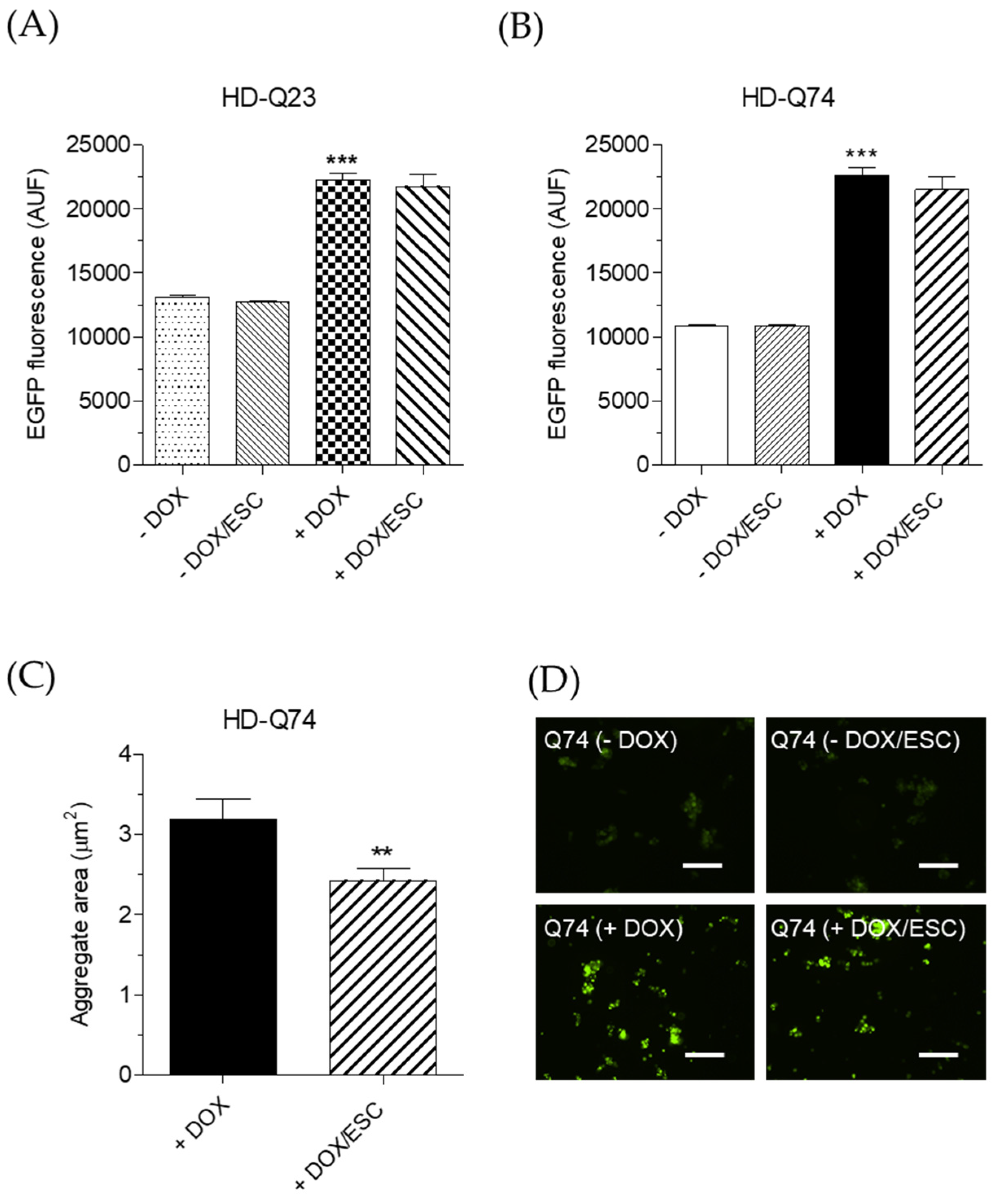
2.2. Effects of ESC on mHTT Aggregation in HD-Q74 Cells
2.3. Neuroprotective Effects of ESC against mHTT Toxicity in HD-Q74 Cells
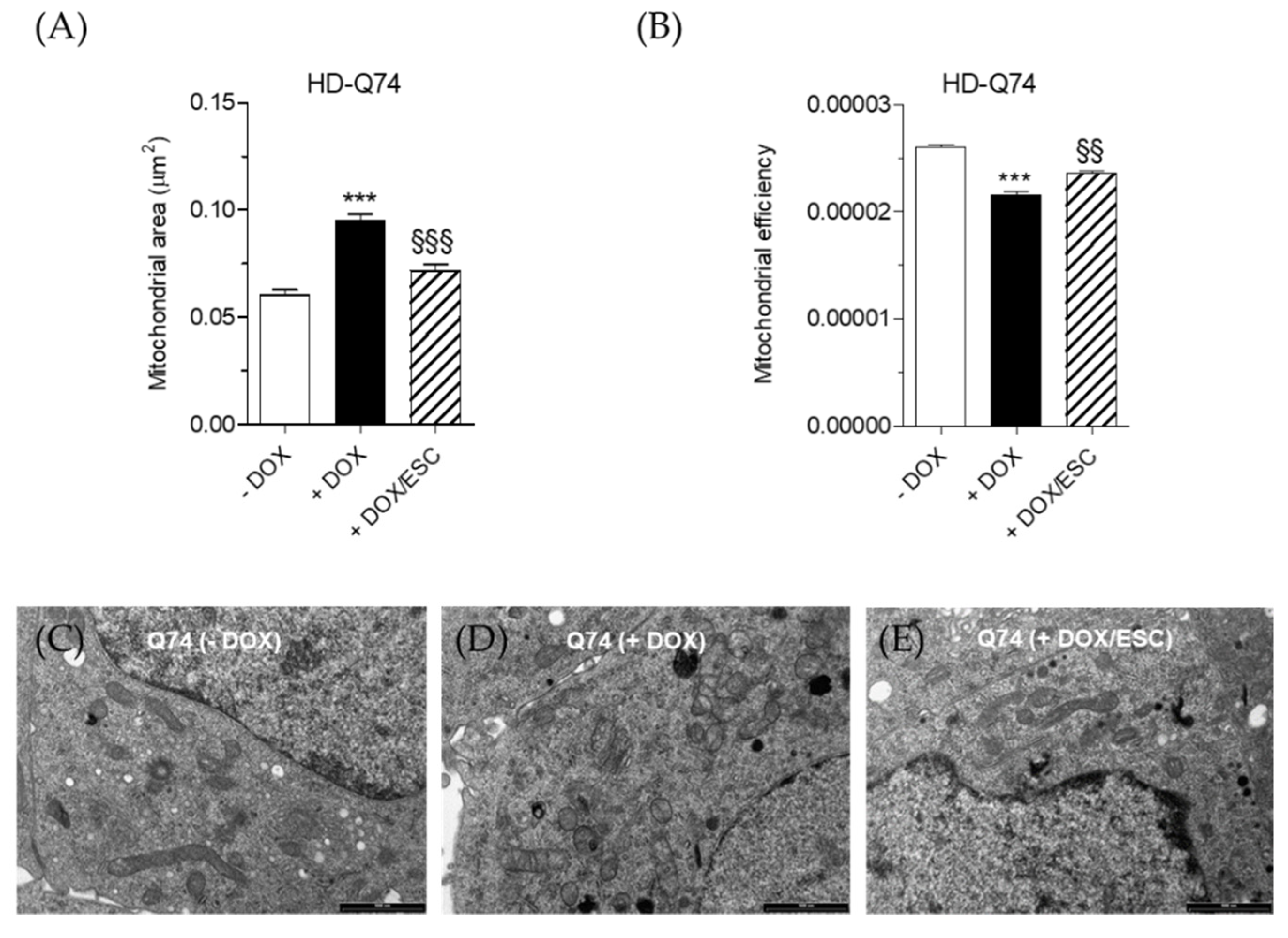
2.4. Effects of ESC on Mitochondrial Activity Altered by mHTT
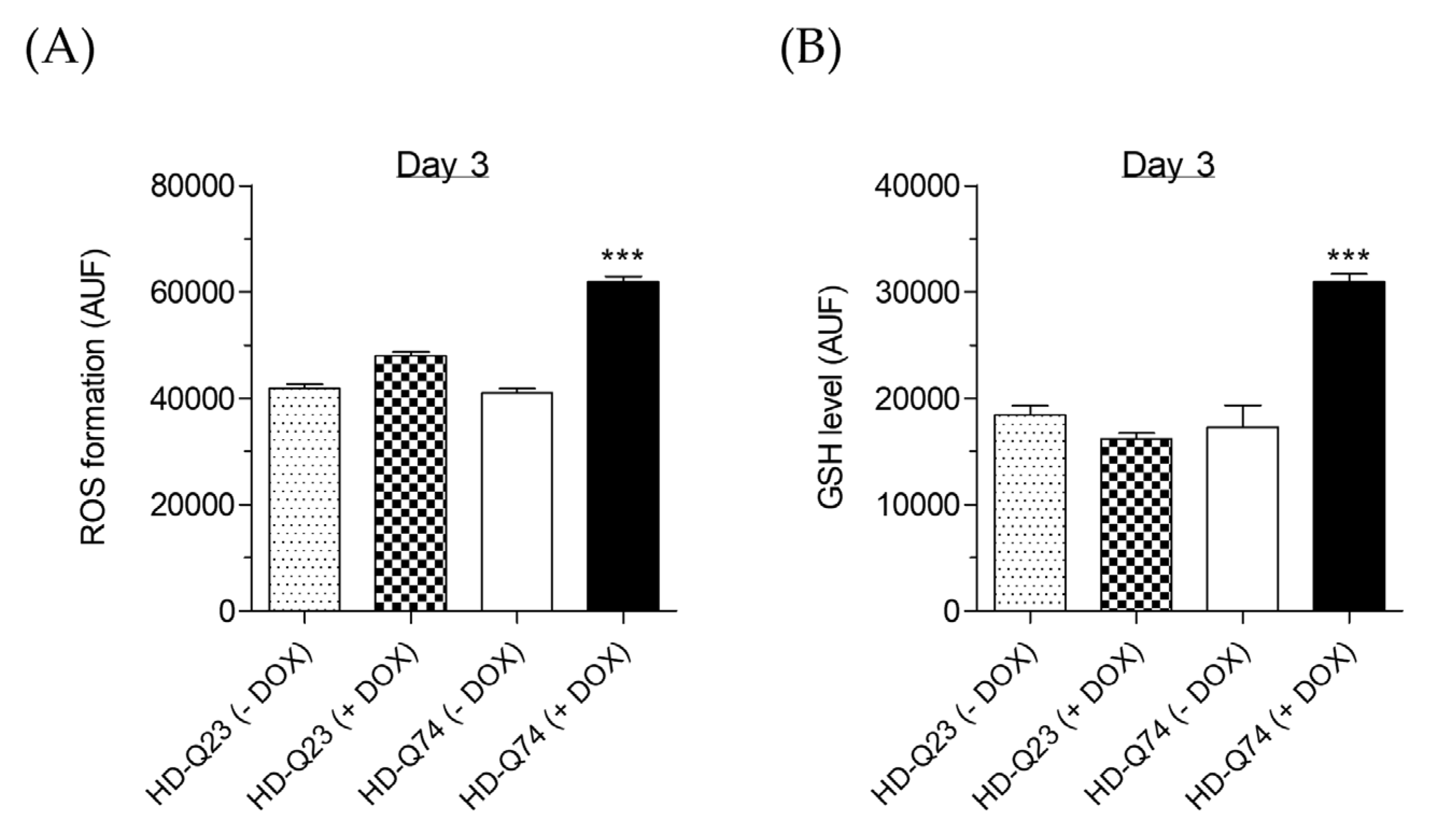
2.5. Effects of ESC on Cellular Redox Status Altered by mHTT
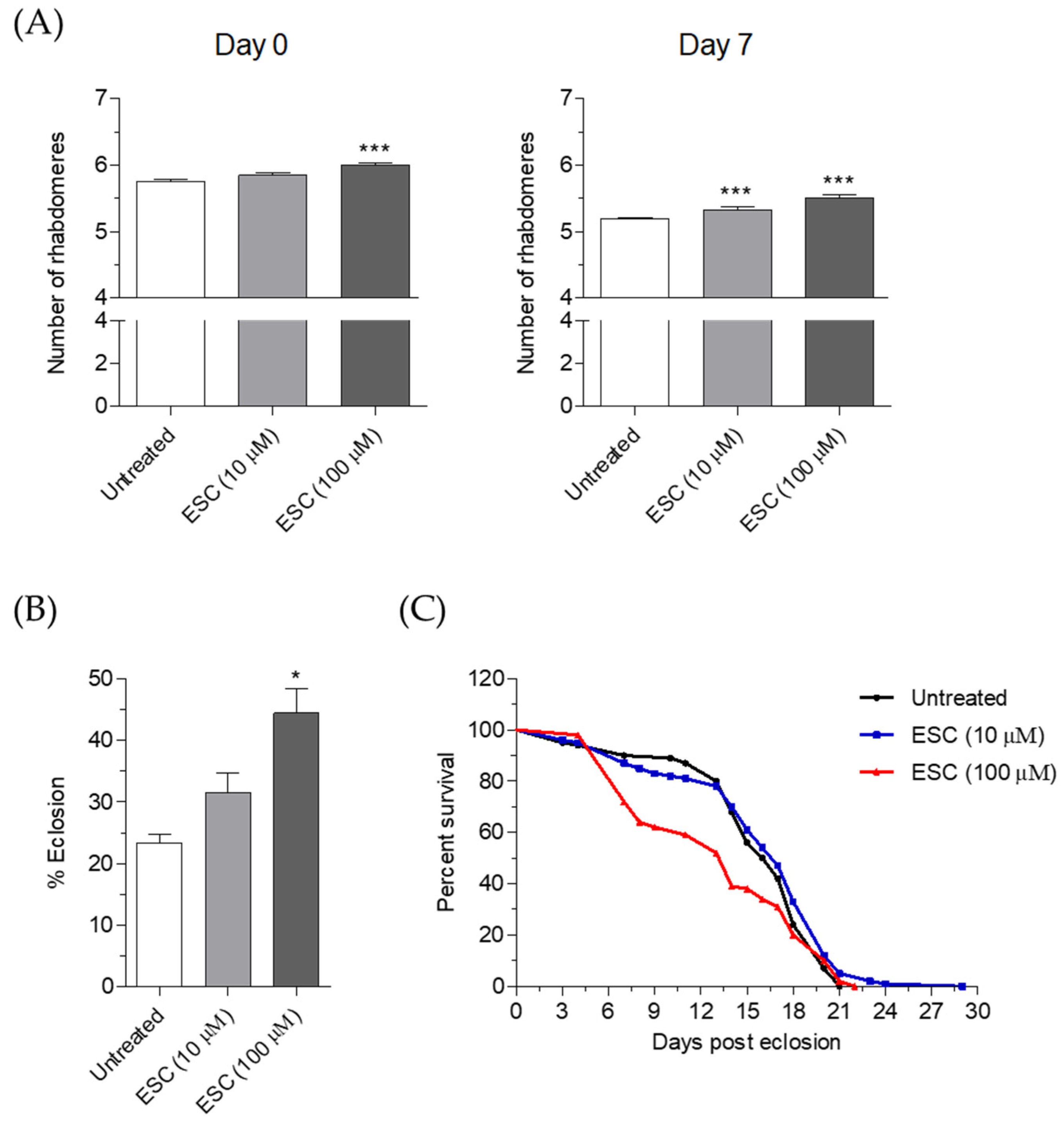
2.6. Neuroprotective Effects of ESC in HTT93Q Transgenic Drosophila
3. Materials and Methods
3.1. Chemicals
3.2. Cell Cultures
3.3. Determination of Cell Proliferation and Neuronal Death
3.4. Determination of TH Protein Level
3.5. Determination of mHTT Protein Aggregation
3.6. Determination of ROS Formation
3.7. Determination of Intracellular GSH Levels
3.8. Determination of TH and GCLC Gene Expression
3.9. Determination of ATP Levels
3.10. Ultrastructure Analysis by TEM
3.11. Drosophila Melanogaster
3.12. Drosophila Compound Feeding
3.12.1. Pseudopupil Analysis
3.12.2. Eclosion Analysis
3.12.3. Longevity Analysis
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Prim. 2015, 1, 15005. [Google Scholar] [CrossRef]
- Podvin, S.; Reardon, H.T.; Yin, K.; Mosier, C.; Hook, V. Multiple clinical features of Huntington’s disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration. J. Neurol. 2019, 266, 551–564. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Shacham, T.; Sharma, N.; Lederkremer, G.Z. Protein Misfolding and ER Stress in Huntington’s Disease. Front. Mol. Biosci. 2019, 6, 20. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. Huntington’s disease: Underlying molecular mechanisms and emerging concepts. Trends Biochem. Sci. 2013, 38, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Van Roon-Mom, W.M.; Pepers, B.A.; ’t Hoen, P.A.; Verwijmeren, C.A.; den Dunnen, J.T.; Dorsman, J.C.; van Ommen, G.B. Mutant huntingtin activates Nrf2-responsive genes and impairs dopamine synthesis in a PC12 model of Huntington’s disease. BMC Mol. Biol. 2008, 9, 84. [Google Scholar] [CrossRef]
- Hwang, S.; Disatnik, M.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef]
- Fukui, H.; Moraes, C.T. Extended polyglutamine repeats trigger a feedback loop involving the mitochondrial complex III, the proteasome and huntingtin aggregates. Hum. Mol. Genet. 2007, 16, 783–797. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial Medicine for Aging and Neurodegenerative Diseases. NeuroMol. Med. 2008, 10, 291–315. [Google Scholar] [CrossRef]
- Reddy, P.H.; Shirendeb, U.P. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim. Biophys. Acta—Mol. Basis Dis. 2012, 1822, 101–110. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Fão, L.; Rego, A.C. Mitochondrial and Redox-Based Therapeutic Strategies in Huntington’s Disease. Antioxid. Redox Signal. 2021, 34, 650–673. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Qureshi, M.S.; Manan, A.; Pushparaj, P.N.; Asif, M.; Qazi, M.H.; Qazi, A.M.; Kamal, M.A.; Gan, S.H.; et al. Recent Updates in the Treatment of Neurodegenerative Disorders Using Natural Compounds. Evid.-Based Complement. Altern. Med. 2014, 2014, 979730. [Google Scholar] [CrossRef]
- Wang, C.; Pei, A.; Chen, J.; Yu, H.; Sun, M.L.; Liu, C.F.; Xu, X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J. Neurochem. 2012, 121, 1007–1013. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Ellis, E.M. Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 453–461. [Google Scholar] [CrossRef]
- Ali, Y.; Jannat, S.; Jung, H.A.; Choi, R.J.; Roy, A.; Choi, J.S. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac. J. Trop. Med. 2016, 9, 103–111. [Google Scholar] [CrossRef]
- Wyttenbach, A. Polyglutamine expansions cause decreased CRE-mediated transcription and early gene expression changes prior to cell death in an inducible cell model of Huntington’s disease. Hum. Mol. Genet. 2001, 10, 1829–1845. [Google Scholar] [CrossRef]
- Van Hagen, M.; Piebes, D.G.E.; de Leeuw, W.C.; Vuist, I.M.; van Roon-Mom, W.M.C.; Moerland, P.D.; Verschure, P.J. The dynamics of early-state transcriptional changes and aggregate formation in a Huntington’s disease cell model. BMC Genom. 2017, 18, 373. [Google Scholar] [CrossRef][Green Version]
- Steffan, J.S.; Bodai, L.; Pallos, J.; Poelman, M.; McCampbell, A.; Apostol, B.L.; Kazantsev, A.; Schmidt, E.; Zhu, Y.Z.; Greenwald, M.; et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001, 413, 739–743. [Google Scholar] [CrossRef]
- Kazantsev, A.; Preisinger, E.; Dranovsky, A.; Goldgaber, D.; Housman, D. Insoluble detergent-resistant aggregates form between pathological and nonpathological lengths of polyglutamine in mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 11404–11409. [Google Scholar] [CrossRef] [PubMed]
- Machiela, E.; Jeloka, R.; Caron, N.S.; Mehta, S.; Schmidt, M.E.; Baddeley, H.J.E.; Tom, C.M.; Polturi, N.; Xie, Y.; Mattis, V.B.; et al. The Interaction of Aging and Cellular Stress Contributes to Pathogenesis in Mouse and Human Huntington Disease Neurons. Front. Aging Neurosci. 2020, 12, 524369. [Google Scholar] [CrossRef] [PubMed]
- Bono-Yagüe, J.; Gómez-Escribano, A.P.; Millán, J.M.; Vázquez-Manrique, R.P. Reactive Species in Huntington Disease: Are They Really the Radicals You Want to Catch? Antioxidants 2020, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Karandikar, A.; Thangarajan, S. Comparative binding analysys of esculetin and glycyrrhizin to huntingtin N-terminal fragment. Int. J. Pharm. Biol. Sci. 2018, 8, 564–576. [Google Scholar]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Dong, Y.N.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch. Biochem. Biophys. 2021, 702, 108698. [Google Scholar] [CrossRef] [PubMed]
- Gohel, D.; Singh, R. Mitohormesis; Potential implications in neurodegenerative diseases. Mitochondrion 2021, 56, 40–46. [Google Scholar] [CrossRef]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a Bifunctional Antioxidant Prevents and Counteracts the Oxidative Stress and Neuronal Death Induced by Amyloid Protein in SH-SY5Y Cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef]
- Nakano, M.; Imamura, H.; Sasaoka, N.; Yamamoto, M.; Uemura, N.; Shudo, T.; Fuchigami, T.; Takahashi, R.; Kakizuka, A. ATP Maintenance via Two Types of ATP Regulators Mitigates Pathological Phenotypes in Mouse Models of Parkinson’s Disease. EBioMedicine 2017, 22, 225–241. [Google Scholar] [CrossRef]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A Mitochondria-Associated Oxidative Stress Perspective on Huntington’s Disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Kaneko, T.; Baba, N.; Matsuo, M. Protection of coumarins against linoleic acid hydroperoxide-induced cytotoxicity. Chem. Biol. Interact. 2003, 142, 239–254. [Google Scholar] [CrossRef]
- Veselinović, J.B.; Veselinović, A.M.; Vitnik, Ž.J.; Vitnik, V.D.; Nikolić, G.M. Antioxidant properties of selected 4-phenyl hydroxycoumarins: Integrated in vitro and computational studies. Chem. Biol. Interact. 2014, 214, 49–56. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Forward/Reverse | 5′ to 3′ Sequence |
|---|---|---|
| Tyrosine Hydroxylase | For | GGAACGGTACTGTGGCTACC |
| Rev | TTCAAGAAGCGGGACACG | |
| Glutamate–Cysteine Ligase Catalytic | For | AAGCCTCCTCCTCCAAACTC |
| Rev | TACCTCCATTGGTCGGAACT | |
| Β-Actin | For | CTGGCTCCTAGCACCATGA |
| Rev | TAGAGCCACCAATCCACACA | |
| Ywhaz | For | AAATGAGCTGGTGCAGAAGG |
| Rev | GGCTGCCATGTCATCGTAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruccoli, L.; Breda, C.; Teti, G.; Falconi, M.; Giorgini, F.; Tarozzi, A. Esculetin Provides Neuroprotection against Mutant Huntingtin-Induced Toxicity in Huntington’s Disease Models. Pharmaceuticals 2021, 14, 1044. https://doi.org/10.3390/ph14101044
Pruccoli L, Breda C, Teti G, Falconi M, Giorgini F, Tarozzi A. Esculetin Provides Neuroprotection against Mutant Huntingtin-Induced Toxicity in Huntington’s Disease Models. Pharmaceuticals. 2021; 14(10):1044. https://doi.org/10.3390/ph14101044
Chicago/Turabian StylePruccoli, Letizia, Carlo Breda, Gabriella Teti, Mirella Falconi, Flaviano Giorgini, and Andrea Tarozzi. 2021. "Esculetin Provides Neuroprotection against Mutant Huntingtin-Induced Toxicity in Huntington’s Disease Models" Pharmaceuticals 14, no. 10: 1044. https://doi.org/10.3390/ph14101044
APA StylePruccoli, L., Breda, C., Teti, G., Falconi, M., Giorgini, F., & Tarozzi, A. (2021). Esculetin Provides Neuroprotection against Mutant Huntingtin-Induced Toxicity in Huntington’s Disease Models. Pharmaceuticals, 14(10), 1044. https://doi.org/10.3390/ph14101044