The Inhibitory Effects of Terminalia catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells
Abstract
1. Introduction
2. Results
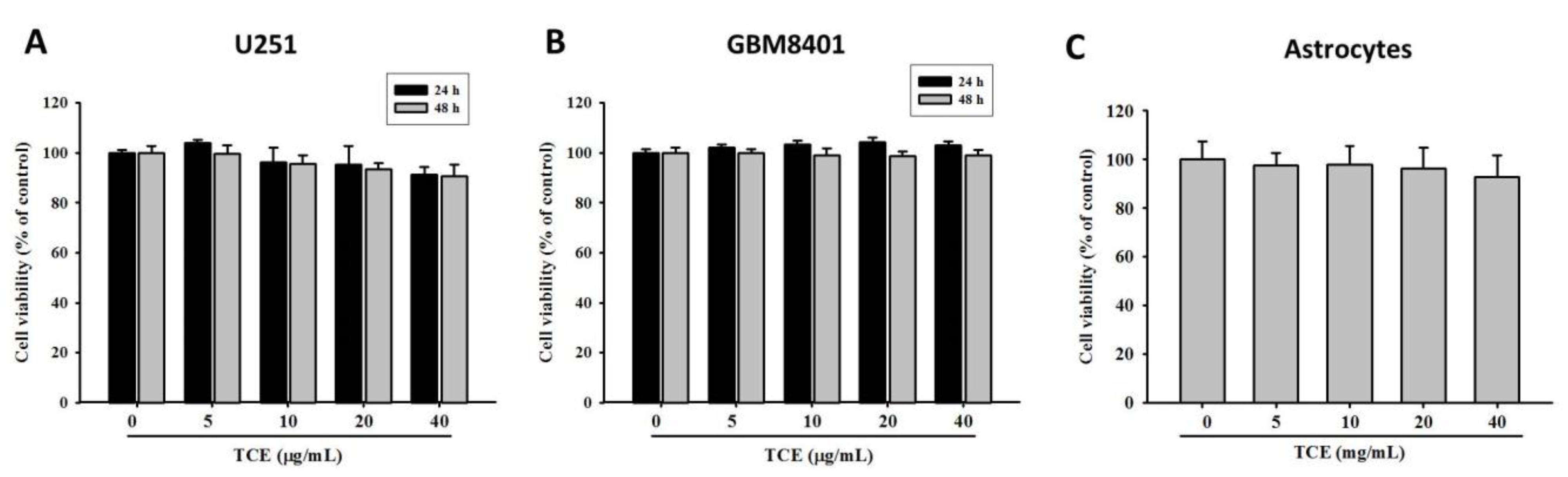
2.1. Effects of TCE on Cell Viability in U251 and GBM8401 Cell Lines
2.2. Effects of TCE on Migration and Invasion of U251 and GBM8401 Cell Lines
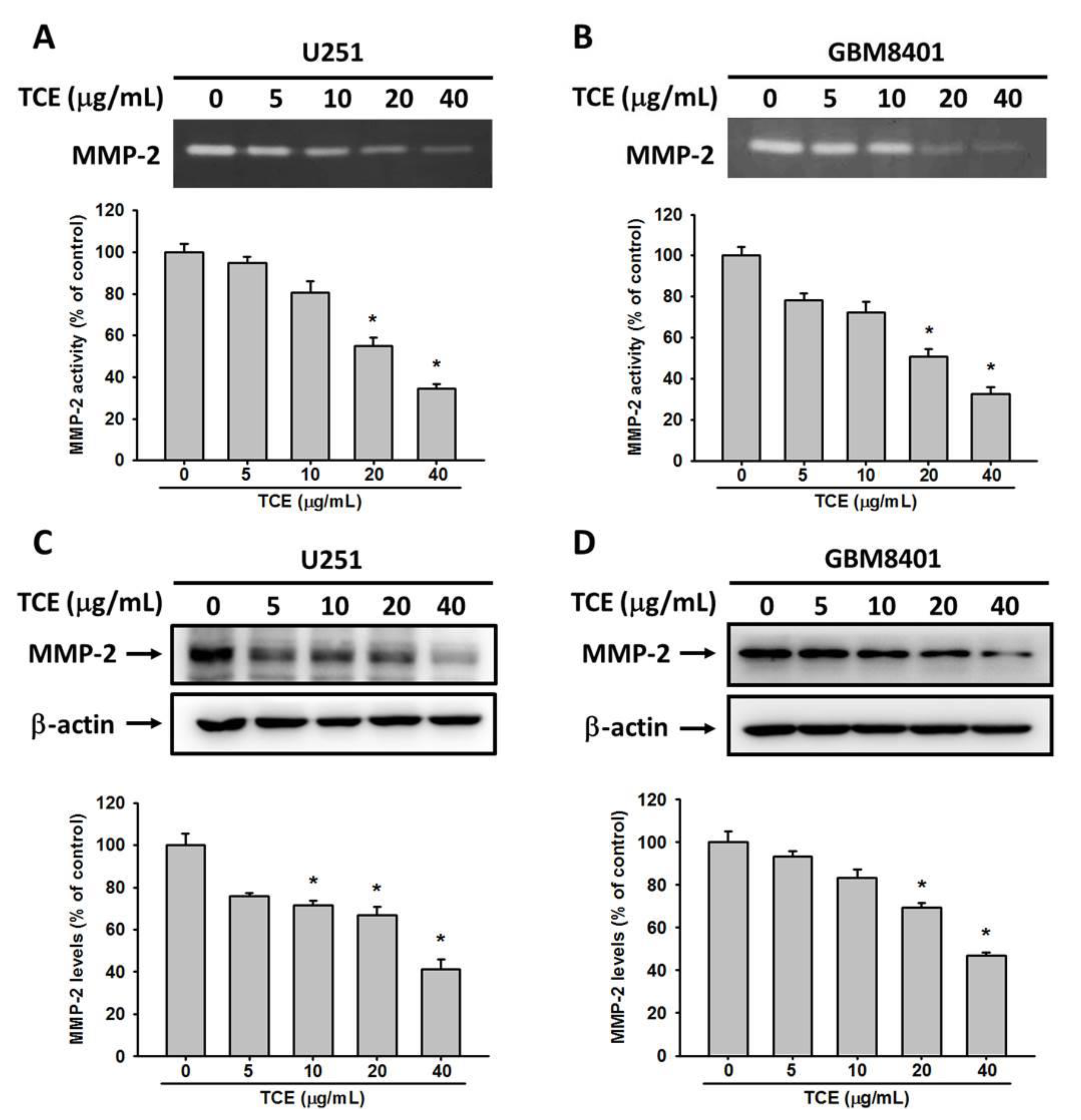
2.3. TCE Inhibits the Enzyme Activity and Protein Expression of MMP-2
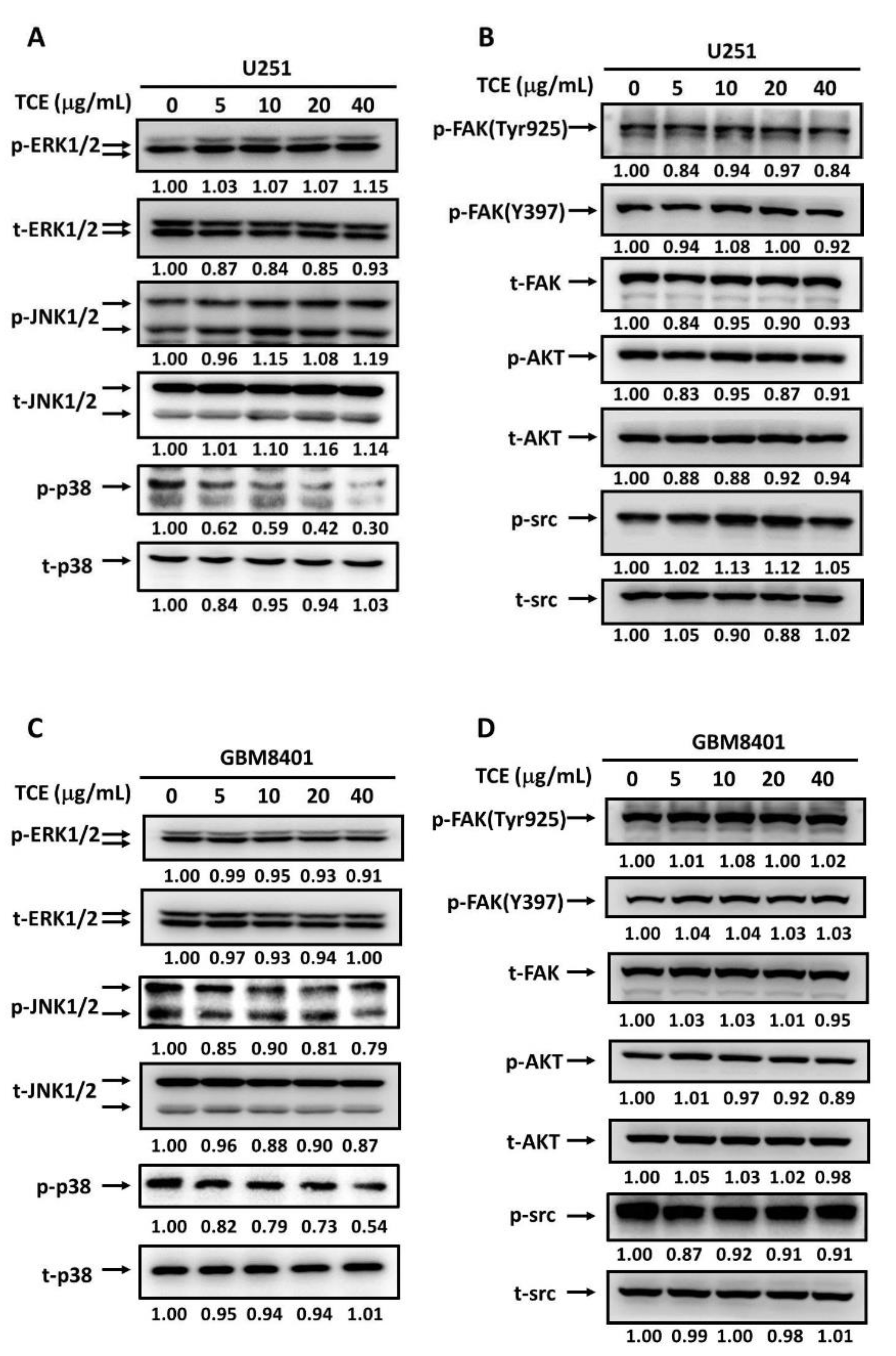
2.4. Effects of TCE on Signaling Cascades in U251 and GBM8401 Cell Lines
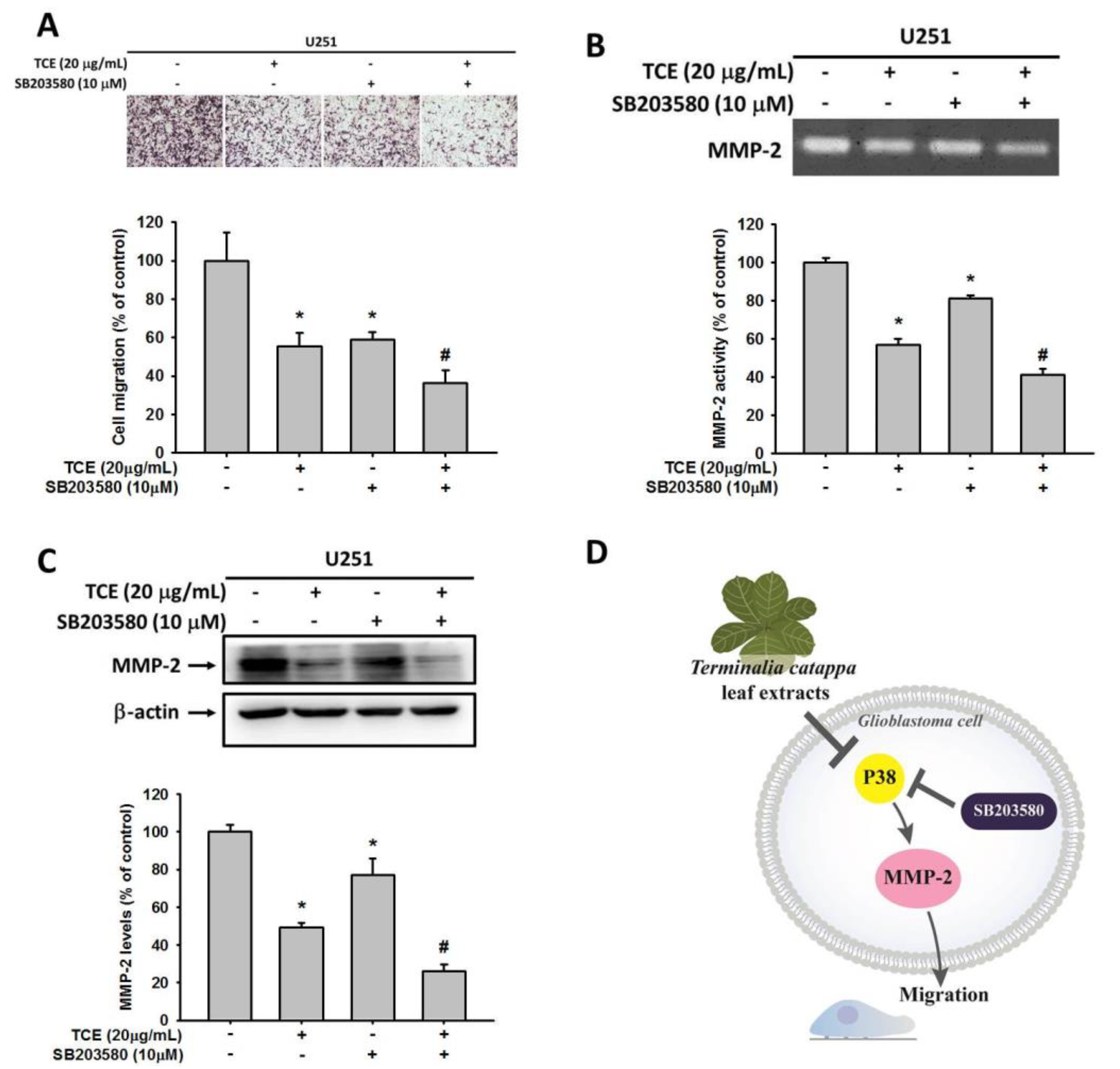
2.5. Effects of TCE and p38 Inhibition in U251 Cell Lines
3. Discussion
4. Materials and Methods
4.1. Preparation of T. catappa L. Extract (TCE)
4.2. Cell Lines and TCE Treatment
4.3. Cell Viability Assay
4.4. In Vitro Wound Closure
4.5. Migration and Invasion Assays
4.6. Assessment of MMP-2 by Gelatin Zymography
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dapash, M.; Castro, B.; Hou, D.; Lee-Chang, C. Current immunotherapeutic strategies for the treatment of glioblastoma. Cancers 2021, 13, 4548. [Google Scholar] [CrossRef]
- Bahadur, S.; Sahu, A.K.; Baghel, P.; Saha, S. Current promising treatment strategy for glioblastoma multiform: A review. Oncol. Rev. 2019, 13, 417. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, Q.; Ren, L.; Liu, J.; Li, W.; Fu, W.; Wang, J.; Du, G. A narrative review of research progress on drug therapies for glioblastoma multiforme. Ann. Transl. Med. 2021, 9, 943. [Google Scholar] [CrossRef]
- Esteyrie, V.; Dehais, C.; Martin, E.; Carpentier, C.; Uro-Coste, E.; Figarella-Branger, D.; Bronniman, C.; Pouessel, D.; Ciron, D.L.; Ducray, F.; et al. Radiotherapy plus procarbazine, lomustine, and vincristine versus radiotherapy plus temozolomide for idh-mutant anaplastic astrocytoma: A retrospective multicenter analysis of the french pola cohort. Oncologist 2021, 26, e838–e846. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, L.; Ai, P.; He, Y.; Guan, H.; Wei, Z.; He, L.; Mu, X.; Liu, Y.; Peng, X. Observation versus radiotherapy with or without temozolomide in postoperative who grade ii high-risk low-grade glioma: A retrospective cohort study. Neurosurg. Rev. 2021, 44, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef]
- Fletcher-Sananikone, E.; Kanji, S.; Tomimatsu, N.; Macedo Di Cristofaro, L.F.; Kollipara, R.K.; Saha, D.; Floyd, J.R.; Sung, P.; Hromas, R.; Burns, T.C.; et al. Elimination of radiation-induced senescence in the brain tumor microenvironment attenuates glioblastoma recurrence. Cancer Res. 2021, in press. [Google Scholar] [CrossRef]
- Tang, Z.; Dokic, I.; Knoll, M.; Ciamarone, F.; Schwager, C.; Klein, C.; Cebulla, G.; Hoffmann, D.C.; Schlegel, J.; Seidel, P.; et al. Radioresistance and transcriptional reprograming of invasive glioblastoma cells. Int. J. Radiat. Oncol. Biol. Phys. 2021, in press. [Google Scholar]
- Afshari, A.R.; Mollazadeh, H.; Henney, N.C.; Jamialahmad, T.; Sahebkar, A. Effects of statins on brain tumors: A review. Semin. Cancer Biol. 2021, 73, 116–133. [Google Scholar] [CrossRef]
- Lookian, P.P.; Zhao, D.; Medina, R.; Wang, H.; Zenka, J.; Gilbert, M.R.; Pacak, K.; Zhuang, Z. Mannan-bam, tlr ligands, anti-cd40 antibody (mbta) vaccine immunotherapy: A review of current evidence and applications in glioblastoma. Int. J. Mol. Sci. 2021, 22, 3455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Katsakhyan, L.; LiVolsi, V.A.; Roth, J.J.; Rassekh, C.H.; Bagley, S.J.; Nasrallah, M.P. Tp53 mutation and extraneural metastasis of glioblastoma: Insights from an institutional experience and comprehensive literature review. Am. J. Surg. Pathol. 2021, 45, 1516–1526. [Google Scholar] [CrossRef]
- Marozzi, M.; Parnigoni, A.; Negri, A.; Viola, M.; Vigetti, D.; Passi, A.; Karousou, E.; Rizzi, F. Inflammation, extracellular matrix remodeling, and proteostasis in tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 8102. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The role of tumor microenvironment in cancer metastasis: Molecular mechanisms and therapeutic opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Zheng, Y.; Chen, Y.; Fei, W.; Wang, F.; Zheng, C. Extracellular matrix: Emerging roles and potential therapeutic targets for breast cancer. Front. Oncol. 2021, 11, 650453. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Hold on or cut? Integrin- and mmp-mediated cell-matrix interactions in the tumor microenvironment. Int. J. Mol. Sci. 2020, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and their inhibitors: Potential for the development of new therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Laing, T.D.; Gibson, A.W.; Rewcastle, N.B.; Brasher, P.; Sutherland, G.; Johnston, R.N.; Edwards, D.R. High levels of gelatinase-b and active gelatinase-a in metastatic glioblastoma. J. Neuro-Oncol. 1998, 36, 21–29. [Google Scholar] [CrossRef]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.G.; Kim, P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 2018, 8, 4608. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Kuo, F.H.; Chen, P.N.; Hsieh, Y.H.; Yu, N.Y.; Yang, W.E.; Hsieh, M.J.; Yang, S.F. Andrographolide suppresses the migratory ability of human glioblastoma multiforme cells by targeting erk1/2-mediated matrix metalloproteinase-2 expression. Oncotarget 2017, 8, 105860–105872. [Google Scholar] [CrossRef]
- Chao, R.; Chow, J.M.; Hsieh, Y.H.; Chen, C.K.; Lee, W.J.; Hsieh, F.K.; Yu, N.Y.; Chou, M.C.; Cheng, C.W.; Yang, S.F.; et al. Tricetin suppresses the migration/invasion of human glioblastoma multiforme cells by inhibiting matrix metalloproteinase-2 through modulation of the expression and transcriptional activity of specificity protein 1. Expert Opin. Ther. Targets 2015, 19, 1293–1306. [Google Scholar] [CrossRef]
- Le Joncour, V.; Guichet, P.O.; Dembélé, K.P.; Mutel, A.; Campisi, D.; Perzo, N.; Desrues, L.; Modzelewski, R.; Couraud, P.O.; Honnorat, J.; et al. Targeting the urotensin ii/ut g protein-coupled receptor to counteract angiogenesis and mesenchymal hypoxia/necrosis in glioblastoma. Front. Cell Dev. Biol. 2021, 9, 652544. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, J.; Cui, Y.; Chen, Y.; Dong, T.; An, H.; Liu, P. Msh6 aggravates the hypoxic microenvironment via regulating hif1a to promote the metastasis of glioblastoma multiforme. DNA Cell Biol. 2021, 40, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, Y.L.; Lin, J.M.; Ujiie, T. Evaluation of the antioxidant and hepatoprotective activity of Terminalia catappa. Am. J. Chin. Med. 1997, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Kuo, Y.-H.; Shiao, M.-S.; Chen, C.-C.; Ou, J.-C. Flavonoid glycosides from Terminalia catappa L. J. Chin. Chem. Soc. 2000, 47, 253–256. [Google Scholar] [CrossRef]
- Gao, J.; Tang, X.; Dou, H.; Fan, Y.; Zhao, X.; Xu, Q. Hepatoprotective activity of Terminalia catappa L. Leaves and its two triterpenoids. J. Pharm. Pharmacol. 2004, 56, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, F.L.; Hsu, H.Y. Antioxidant and hepatoprotective activity of punicalagin and punicalin on carbon tetrachloride-induced liver damage in rats. J. Pharm. Pharmacol. 1998, 50, 789–794. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L. A comprehensive review of the structure elucidation of tannins from terminalia linn. Evid. Based Complement. Altern. Med. 2019, 2019, 8623909. [Google Scholar] [CrossRef]
- Yang, S.F.; Chen, M.K.; Hsieh, Y.S.; Yang, J.S.; Zavras, A.I.; Hsieh, Y.H.; Su, S.C.; Kao, T.Y.; Chen, P.N.; Chu, S.C. Antimetastatic effects of Terminalia catappa L. On oral cancer via a down-regulation of metastasis-associated proteases. Food Chem. Toxicol. 2010, 48, 1052–1058. [Google Scholar] [CrossRef]
- Tang, X.; Gao, J.; Wang, Y.; Fan, Y.M.; Xu, L.Z.; Zhao, X.N.; Xu, Q.; Qian, Z.M. Effective protection of Terminalia catappa L. Leaves from damage induced by carbon tetrachloride in liver mitochondria. J. Nutr. Biochem. 2006, 17, 177–182. [Google Scholar] [CrossRef]
- Tang, X.H.; Gao, J.; Dou, H.; Wang, Y.P.; Xu, L.Z.; Zhu, Z.R.; Xu, Q. Protective effect of the extract of Terminalia catappa leaves on acute liver injury induced by d-galn in mice. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2004, 29, 1069–1073. (In Chinese) [Google Scholar]
- Chu, S.C.; Yang, S.F.; Liu, S.J.; Kuo, W.H.; Chang, Y.Z.; Hsieh, Y.S. In vitro and in vivo antimetastatic effects of Terminalia catappa L. Leaves on lung cancer cells. Food Chem. 2007, 45, 1194–1201. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 who classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Gandhi, A.K.; Rath, G.K. Therapeutic approach beyond conventional temozolomide for newly diagnosed glioblastoma: Review of the present evidence and future direction. Indian J. Med Paediatr. Oncol. 2015, 36, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Pandya, N.B.; Tigari, P.; Dupadahalli, K.; Kamurthy, H.; Nadendla, R.R. Antitumor and antioxidant status of Terminalia catappa against ehrlich ascites carcinoma in swiss albino mice. Indian J. Pharmacol. 2013, 45, 464–469. [Google Scholar] [PubMed]
- Yeh, C.B.; Yu, Y.L.; Lin, C.W.; Chiou, H.L.; Hsieh, M.J.; Yang, S.F. Terminalia catappa attenuates urokinase-type plasminogen activator expression through erk pathways in hepatocellular carcinoma. BMC Complement. Altern. Med. 2014, 14, 141. [Google Scholar] [CrossRef][Green Version]
- Yeh, C.B.; Hsieh, M.J.; Hsieh, Y.S.; Chien, M.H.; Lin, P.Y.; Chiou, H.L.; Yang, S.F. Terminalia catappa exerts antimetastatic effects on hepatocellular carcinoma through transcriptional inhibition of matrix metalloproteinase-9 by modulating nf-κb and ap-1 activity. Evid. Based Complement. Altern. Med. 2012, 2012, 595292. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.M.; Xu, L.Z.; Gao, J.; Wang, Y.; Tang, X.H.; Zhao, X.N.; Zhang, Z.X. Phytochemical and antiinflammatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef]
- Conway, G.E.; Zizyte, D.; Mondala, J.R.M.; He, Z.; Lynam, L.; Lecourt, M.; Barcia, C.; Howe, O.; Curtin, J.F. Ursolic acid inhibits collective cell migration and promotes jnk-dependent lysosomal associated cell death in glioblastoma multiforme cells. Pharmaceuticals 2021, 14, 91. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Ding, F.; Guo, S.; Ying, G.; Yan, Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating o(6)-methylguanine-DNA methyltransferase (mgmt) expression. Am. J. Transl. Res. 2016, 8, 3299–3308. [Google Scholar]
- Ansardamavandi, A.; Tafazzoli-Shadpour, M. The functional cross talk between cancer cells and cancer associated fibroblasts from a cancer mechanics perspective. Biochim. Biophys. Acta. Mol. Cell Res. 2021, 1868, 119103. [Google Scholar] [CrossRef]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef]
- Vasilaki, D.; Bakopoulou, A.; Tsouknidas, A.; Johnstone, E.; Michalakis, K. Biophysical interactions between components of the tumor microenvironment promote metastasis. Biophys. Rev. 2021, 13, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Demuth, T.; Berens, M.E. Molecular mechanisms of glioma cell migration and invasion. J. Neuro-Oncol. 2004, 70, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Gao, Y.; Johnson, N.W.; Gao, J. Immunoexpression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in the metastasis of squamous cell carcinoma of the human tongue. Aust. Dent. J. 2010, 55, 385–389. [Google Scholar] [CrossRef]
- Vartio, T.; Vaheri, A. A gelatin-binding 70,000-dalton glycoprotein synthesized distinctly from fibronectin by normal and malignant adherent cells. J. Biol. Chem. 1981, 256, 13085–13090. [Google Scholar] [CrossRef]
- Anuar, N.N.M.; Zulkafali, N.I.N.; Ugusman, A. Modulation of matrix metalloproteinases by plant-derived products. Curr. Cancer Drug Targets 2021, 21, 91–106. [Google Scholar] [CrossRef]
- Mahecha, A.M.; Wang, H. The influence of vascular endothelial growth factor-a and matrix metalloproteinase-2 and -9 in angiogenesis, metastasis, and prognosis of endometrial cancer. OncoTargets Ther. 2017, 10, 4617–4624. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Baidya, S.K.; Amin, S.A.; Jha, T. Outline of gelatinase inhibitors as anti-cancer agents: A patent mini-review for 2010-present. Eur. J. Med. Chem. 2021, 213, 113044. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuñiga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernández-Tilapa, G.; Mendoza-Catalán, M.A.; Zacapala-Gómez, A.E.; Ortiz-Ortiz, J.; Ortuño-Pineda, C.; Navarro-Tito, N. Natural isoflavonoids in invasive cancer therapy: From bench to bedside. Phytother. Res. PTR 2021, 35, 4092–4110. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tu, Y.; Liu, Q.; Ouyang, Y.; He, M.; Luo, M.; Chen, J.; Pi, R.; Liu, A. Pt93, a novel caffeic acid amide derivative, suppresses glioblastoma cells migration, proliferation and mmp-2/-9 expression. Oncol. Lett. 2017, 13, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yoshida, D.; Liu, S.; Teramoto, A. Inhibition of cell invasion by indomethacin on glioma cell lines: In vitro study. J. Neuro-Oncol. 2005, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, Y.; Biswas, A.; Kumar, U.; Das, S.; Banerjee, I.; Banik, P.; Bharti, R.; Nayak, S.; Ghosh, S.K.; Mandal, M. Targeting nfe2l2, a transcription factor upstream of mmp-2: A potential therapeutic strategy for temozolomide resistant glioblastoma. Biochem. Pharmacol. 2019, 164, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Bhushan, A. Natural bioactive compounds: Alternative approach to the treatment of glioblastoma multiforme. Biomed. Res. Int. 2017, 2017, 9363040. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Cheng, H.-Y.; Kuo, C.-L.; Way, T.-D.; Lien, J.-C.; Chueh, F.-S.; Lin, Y.-L.; Chung, J.-G. Tetrandrine inhibits human brain glioblastoma multiforme gbm 8401 cancer cell migration and invasion in vitro. Environ. Toxicol. 2019, 34, 364–374. [Google Scholar] [CrossRef]
- Chen, X.; Hao, A.; Li, X.; Ye, K.; Zhao, C.; Yang, H.; Ma, H.; Hu, L.; Zhao, Z.; Hu, L.; et al. Activation of jnk and p38 mapk mediated by zdhhc17 drives glioblastoma multiforme development and malignant progression. Theranostics 2020, 10, 998–1015. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Bhaskara, V.K.; Babu, P.P. Implications of mitogen-activated protein kinase signaling in glioma. J. Neurosci. Res. 2016, 94, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheng, C.D.; Wu, H.; Wang, Z.W.; Wang, L.; Jiang, Z.R.; Wang, A.L.; Hu, C.; Dong, Y.F.; Niu, W.X.; et al. Osimertinib successfully combats egfr-negative glioblastoma cells by inhibiting the mapk pathway. Acta Pharmacol. Sin. 2021, 42, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Luo, C.; Hameed, N.U.F.; Wang, Y.; Zhuang, D. Ucp2 silencing in glioblastoma reduces cell proliferation and invasiveness by inhibiting p38 mapk pathway. Exp. Cell Res. 2020, 394, 112110. [Google Scholar] [CrossRef]
- Terças, A.G.; Monteiro, A.S.; Moffa, E.B.; Dos Santos, J.R.A.; de Sousa, E.M.; Pinto, A.R.B.; Costa, P.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical characterization of Terminalia catappa linn. Extracts and their antifungal activities against candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Chen, P.S.; Li, J.H.; Liu, T.Y.; Lin, T.C. Folk medicine Terminalia catappa and its major tannin component, punicalagin, are effective against bleomycin-induced genotoxicity in chinese hamster ovary cells. Cancer Lett. 2000, 152, 115–122. [Google Scholar] [CrossRef]
- Mininel, F.J.; Leonardo Junior, C.S.; Espanha, L.G.; Resende, F.A.; Varanda, E.A.; Leite, C.Q.F.; Vilegas, W.; dos Santos, L.C. Characterization and quantification of compounds in the hydroalcoholic extract of the leaves from Terminalia catappa linn. (combretaceae) and their mutagenic activity. Evid. Based Complementary Altern. Med. 2014, 2014, 676902. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-H.; Hsieh, M.-J.; Hsieh, Y.-S.; Chen, P.-N.; Ko, C.-P.; Yu, N.-Y.; Lin, C.-W.; Yang, S.-F. The Inhibitory Effects of Terminalia catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells. Pharmaceuticals 2021, 14, 1183. https://doi.org/10.3390/ph14111183
Chung H-H, Hsieh M-J, Hsieh Y-S, Chen P-N, Ko C-P, Yu N-Y, Lin C-W, Yang S-F. The Inhibitory Effects of Terminalia catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells. Pharmaceuticals. 2021; 14(11):1183. https://doi.org/10.3390/ph14111183
Chicago/Turabian StyleChung, Hsiao-Hang, Ming-Ju Hsieh, Yih-Shou Hsieh, Pei-Ni Chen, Chung-Po Ko, Nuo-Yi Yu, Chiao-Wen Lin, and Shun-Fa Yang. 2021. "The Inhibitory Effects of Terminalia catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells" Pharmaceuticals 14, no. 11: 1183. https://doi.org/10.3390/ph14111183
APA StyleChung, H.-H., Hsieh, M.-J., Hsieh, Y.-S., Chen, P.-N., Ko, C.-P., Yu, N.-Y., Lin, C.-W., & Yang, S.-F. (2021). The Inhibitory Effects of Terminalia catappa L. Extract on the Migration and Invasion of Human Glioblastoma Multiforme Cells. Pharmaceuticals, 14(11), 1183. https://doi.org/10.3390/ph14111183







