Ten-Year Outcomes of Intravitreal Bevacizumab for Myopic Choroidal Neovascularization: Analysis of Prognostic Factors
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Data
2.2. BCVA Outcomes
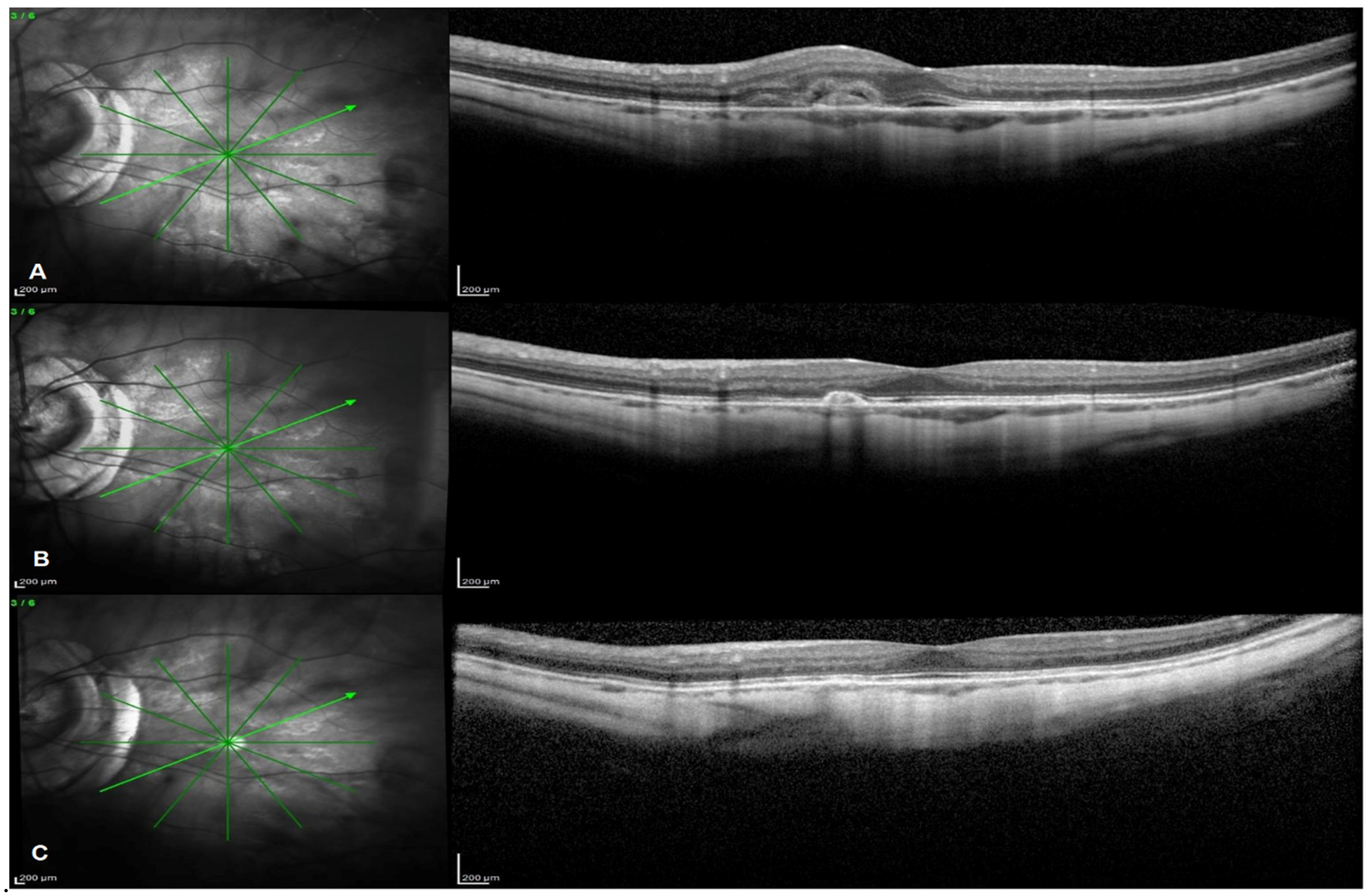
2.3. OCT and FA Outcomes
3. Discussion
4. Methods
4.1. Patients
4.2. BCVA
4.3. OCT Evaluations
4.4. Colour Fundus Photography and FA
4.5. Treatments
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohno-Matsui, K.; Yoshida, T.; Futagami, S.; Yasuzumi, K.; Shimada, N.; Kojima, A.; Tokoro, T.; Mochizuki, M. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br. J. Ophthalmol. 2003, 87, 570–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Ohno-Matsui, K.; Yasuzumi, K.; Kojima, A.; Shimada, N.; Futagami, S.; Tokoro, T.; Mochizuki, M. Myopic choroidal neovascularization: A 10-year follow-up. Ophthalmology 2003, 110, 1297–1305. [Google Scholar] [CrossRef]
- Cohen, S.Y. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina 2009, 29, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Giustolisi, R.; Marchiori, J.; Bruscolini, A.; Mallone, F.; Fameli, V.; Nebbioso, M.; Abdolrahimzadeh, S. Comparison of Short-Term Choroidal Thickness and Retinal Morphological Changes after Intravitreal Anti-VEGF Therapy with Ranibizumab or Aflibercept in Treatment-Naive Eyes. Curr. Eye Res. 2018, 43, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Varano, M.; Iacono, P.; Giorno, P.; Chiaravalloti, A.; Parravano, M. Photodynamic therapy in subfoveal and juxtafoveal myopic choroidal neovascularization: A 10-year retrospective analysis. Ophthalmologica 2014, 231, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-M.; Lai, T.; Liu, D.T.L.; Lam, D.S.C. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br. J. Ophthalmol. 2009, 93, 150–154. [Google Scholar] [CrossRef]
- Gharbiya, M.; Allievi, F.; Mazzeo, L.; Gabrieli, C.B. Intravitreal Bevacizumab Treatment for Choroidal Neovascularization in Pathologic Myopia: 12-month Results. Am. J. Ophthalmol. 2009, 147, 84–93.e1. [Google Scholar] [CrossRef]
- Gharbiya, M.; Giustolisi, R.; Allievi, F.; Fantozzi, N.; Mazzeo, L.; Scavella, V.; Gabrieli, C.B. Choroidal Neovascularization in Pathologic Myopia: Intravitreal Ranibizumab Versus Bevacizumab-A Randomized Controlled Trial. Am. J. Ophthalmol. 2010, 149, 458–464.e1. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Gomez-Ulla, F.; Montero, J.A.; Ares, S.; Lopez-Lopez, F.; Rodriguez, M.; Fernandez, M. Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: Short-term results. Eye 2009, 23, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moreno, J.M.; Montero, J.A.; Arias, L.; Araiz, J.; Gomez-Ulla, F.; Silva, R.; Piñero, D.P. Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina 2010, 30, 1609–1615. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Montero, J.A. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-Year outcome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 937–941. [Google Scholar] [CrossRef]
- Ikuno, Y.; Nagai, Y.; Matsuda, S.; Arisawa, A.; Sho, K.; Oshita, T.; Takahashi, K.; Yasutaka, U.; Gomi, F. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am. J. Ophthalmol. 2010, 149, 140–146.e1. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimada, N.; Moriyama, M.; Hayashi, W.; Tokoro, T.; Ohno-Matsui, K. Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina 2012, 32, 687–695. [Google Scholar] [CrossRef]
- Gharbiya, M.; Allievi, F.; Conflitti, S.; Esposito, M.; Scavella, V.; Moramarco, A.; Cruciani, F. Intravitreal bevacizumab for treatment of myopic choroidal neovascularization: The second year of a prospective study. Clin. Ther. 2010, 161, 87–93. [Google Scholar]
- Nakanishi, H.; Tsujikawa, A.; Yodoi, Y.; Ojima, Y.; Otani, A.; Tamura, H.; Yamashiro, K.; Ooto, S.; Yoshimura, N. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye 2011, 25, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Gharbiya, M.; Cruciani, F.; Parisi, F.; Cuozzo, G.; Altimari, S.; Abdolrahimzadeh, S. Long-term results of intravitreal bevacizumab for choroidal neovascularisation in pathological myopia. Br. J. Ophthalmol. 2012, 96, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiretti, E.; Vinci, M.; Fossarello, M. Intravitreal bevacizumab as a treatment for choroidal neovascularisation secondary to myopia: 4-Year study results. Can. J. Ophthalmol. 2012, 47, 28–33. [Google Scholar] [CrossRef]
- Oishi, A.; Yamashiro, K.; Tsujikawa, A.; Ooto, S.; Tamura, H.; Nakata, I.; Miyake, M.; Yoshimura, N. Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarao, V.; Veritti, D.; Macor, S.; Lanzetta, P. Intravitreal bevacizumab for choroidal neovascularization due to pathologic myopia: Long-term outcomes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 445–454. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Arias, L.; Montero, J.A.; Carneiro, Â.; Silva, R. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br. J. Ophthalmol. 2013, 97, 1447–1450. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moreno, J.M.; Montero, J.A.; Araiz, J.; Arias, L.; García-Layana, A.; Carneiro, A.; Figueroa, M.S.; Silva, R. Intravitreal anti-vascular endothelial growth factor therapy for choroidal neovascularization secondary to pathologic myopia: Six years outcome. Retina 2015, 35, 2450–2456. [Google Scholar] [CrossRef]
- Chhablani, J.; Paulose, R.M.; Lasave, A.; Wu, L.; Carpentier, C.; Maia, M.; Lujan, S.; Rojas, S.; Serrano, M.; Berrocal, M.H.; et al. Intravitreal bevacizumab monotherapy in myopic choroidal neovascularisation: 5-year outcomes for the PAN-American Collaborative Retina Study Group. Br. J. Ophthalmol. 2018, 102, 455–459. [Google Scholar] [CrossRef]
- Traversi, C.; Nuti, E.; Marigliani, D.; Cevenini, G.; Balestrazzi, A.; Martone, G.; Caporossi, T.; Tosi, G.M. Forty-two-month outcome of intravitreal bevacizumab in myopic choroidal neovascularization. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 511–517. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, J.G.; Kim, J.T.; Joe, S.G. Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after intravitreal bevacizumab. Am. J. Ophthalmol. 2013, 156, 1201–1210.e2. [Google Scholar] [CrossRef]
- Ahn, S.J.; Woo, S.J.; Kim, K.E.; Park, K.H. Association between choroidal morphology and anti-vascular endothelial growth factor treatment outcome in myopic choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, K.; Moriyama, M.; Morohoshi, K.; Yoshida, T.; Simada, N.; Nagaoka, N.; Yokoi, T.; Shinohara, K.; Kaneko, Y.; Suga, M.; et al. Six-year outcomes of intravitreal bevacizumab for choroidal neovascularization in patients with pathologic myopia. Retina 2017, 37, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.Y.; Luk, F.O.J.; Lee, G.K.Y.; Lam, D.S.C. Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic. Eye 2012, 26, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Zweifel, S.A.; Engelbert, M. Edirorial: Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010, 30, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Montero, J.A.; Amat-Peral, P. Myopic choroidal neovascularization treated by intravitreal bevacizumab: Comparison of two different initial doses. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 595–599. [Google Scholar] [CrossRef]
- Ach, T.; Hoeh, A.E.; Ruppenstein, M.; Kretz, F.T.; Dithmar, S. Intravitreal bevacizumab in vascular pigment epithelium detachment as a result of subfoveal occult choroidal neovascularization in age-related macular degeneration. Retina 2010, 30, 1420–1425. [Google Scholar] [CrossRef]
- Aghdam, K.A.; Seidensticker, F.; Pielen, A.; Framme, C.; Junker, B. The short-term effects of aflibercept on the size of choroidal neovascularization lesion in treatment-resistant neovascular age-related macular degeneration as. Lasers Surg. Med. 2016, 48, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimzadeh, S.; Parisi, F.; Marcelli, M.; Giustolisi, R.; Gharbiya, M. Optical coherence tomography evidence of macular ganglion cell-inner plexiform layer thinning in eyes with subretinal drusenoid deposits. Eye 2019, 33, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, T.; Wang, K.; Xu, G.; Huang, X. Changes in choroidal thickness after panretinal photocoagulation in patients with type 2 diabetes. Retina 2015, 35, 695–703. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (30 Patients/30 Eyes) |
|---|---|
| Age (years) | 63.7 ± 7.6 |
| Gender (F/M) | F20/M10 |
| Caucasic | 30/30 (100%) |
| Axial length (mm) | 30.7 ± 1.2 |
| Baseline BCVA (ETDRS in logMAR) | 0.47 ± 0.42 |
| Phakic/pseudophakic/aphakic | 14(46.7%)/14(46.7%)/2(6.7%) |
| Subfoveal CNV | 6/30 (20%) |
| Juxtafoveal CNV | 14/30 (46.7%) |
| Extrafoveal CNV | 10/30(33.3%) |
| Retreated CNV | 14/30 (46.7%) |
| Complete lesion regression | 12/30 (40%) |
| Total n. injections | 6.8 ± 6.31 |
| Fellow eye involvement | 8/30 (26.7%) |
| Outcome | 1 Year | 2 Years | 3 Years | 5 Years | 10 Years | Total Number |
|---|---|---|---|---|---|---|
| N° of injections (mean ± SD) | 5.1 ± 3.36 | 1.0 ± 2.13 | 0.7 ± 0.82 | 0 | 0 | 6.8 ± 6.31 |
| CRA around mCNV (N° eyes/%) | 7/30 (23%) | 20/30 (66%) | 24/30 (80%) | 30/30 (100%) | 30/30 (100%) |
| Outcome | Baseline | 2 Years | 4 Years | 6 Years | 8 Years | 10 Years |
|---|---|---|---|---|---|---|
| BCVA (ETDRS in logMAR) | 0.47 ± 0.42 | 0.28 ± 0.33 | 0.19 ± 0.28 | 0.22 ± 0.26 | 0.18 ± 0.25 | 0.21 ± 0.29 |
| BCVA change (ETDRS in logMAR) | 0.19 ± 0.15 | 0.28 ± 0.14 | 0.25 ± 0.16 | 0.29 ± 0.17 | 0.26 ± 0.22 | |
| p value | <0.0001 * | 0.001 * |
| BVCA at 10 Years | |||
|---|---|---|---|
| Factor | B | β | p Value |
| BCVA T0 (No of ETDRS letters in logMAR) | 0.602 | 0.876 | <0.0001 |
| Age (years) | 0.044 | 0.796 | 0.851 |
| CSF CT T0 | −0.125 | 0.481 | 0.754 |
| CSF RT T0 | 0.138 | 0.368 | 0.994 |
| CNV A T0 | 0.005 | 0.978 | 0.929 |
| CNV LOC | −0.032 | 0.847 | 0.892 |
| Adjusted R2 | 0.747 | ||
| Outcome | Baseline | 5 Years | 10 Years | p Value 5 y | p Value 10 y |
|---|---|---|---|---|---|
| CNV A | 0.12 ± 0.11 | 0.054 ± 0.06 | 0.044 ± 0.06 | p = 0.017 * | p = 0.003 * |
| CNV H | 165.53 ± 108.27 | 72.4 ± 67.44 | 59.53 ± 61.29 | p < 0.0001 * | p < 0.0001 * |
| CSF CT | 76.53 ± 39.35 | 58.53 ± 29.60 | 55.2 ± 31.81 | p = 0.029 * | p = 0.036 * |
| CT3mm | 70.43 ± 32.29 | 58.91 ± 29.43 | 60.18 ± 30.13 | p = 0.004 * | p = 0.084 * |
| CT6mm | 70.65 ± 27.14 | 61.56 ± 28.47 | 58.43 ± 26.74 | p = 0.03 * | p = 0.002 * |
| CSF RT | 277.2 ± 41.40 | 250.2 ± 38.09 | 218.06 ± 74.46 | p = 0.01 * | p = 0.007 * |
| RT3mm | 292.91 ± 33.22 | 271.12 ± 32.31 | 242.01 ± 61.69 | p = 0.014 * | p = 0.003 * |
| RT6mm | 250.5 ± 31.22 | 237.31 ± 30.12 | 203.57 ± 57.1 | p = 0.145 * | p = 0.011 * |
| Outcome | Baseline CNV | Baseline no CNV | p Value Baseline | 5 Years CNV | 5 Years no CNV | p Value 5 Years | 10 Years CNV | 10 Years no CNV | p Value 10 Years |
|---|---|---|---|---|---|---|---|---|---|
| CSF CT | 76.53 ± 39.35 | 65.25 ± 23.66 | 0.469 * | 58.53 ± 29.60 | 46.63 ± 27.34 | 0.357 * | 55.2 ± 31.81 | 32.62 ± 22.24 | 0.090 * |
| CSF CT change from baseline | −21.33 ± 35.56 | −32.62 ± 20.74 | 0.421 ** | ||||||
| CT3mm | 70.43 ± 32.29 | 67.31 ± 35.05 | 0.832 * | 58.91 ± 29.43 | 50.81 ± 28.63 | 0.533 * | 60.18 ± 30.13 | 38.94 ± 22.67 | 0.096 * |
| CT3mm change from baseline | −10.25 ± 21.36 | −28.37 ± 20.38 | 0.062 ** | ||||||
| CT6mm | 70.65 ± 27.14 | 69.31 ± 29.76 | 0.914 * | 61.56 ± 28.47 | 50.12 ± 16.69 | 0.311 * | 58.40 ± 26.74 | 36.57 ± 14.29 | 0.045 * |
| CT6mm change from baseline | −12.25 ± 12.56 | −32.74 ± 28.57 | 0.088 ** | ||||||
| CSF RT | 277.2 ± 41.40 | 274.13 ± 39.70 | 0.865 * | 250.2 ± 38.09 | 254.13 ± 50.58 | 0.836 * | 218.06 ± 74.46 | 232.5 ± 39.62 | 0.617 * |
| CSF RT change from baseline | −59.13 ± 72.36 | −41.62 ± 42.62 | 0.539 ** | ||||||
| RT3mm | 299.58 ± 36.97 | 291.53 ± 26.07 | 0.591 * | 271.12 ± 32.31 | 263.1 ± 44.11 | 0.621 * | 242.01 ± 61.69 | 248.03 ± 36.25 | 0.804 * |
| RT3mm change from baseline | −57.56 ± 50.14 | −43.5 ± 27.68 | 0.473 ** | ||||||
| RT6mm | 250.5 ± 31.22 | 242.69 ± 35.21 | 0.590 * | 237.32 ± 30.12 | 208.9 ± 53.97 | 0.117 * | 203.57 ± 57.1 | 188.91 ± 70.07 | 0.593 * |
| RT6mm change from baseline | −46.93 ± 62.27 | −53.78 ± 63.50 | 0.805 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallone, F.; Giustolisi, R.; Franzone, F.; Marenco, M.; Plateroti, R.; Nebbioso, M.; Lambiase, A.; Gharbiya, M. Ten-Year Outcomes of Intravitreal Bevacizumab for Myopic Choroidal Neovascularization: Analysis of Prognostic Factors. Pharmaceuticals 2021, 14, 1042. https://doi.org/10.3390/ph14101042
Mallone F, Giustolisi R, Franzone F, Marenco M, Plateroti R, Nebbioso M, Lambiase A, Gharbiya M. Ten-Year Outcomes of Intravitreal Bevacizumab for Myopic Choroidal Neovascularization: Analysis of Prognostic Factors. Pharmaceuticals. 2021; 14(10):1042. https://doi.org/10.3390/ph14101042
Chicago/Turabian StyleMallone, Fabiana, Rosalia Giustolisi, Federica Franzone, Marco Marenco, Rocco Plateroti, Marcella Nebbioso, Alessandro Lambiase, and Magda Gharbiya. 2021. "Ten-Year Outcomes of Intravitreal Bevacizumab for Myopic Choroidal Neovascularization: Analysis of Prognostic Factors" Pharmaceuticals 14, no. 10: 1042. https://doi.org/10.3390/ph14101042
APA StyleMallone, F., Giustolisi, R., Franzone, F., Marenco, M., Plateroti, R., Nebbioso, M., Lambiase, A., & Gharbiya, M. (2021). Ten-Year Outcomes of Intravitreal Bevacizumab for Myopic Choroidal Neovascularization: Analysis of Prognostic Factors. Pharmaceuticals, 14(10), 1042. https://doi.org/10.3390/ph14101042








