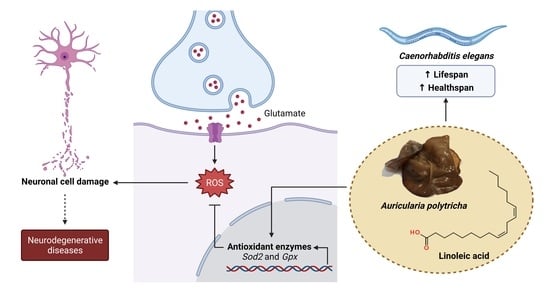
Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts
Abstract
:1. Introduction
2. Results
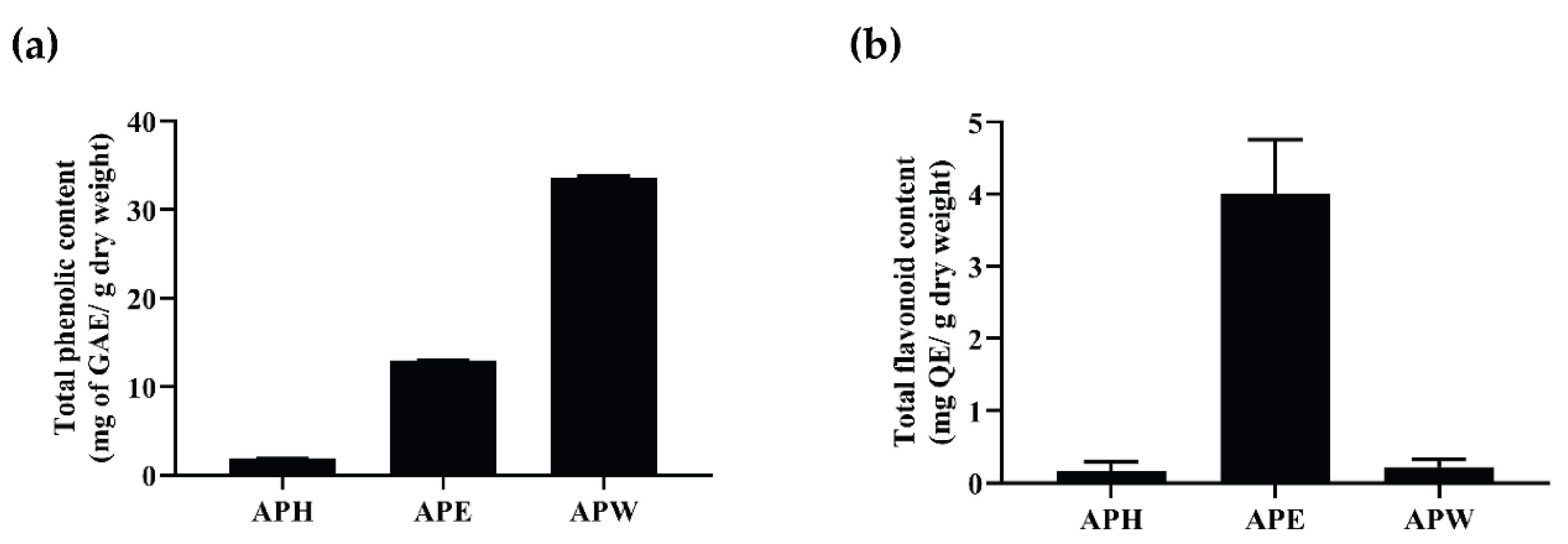
2.1. Total Phenolic and Flavonoid Contents Present in AP Crude Extracts
2.2. Free Radical Scavenging Activity of AP Crude Extracts
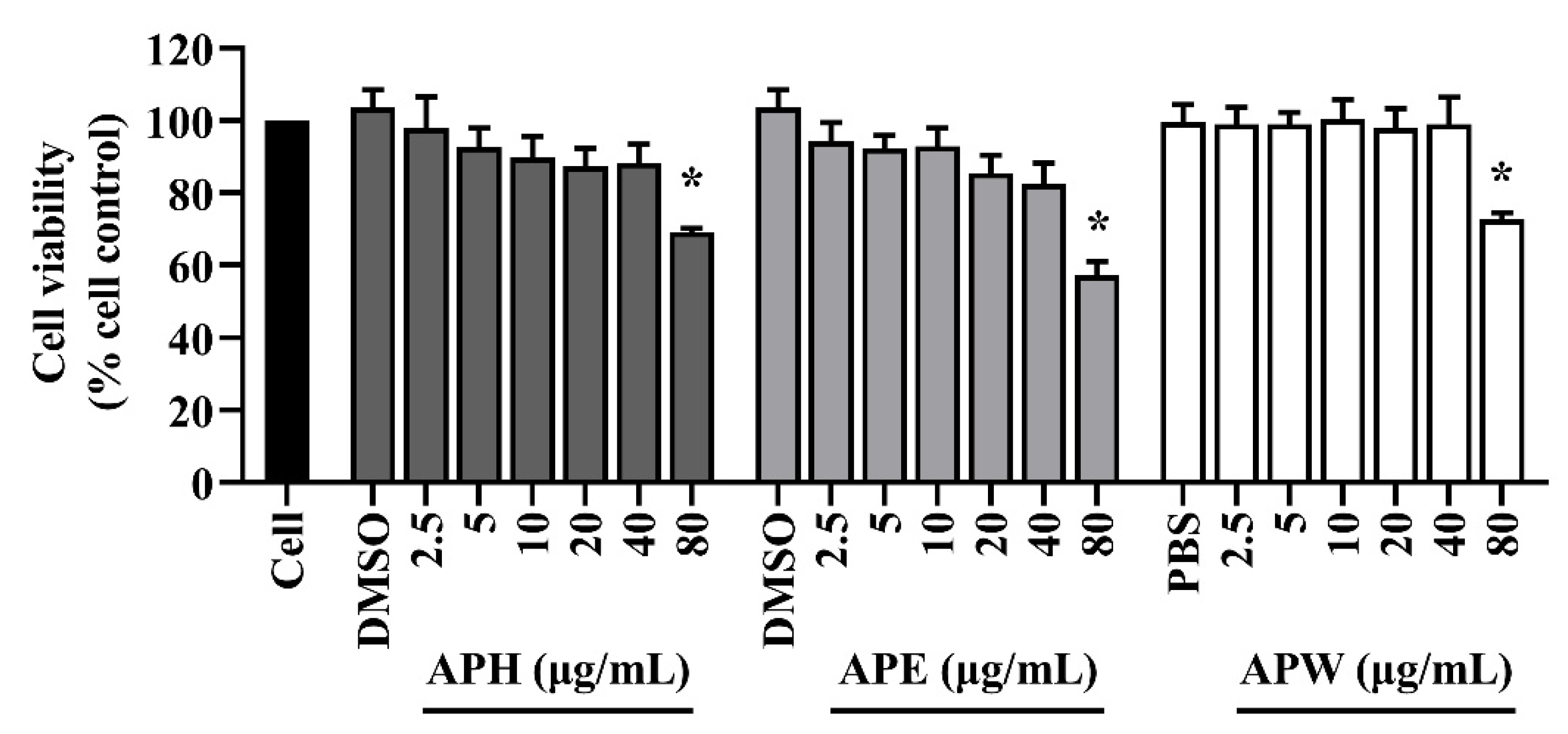
2.3. Cytotoxicity of AP Crude Extracts on HT-22 Mouse Hippocampal Cells
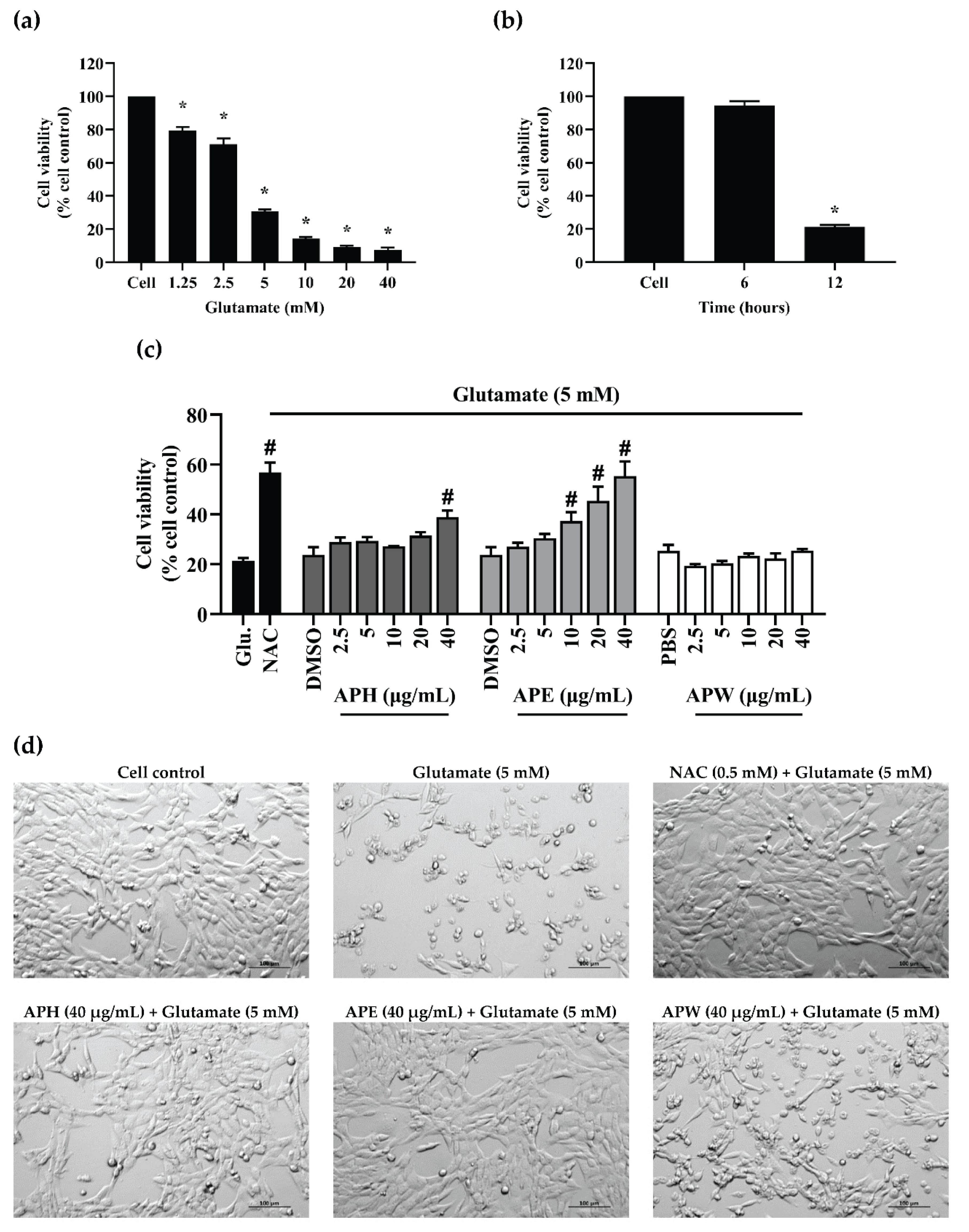
2.4. Glutamate-Induced HT-22 Neurotoxicity and Neuroprotective Effects of AP Crude Extracts
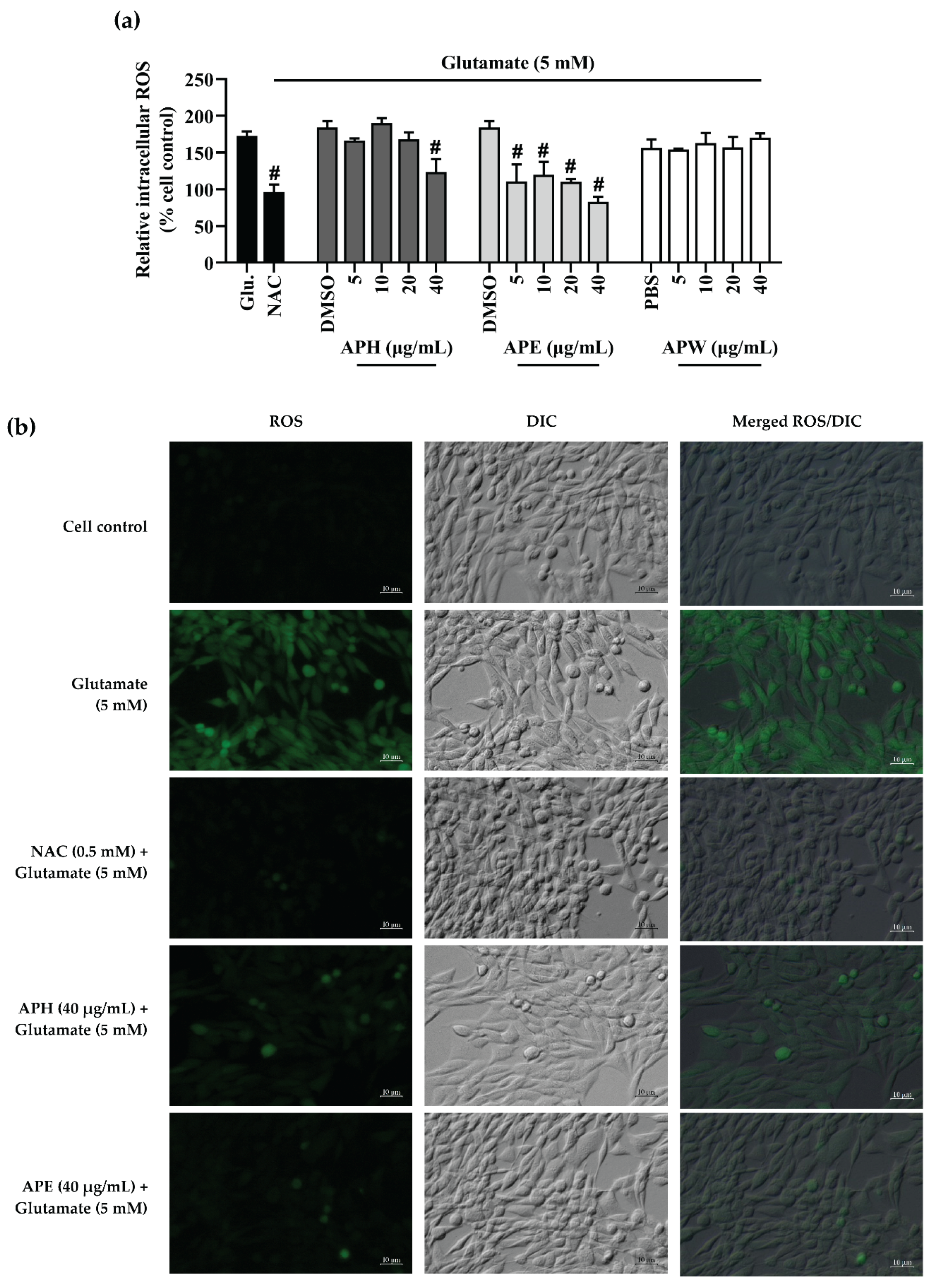
2.5. Intracellular Reactive Oxygen Species (ROS) Inhibitory Effect of AP Crude Extracts on Glutamate-Induced HT-22 Cell Toxicity
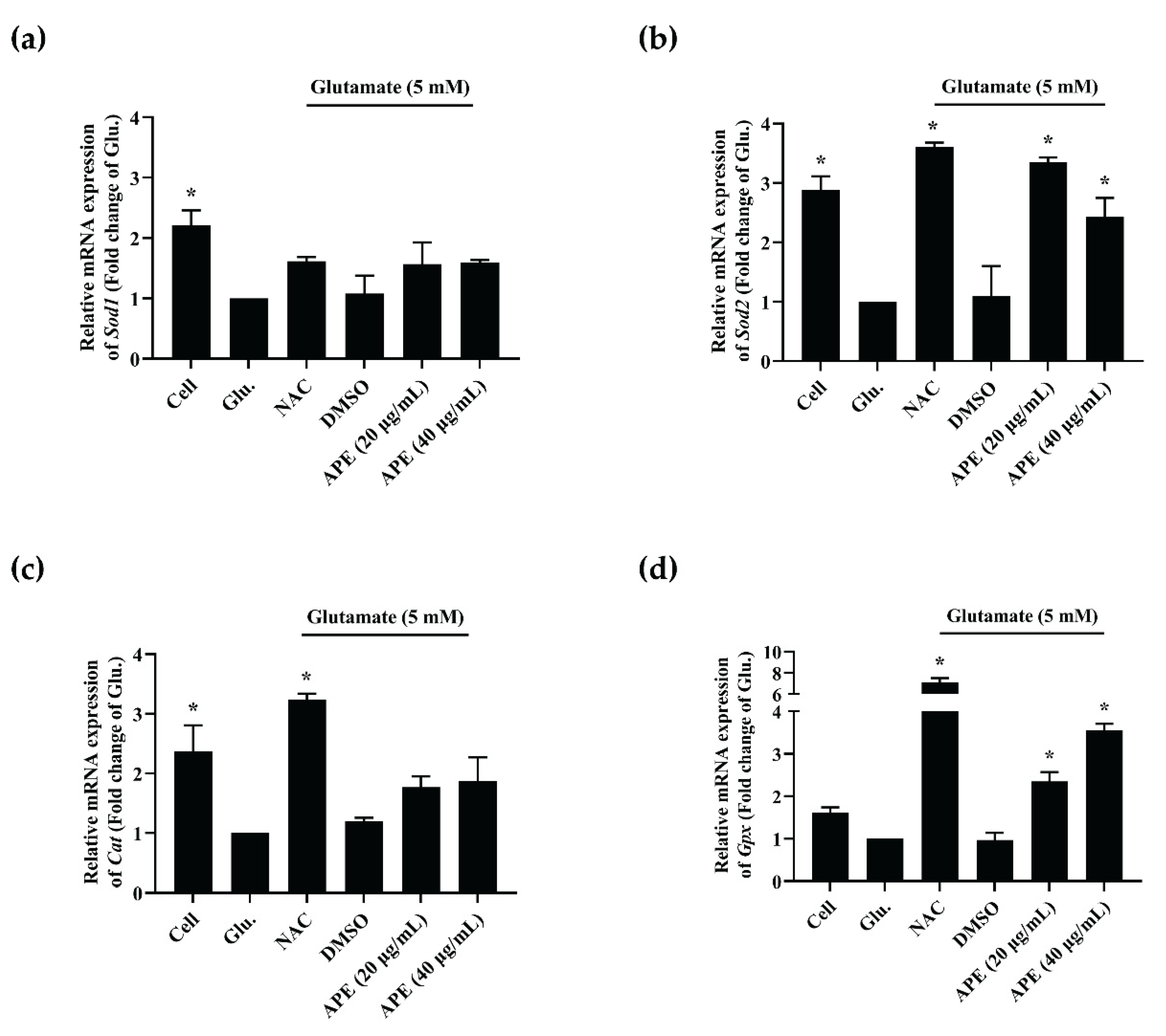
2.6. The Effect of APE Crude Extract on mRNA Expression of Antioxidant Enzymes in Glutamate-Induced HT-22 Cells
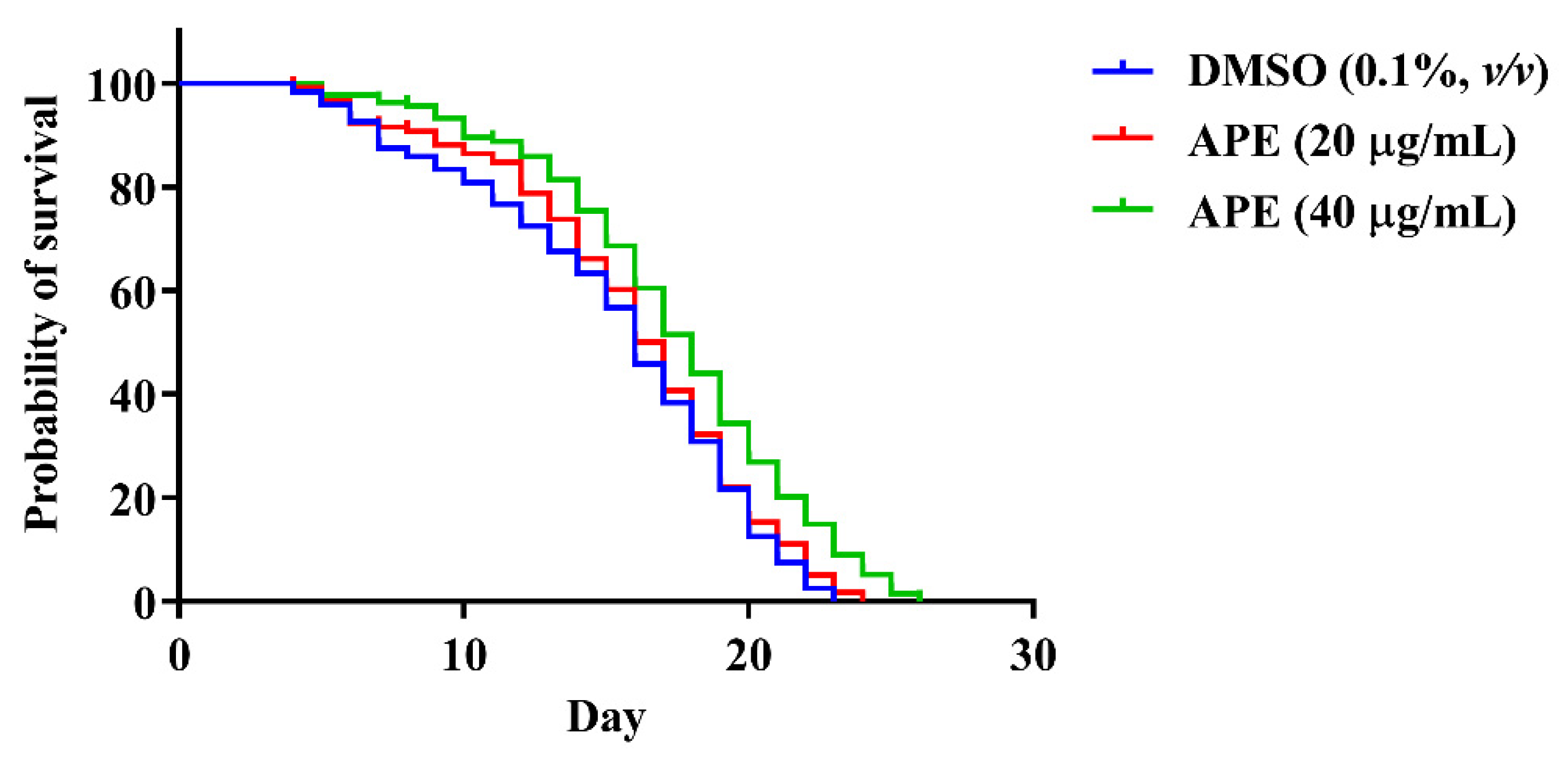
2.7. The Effect of AP Crude Extracts on C. elegans Lifespan
2.8. The Effects of the APE Extract on Age-Related Decline in Pharyngeal Pumping of C. elegans
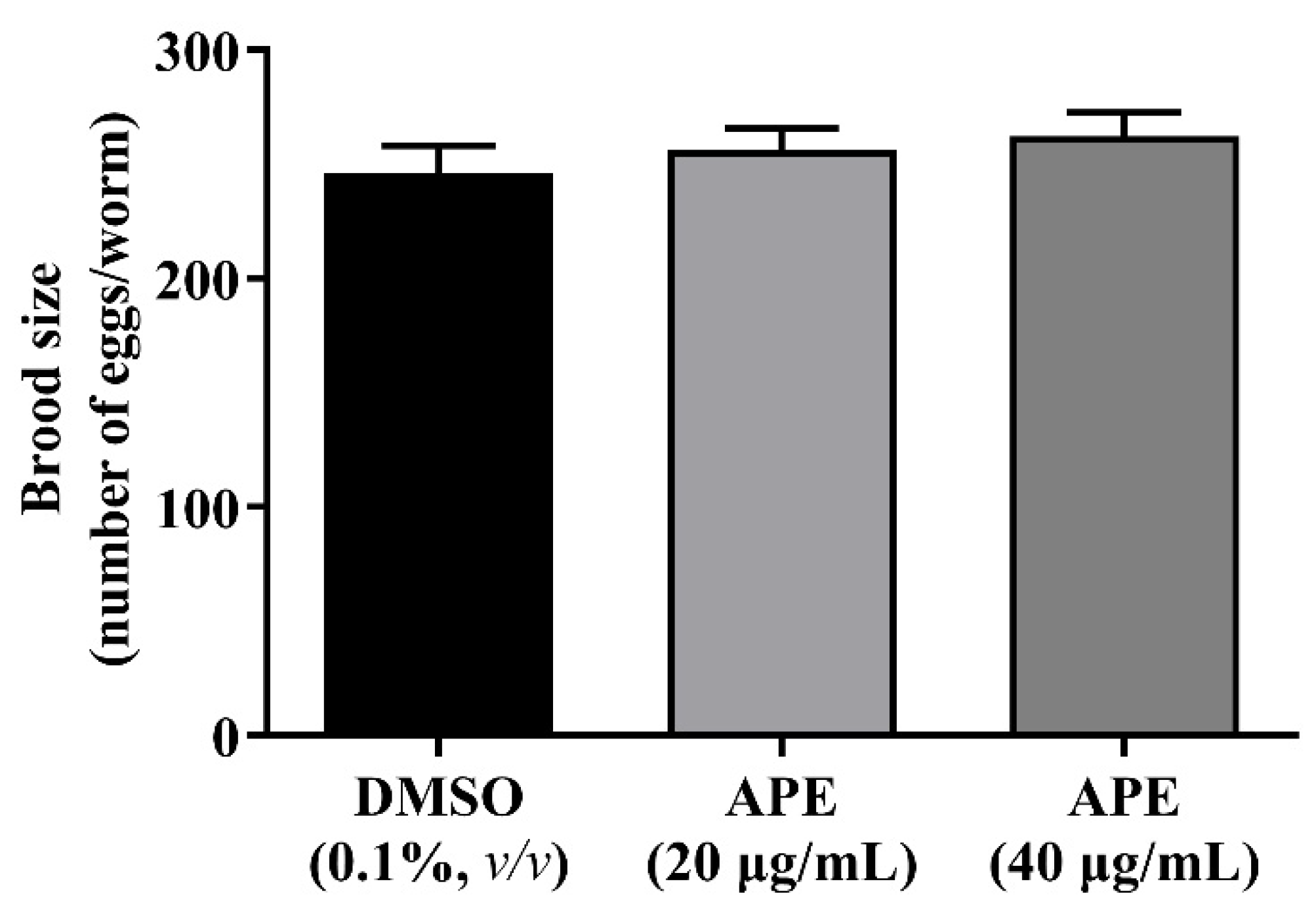
2.9. The Effect of the APE Extract on C. elegans Spawning
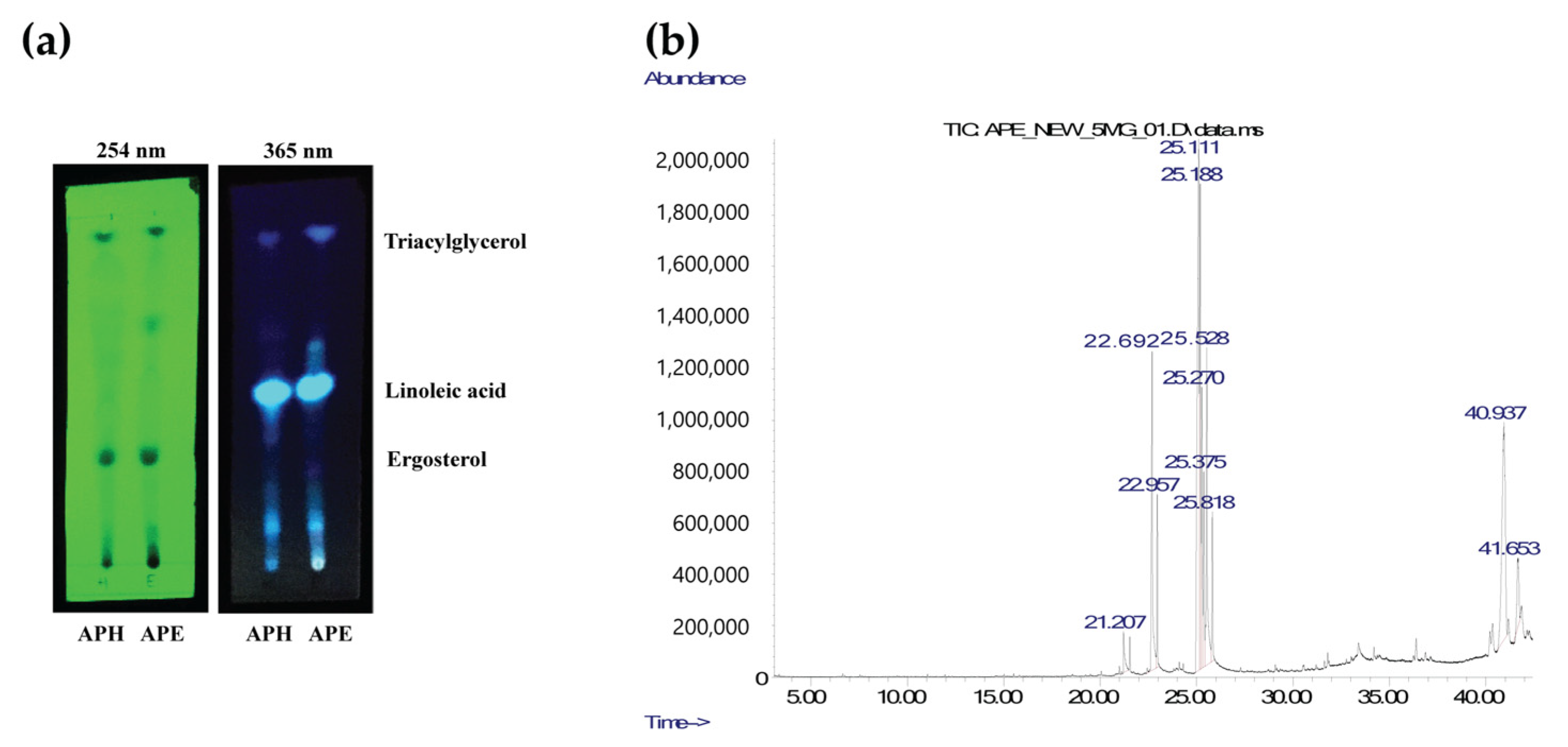
2.10. Analysis of Lipophilic Compounds
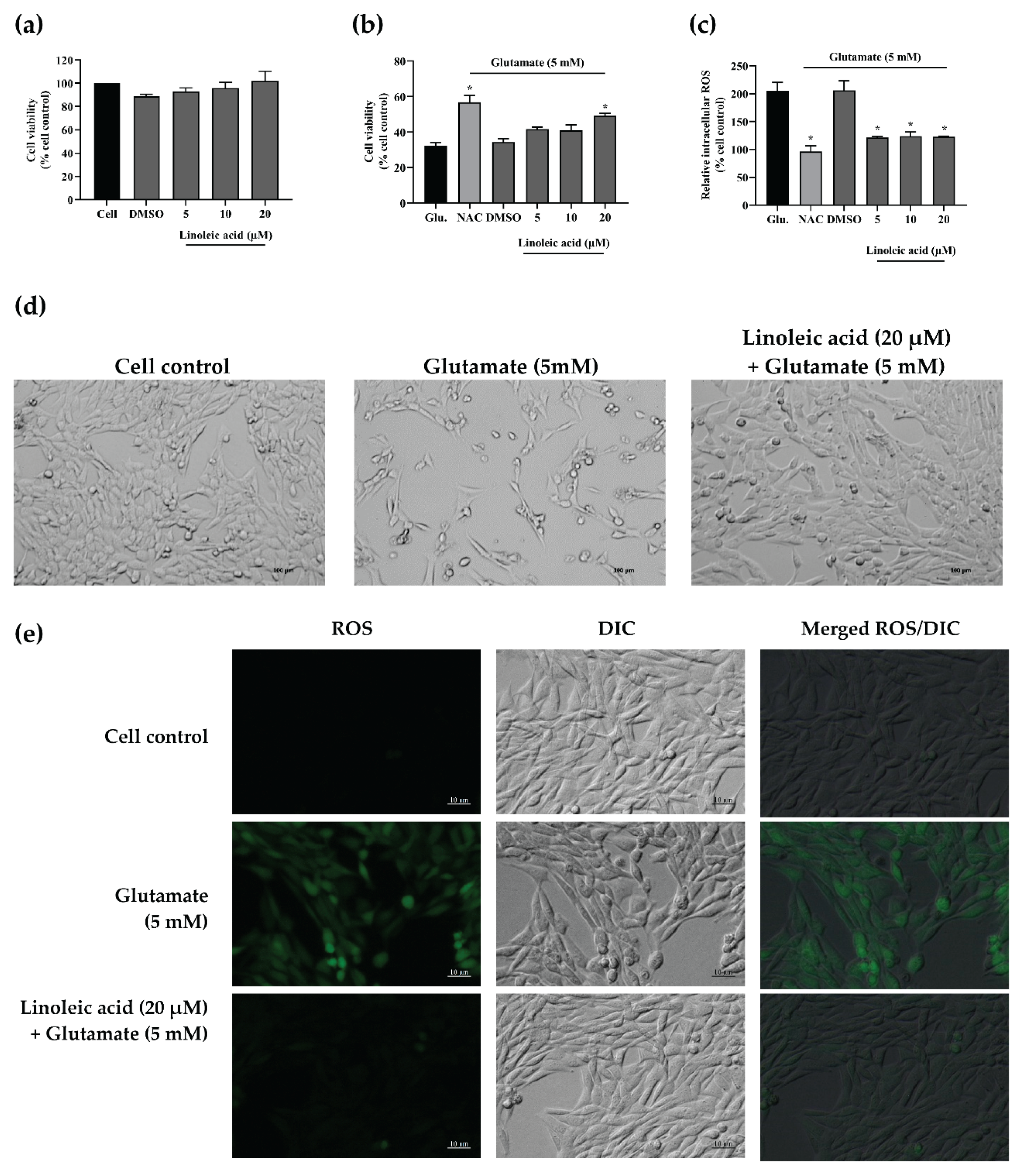
2.11. Neuroprotective Effects of Linoleic Acid on Glutamate-Induced HT-22 Cell Toxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Crude Extract Preparation
4.3. Total Phenolic Content Analysis
4.4. Total Flavonoid Content Analysis
4.5. Free Radical Scavenging Assays
4.5.1. ABTS Assay
4.5.2. DPPH Assay
4.6. Cell Culture
4.7. HT-22 Cytotoxicity of Crude Extracts
4.8. Glutamate-Induced HT-22 Neurotoxicity
4.9. Intracellular Reactive Oxygen Species (ROS) Assay
4.10. RNA Isolation and Reverse Transcription-Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.11. Caenorhabditis elegans Maintenance, Synchronization, and Treatment
4.12. Lifespan Assay
4.13. Pharyngeal Pumping Rate
4.14. Brood Size Assay
4.15. Statistical Analysis
4.16. Thin-Layer Chromatography (TLC)
4.17. Gas Chromatography/Mass Spectrometry (GC/MS)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.P.; Rai, S.N.; Dubey, S.K.; Pandey, A.T.; Tabassum, N.; Chaturvedi, V.K.; Singh, N.B. Biomolecules of mushroom: A recipe of human wellness. Crit. Rev. Biotechnol. 2021, 1–18. [Google Scholar]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complementary Med. 2021, 11, 158–172. [Google Scholar] [CrossRef]
- Teke, N.; Kinge, T.; Bechem, E.; Nji, T.; Ndam, L.; Mih, A. Ethnomycological study in the kilum-ijim mountain forest, northwest region, cameroon. J. Ethnobiol. Ethnomedicine 2018, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Sillapachaiyaporn, C.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S. Anti-HIV-1 protease activity of the crude extracts and isolated compounds from Auricularia polytricha. BMC Complementary Altern. Med. 2019, 19, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Du, Q. Isolation of a polysaccharide with anticancer activity from Auricularia polytricha using high-speed countercurrent chromatography with an aqueous two-phase system. J. Chromatogr. A 2010, 1217, 5930–5934. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, R.; Zhao, Z.; Wang, Y. Auricularia polytricha polysaccharides induce cell cycle arrest and apoptosis in human lung cancer A549 cells. Int. J. Biol. Macromol. 2014, 68, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, X.; Qing, Y.; Luo, X.; Yang, Z.; Zheng, L. Isolation of an anti-tumor polysaccharide from Auricularia polytricha (jew’s ear) and its effects on macrophage activation. Eur. Food Res. Technol. 2009, 228, 477–485. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kwon, K.M.; Lee, S.H. Evaluation of the antioxidant activities and tyrosinase inhibitory property from mycelium culture extracts. Evid.-Based Complementary Altern. Med. 2015, 2015, 616298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.; Billah Prince, M.; Tanvir, E.; Chowdhury, M.; Rahman, M.; Alam, F.; Paul, S.; Saha, M.; Ali, M.; Bhoumik, N.C. Ganoderma lucidum and Auricularia polytricha mushrooms protect against Carbofuran-induced toxicity in rats. Evid.-Based Complementary Altern. Med. 2018, 2018, 6254929. [Google Scholar] [CrossRef] [Green Version]
- K Chellappan, D.; Ganasen, S.; Batumalai, S.; Candasamy, M.; Krishnappa, P.; Dua, K.; Chellian, J.; Gupta, G. The protective action of the aqueous extract of Auricularia polytricha in paracetamol induced hepatotoxicity in rats. Recent Pat. Drug Deliv. Formul. 2016, 10, 72–76. [Google Scholar] [CrossRef]
- Chiu, W.-C.; Yang, H.-H.; Chiang, S.-C.; Chou, Y.-X.; Yang, H.-T. Auricularia polytricha aqueous extract supplementation decreases hepatic lipid accumulation and improves antioxidative status in animal model of nonalcoholic fatty liver. BioMedicine 2014, 4, 29–38. [Google Scholar] [CrossRef]
- Bennett, L.; Sheean, P.; Zabaras, D.; Head, R. Heat-stable components of wood ear mushroom, Auricularia polytricha (higher Basidiomycetes), inhibit in vitro activity of beta secretase (BACE1). Int. J. Med. Mushrooms 2013, 15, 233–249. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Dong, X.-X.; Wang, Y.; Qin, Z.-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Salińska, E.; Danysz, W.; Łazarewicz, J.W. The role of excitotoxicity in neurodegeneration. Folia Neuropathol. 2006, 43, 322–339. [Google Scholar]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Gu, X.; Wink, M.; Tencomnao, T. Vitis vinifera leaf extract protects against glutamate-induced oxidative toxicity in HT22 hippocampal neuronal cells and increases stress resistance properties in Caenorhabditis elegans. Front. Nutr. 2021, 8, 250. [Google Scholar]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complementary Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Sit, K.P.; Wenk, M.R.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Zhang, X.; Su, Z.; Xiao, J.; Lv, M.; Cao, Y.; Chen, Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 5958043. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Hernández-García, S.; García-Carmona, F.; Gandía-Herrero, F. Extension of life-span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans. Food Chem. 2019, 274, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Schlernitzauer, A.; Oiry, C.; Hamad, R.; Galas, S.; Cortade, F.; Chabi, B.; Casas, F.; Pessemesse, L.; Fouret, G.; Feillet-Coudray, C. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE 2013, 8, e78788. [Google Scholar] [CrossRef] [PubMed]
- Avellini, L.; Terracina, L.; Gaiti, A. Linoleic acid passage through the blood-brain barrier and a possible effect of age. Neurochem. Res. 1994, 19, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid transport and metabolism at the blood-brain interface: Implications in health and disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Emre, A.; Cagatay, G.; Avci, G.A.; Suiçmez, M.; Cevher, S.C. An edible mushroom with medicinal significance; Auricularia polytricha. Hittite J. Sci. Eng. 2016, 3, 111–116. [Google Scholar]
- Coyle, J.T.; Puttfarcken, P.J.S. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Lee, I.-K.; Yun, B.-S.; Kim, J.-P.; Ryoo, I.-J.; Kim, Y.-H.; Yoo, I.-D. Neuroprotective activity of p-terphenyl leucomentins from the mushroom Paxillus panuoides. Biosci. Biotechnol. Biochem. 2003, 67, 1813–1816. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Lu, W.; Zhang, S.; Guan, X.; Li, Z.; Wang, D. Anti-oxidative stress activity is essential for Amanita caesarea mediated neuroprotection on glutamate-induced apoptotic HT22 cells and an Alzheimer’s disease mouse model. Int. J. Mol. Sci. 2017, 18, 1623. [Google Scholar] [CrossRef] [Green Version]
- Kittimongkolsuk, P.; Pattarachotanant, N.; Chuchawankul, S.; Wink, M.; Tencomnao, T. Neuroprotective Effects of Extracts from Tiger Milk Mushroom Lignosus rhinocerus Against Glutamate-Induced Toxicity in HT22 Hippocampal Neuronal Cells and Neurodegenerative Diseases in Caenorhabditis elegans. Biology 2021, 10, 30. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Meemon, K.; Sobhon, P.; Tencomnao, T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complementary Altern. Med. 2017, 17, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-S.; Kim, W.-Y.; Jeon, Y.-J.; Lee, S.-K.; Son, C.-G. Aquilariae lignum extract attenuates glutamate-induced neuroexcitotoxicity in HT22 hippocampal cells. Biomed. Pharmacother. 2018, 106, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Francisqueti-Ferron, F.V.; Ferron, A.J.T.; Garcia, J.L.; Silva, C.C.V.d.A.; Costa, M.R.; Gregolin, C.S.; Moreto, F.; Ferreira, A.L.A.; Minatel, I.O.; Correa, C.R. Basic concepts on the role of nuclear factor erythroid-derived 2-like 2 (Nrf2) in age-related diseases. Int. J. Mol. Sci. 2019, 20, 3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Wink, M.; Tencomnao, T. Anacardium occidentale L. Leaf Extracts Protect against Glutamate/H2O2-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein. Front. Pharmacol. 2021, 12, 627738. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T.J.N. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Liu, J.; Zhu, Z.; Hu, Z.J.J.o.R.; Transduction, S. Protective effect of N-acetylcysteine (NAC) on renal ischemia/reperfusion injury through Nrf2 signaling pathway. J. Recept. Signal Transduct. 2014, 34, 396–400. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.; Walder, K.; Berk, M.; Marx, W.; Carvalho, A.; Maes, M.; Puri, B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021, 58, 2158–2182. [Google Scholar] [CrossRef]
- Zhang, S.; Duangjan, C.; Tencomnao, T.; Liu, J.; Lin, J.; Wink, M. Neuroprotective effects of oolong tea extracts against glutamate-induced toxicity in cultured neuronal cells and β-amyloid-induced toxicity in Caenorhabditis elegans. Food Funct. 2020, 11, 8179–8192. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Gu, X.; Wink, M.; Tencomnao, T. Neuroprotective Effects of Glochidion zeylanicum Leaf Extract against H2O2/Glutamate-Induced Toxicity in Cultured Neuronal Cells and Aβ-Induced Toxicity in Caenorhabditis elegans. Biology 2021, 10, 800. [Google Scholar] [CrossRef]
- Jushaj, A.; Churgin, M.; Yao, B.; De La Torre, M.; Fang-Yen, C.; Temmerman, L. Optimized criteria for locomotion-based healthspan evaluation in C. elegans using the WorMotel system. PLoS ONE 2020, 15, e0229583. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zuo, X.; Ouyang, Z.; Qiao, P.; Wang, F. A Systematic Review of Antiaging Effects of 23 Traditional Chinese Medicines. Evid.-Based Complementary Altern. Med. 2021, 2021, 5591573. [Google Scholar]
- Zhang, L.; Gu, B.; Wang, Y. Clove essential oil confers antioxidant activity and lifespan extension in C. elegans via the DAF-16/FOXO transcription factor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108938. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Ferrandino, I.; Boscaino, F.; Cocca, E.; Cigliano, L.; Maurano, F.; Luongo, D.; Spagnuolo, M.S.; Rossi, M.; Bergamo, P. Conjugated linoleic acid prevents age-dependent neurodegeneration in a mouse model of neuropsychiatric lupus via the activation of an adaptive response. J. Lipid Res. 2018, 59, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Shatshat, A.; Pham, A.T.; Rao, P.P. Interactions of polyunsaturated fatty acids with amyloid peptides Aβ40 and Aβ42. Arch. Biochem. Biophys. 2019, 663, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ikram, M.; Park, H.Y.; Jo, M.G.; Ullah, R.; Ahmad, S.; Abid, N.B.; Kim, M.O. Oral administration of alpha linoleic acid rescues Aβ-induced glia-mediated neuroinflammation and cognitive dysfunction in C57BL/6N mice. Cells 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Park, S.H.; Han, J.H.; Hong, Y.K.; Hwang, S.; Lee, S.; Kim, D.; Han, S.Y.; Kim, E.S.; Cho, K.S. The effects of hempseed meal intake and linoleic acid on Drosophila models of neurodegenerative diseases and hypercholesterolemia. Mol. Cells 2011, 31, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Liao, D.-C.; Yu, Y.-T.; Wei, C.-M.; Xuan, L.-Y.; Li, S.; Wang, H.-B. Coix seed oil prolongs lifespan and enhances stress resistance in Caenorhabditis elegans. Biogerontology 2020, 21, 245–256. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbiydzenyuy, N.E.; Ninsiima, H.I.; Valladares, M.B.; Pieme, C.A. Zinc and linoleic acid pre-treatment attenuates biochemical and histological changes in the midbrain of rats with rotenone-induced Parkinsonism. BMC Neurosci. 2018, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, N.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Protective effect of alpha-linoleic acid on Aβ-induced oxidative stress, neuroinflammation, and memory impairment by alteration of α7 nAChR and NMDAR gene expression in the hippocampus of rats. NeuroToxicology 2021, 85, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorganic Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef]
- Weng, Y.; Lu, J.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides C and D, two new anti-aging ergosterols from spores of the medicinal mushroom Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2011, 75, 800–803. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.; Rinker, D.L.; Tsao, R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef]
- Kuete, V.; Karaosmanoğlu, O.; Sivas, H. Anticancer activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–297. [Google Scholar]
- Septisetyani, E.P.; Ningrum, R.A.; Romadhani, Y.; Wisnuwardhani, P.H.; Santoso, A. Optimization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5-dimethylthiazo-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay with wst-1 assay in mcf-7 cells. Indones. J. Pharm. 2014, 25, 245–254. [Google Scholar] [CrossRef]

| Crude Extract | ABTS Scavenging Activity | DPPH Scavenging Activity | ||
|---|---|---|---|---|
| mg VCEAC/g Dry Weight | EC50 (mg/mL) | mg VCEAC/g Dry Weight | EC50 (mg/mL) | |
| APH | 0.92 ± 0.12 a | >8 | 0.99 ± 0.08 a, b | >8 |
| APE | 1.57 ± 0.04 | 3.11 ± 0.05 | 1.27 ± 0.04 a, c | 12.61 ± 3.47 |
| APW | 2.47 ± 0.06 a | 1.06 ± 0.04 | 1.88 ± 0.25 b, c | 3.62 ± 0.35 |
| Treatment | Mean Lifespan | Maximum Lifespan | p-Value (vs. Control) | Number of Worms | ||
|---|---|---|---|---|---|---|
| Day ± SEM | % Increase (vs. Control) | Day ± SEM | % Increase (vs. Control) | |||
| DMSO (0.1%, v/v) | 15.20 ± 0.46 | - | 22.60 ± 0.24 | - | - | N = 120 |
| 20 µg/mL APE | 15.86 ± 0.43 | 4.34% | 23.50 ± 0.20 | 3.98% | 0.4017 | N = 118 |
| 40 µg/mL APE | 17.66 ± 0.41 | 16.18% | 25.33 ± 0.21 | 12.08% | 0.0013 * | N = 131 |
| RT (min) | Identified Compound | Amount (mg/g Lipophilic Content in APE) |
|---|---|---|
| 21.206 | Pentadecanoic acid | 14.7 |
| 22.693 | Palmitic acid | 105.5 |
| 22.958 | Palmitic acid, ethyl ester | 28.7 |
| 25.111 | Linoleic acid | 262.3 |
| 25.187 | cis-Vaccenic acid | 116.7 |
| 25.271 | Linoleic acid, ethyl ester | 69.3 |
| 25.375 | Oleic acid, ethyl ester | 46.3 |
| 25.527 | Stearic acid | 98.4 |
| 25.817 | Stearic acid, ethyl ester | 32.4 |
| 40.936 | Ergosterol | 191.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S.; Tencomnao, T. Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts. Pharmaceuticals 2021, 14, 1001. https://doi.org/10.3390/ph14101001
Sillapachaiyaporn C, Rangsinth P, Nilkhet S, Ung AT, Chuchawankul S, Tencomnao T. Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts. Pharmaceuticals. 2021; 14(10):1001. https://doi.org/10.3390/ph14101001
Chicago/Turabian StyleSillapachaiyaporn, Chanin, Panthakarn Rangsinth, Sunita Nilkhet, Alison T. Ung, Siriporn Chuchawankul, and Tewin Tencomnao. 2021. "Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts" Pharmaceuticals 14, no. 10: 1001. https://doi.org/10.3390/ph14101001
APA StyleSillapachaiyaporn, C., Rangsinth, P., Nilkhet, S., Ung, A. T., Chuchawankul, S., & Tencomnao, T. (2021). Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts. Pharmaceuticals, 14(10), 1001. https://doi.org/10.3390/ph14101001