In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CPC/Hydrogel Composites
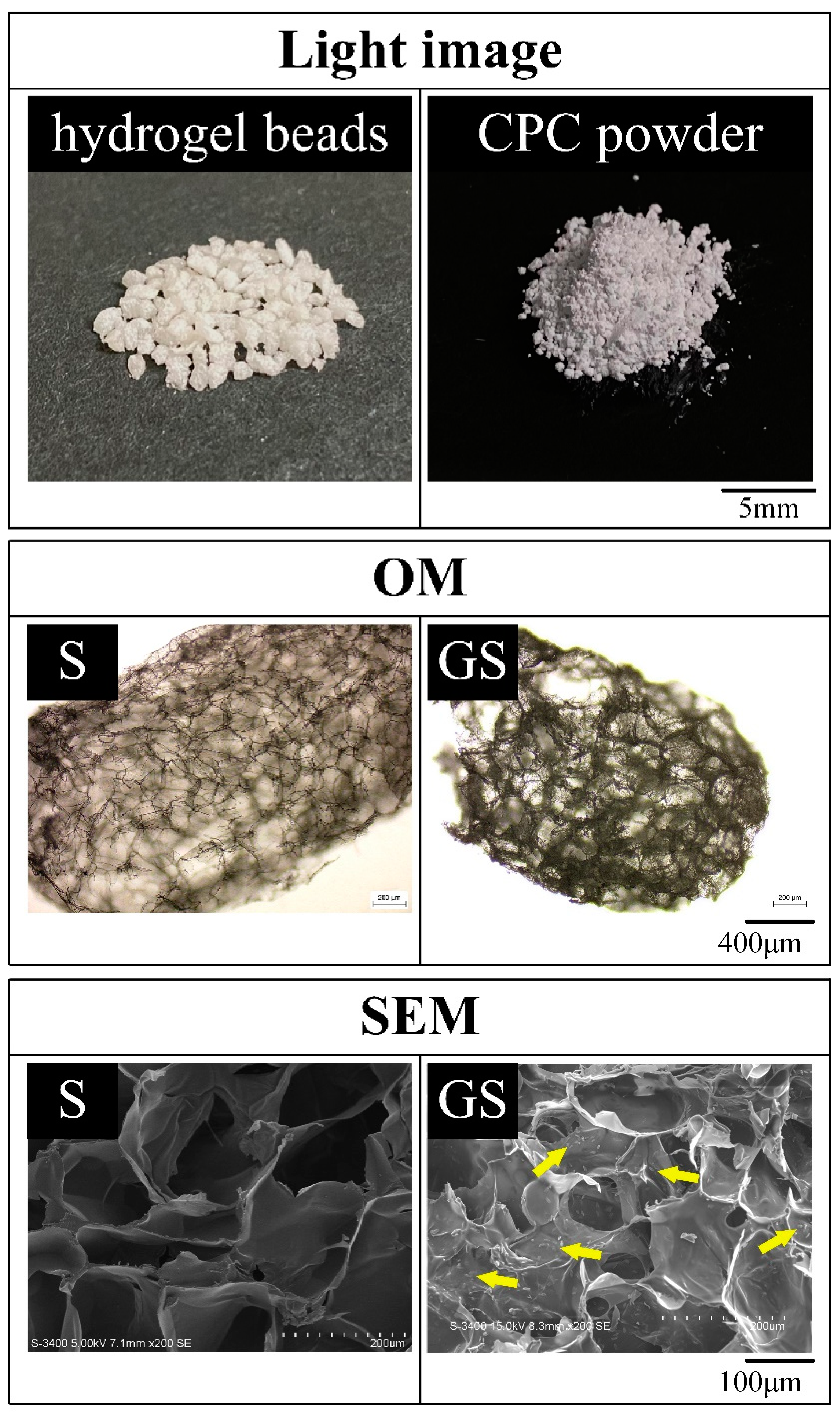
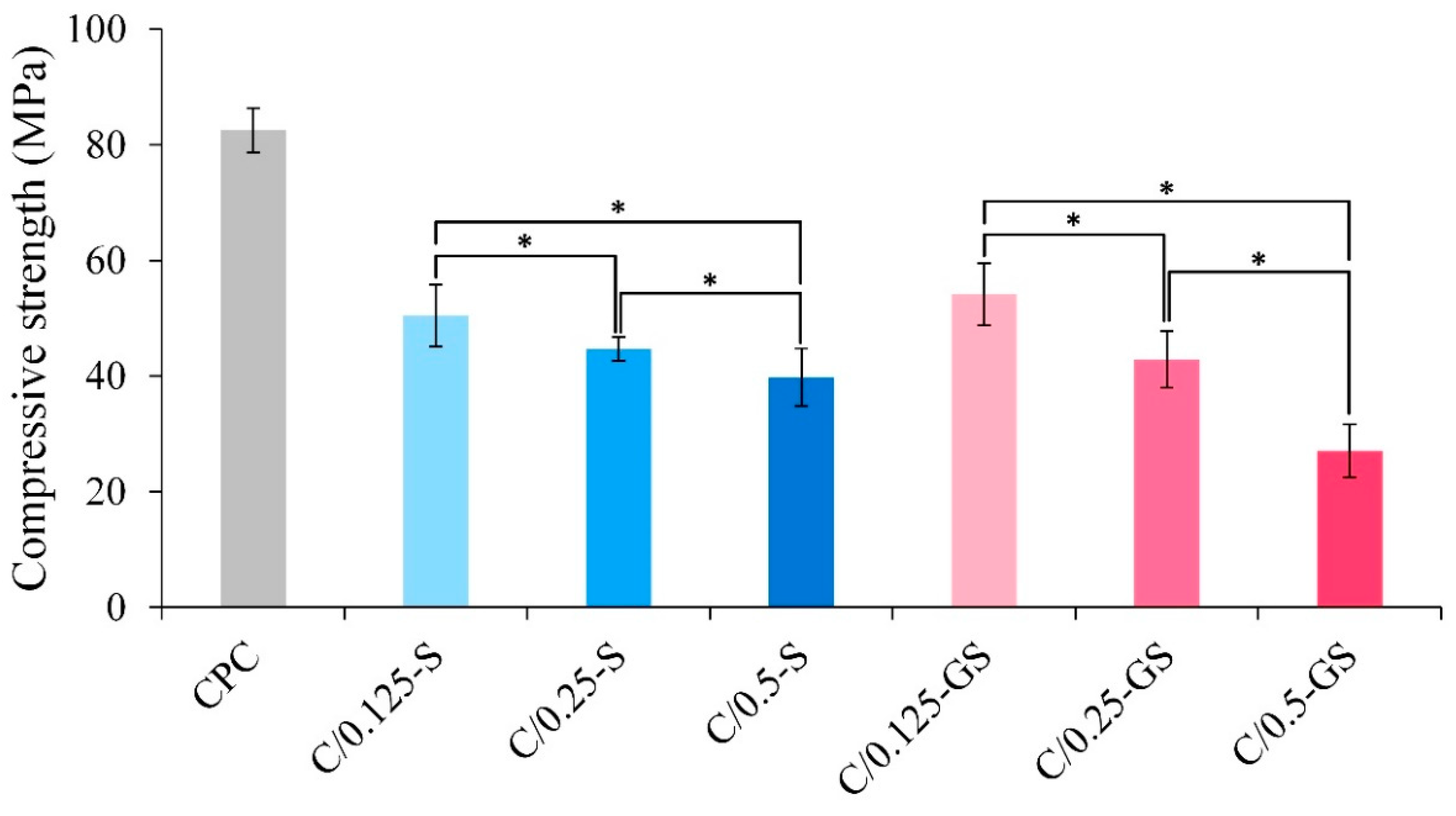
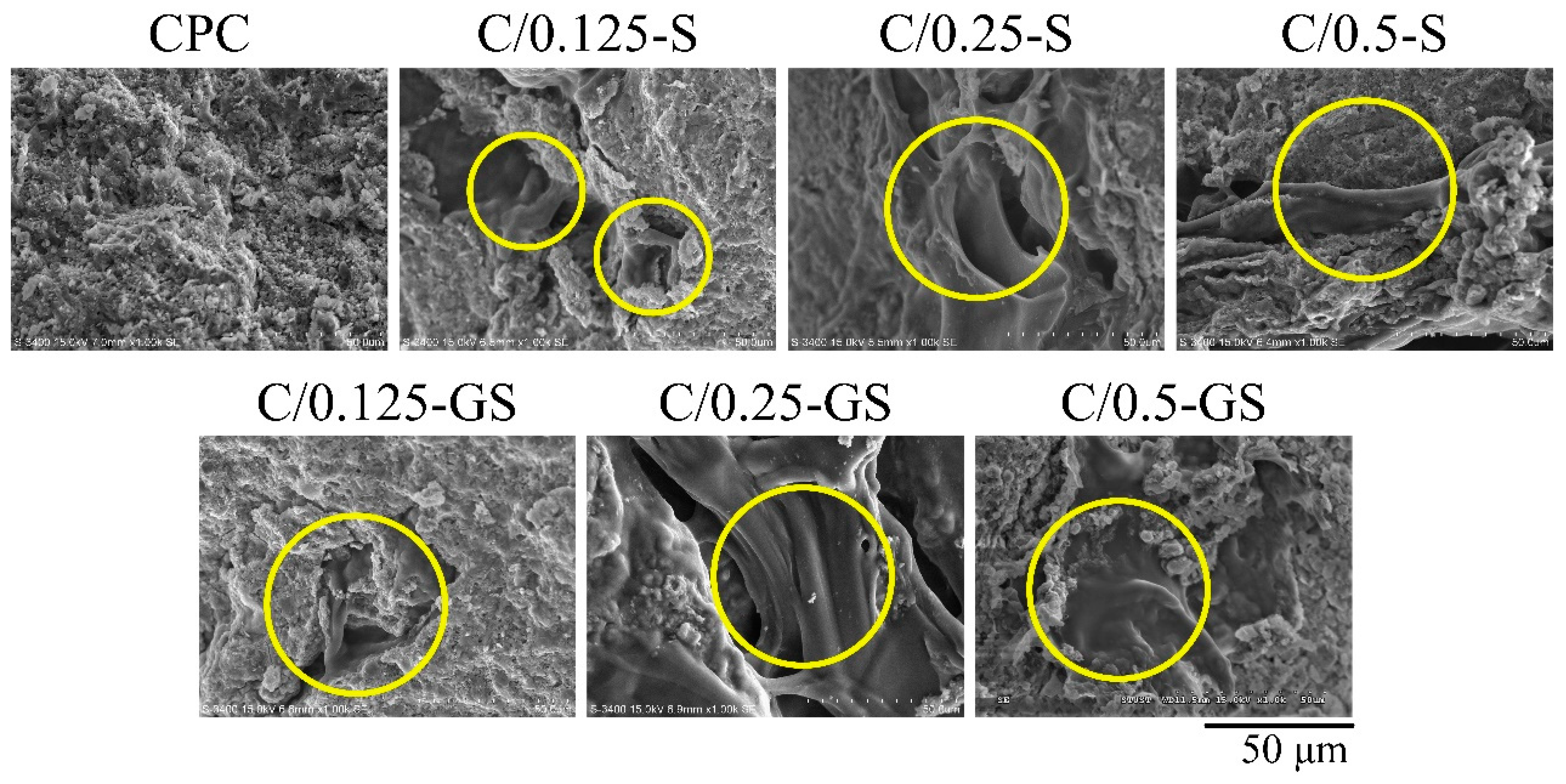
2.1.1. Hydrogel Structures, Compressive Strength, and Fracture Surface of CPC/Hydrogel Composites
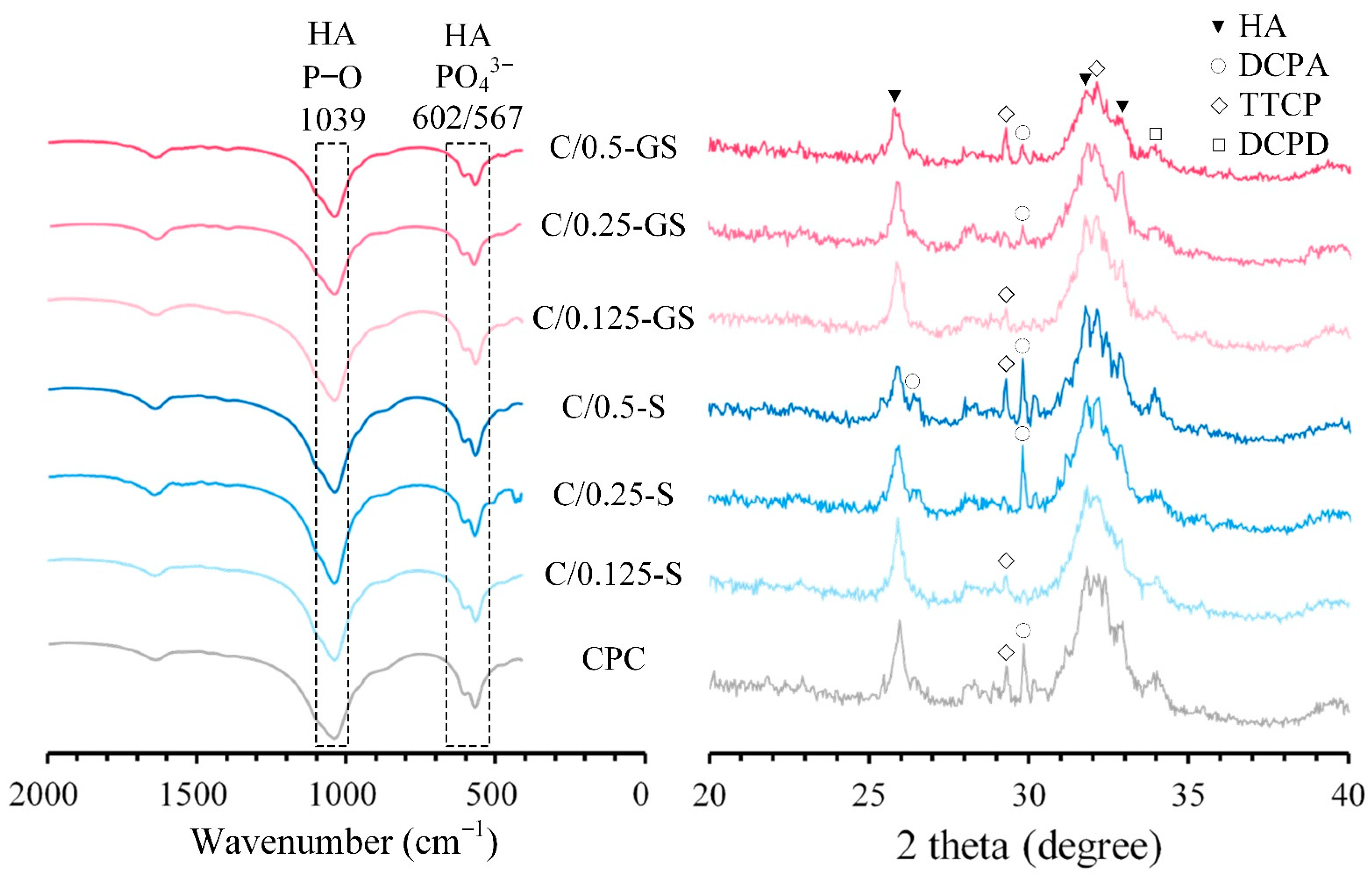
2.1.2. Attenuated Total Reflection–Fourier Transform Infrared (ATR-FTIR) Spectroscopy and X-ray Diffraction (XRD) Patterns
2.1.3. Working/Setting Time, Injectability, and Dispersibility
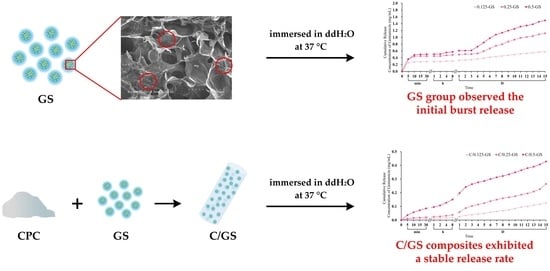
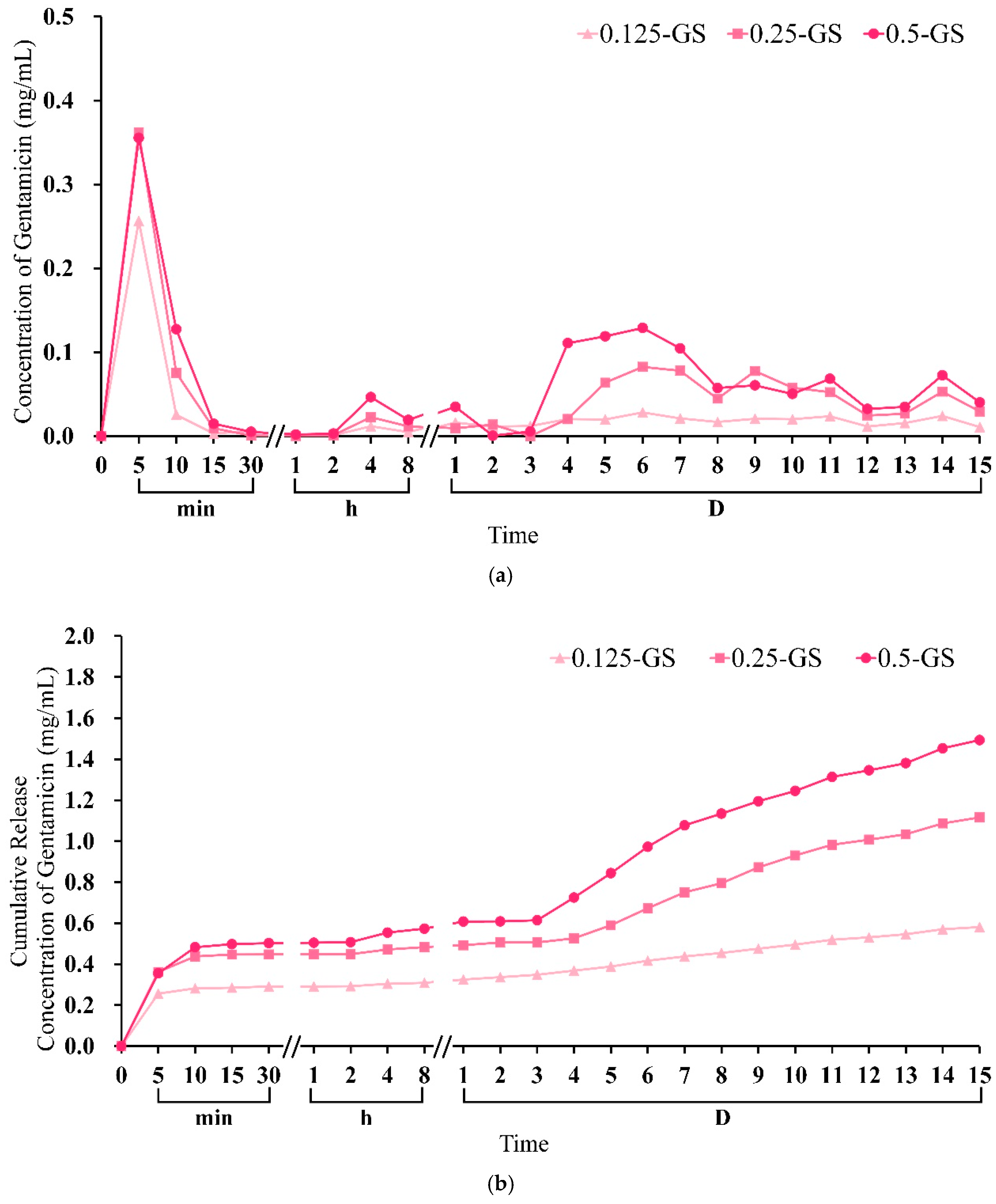
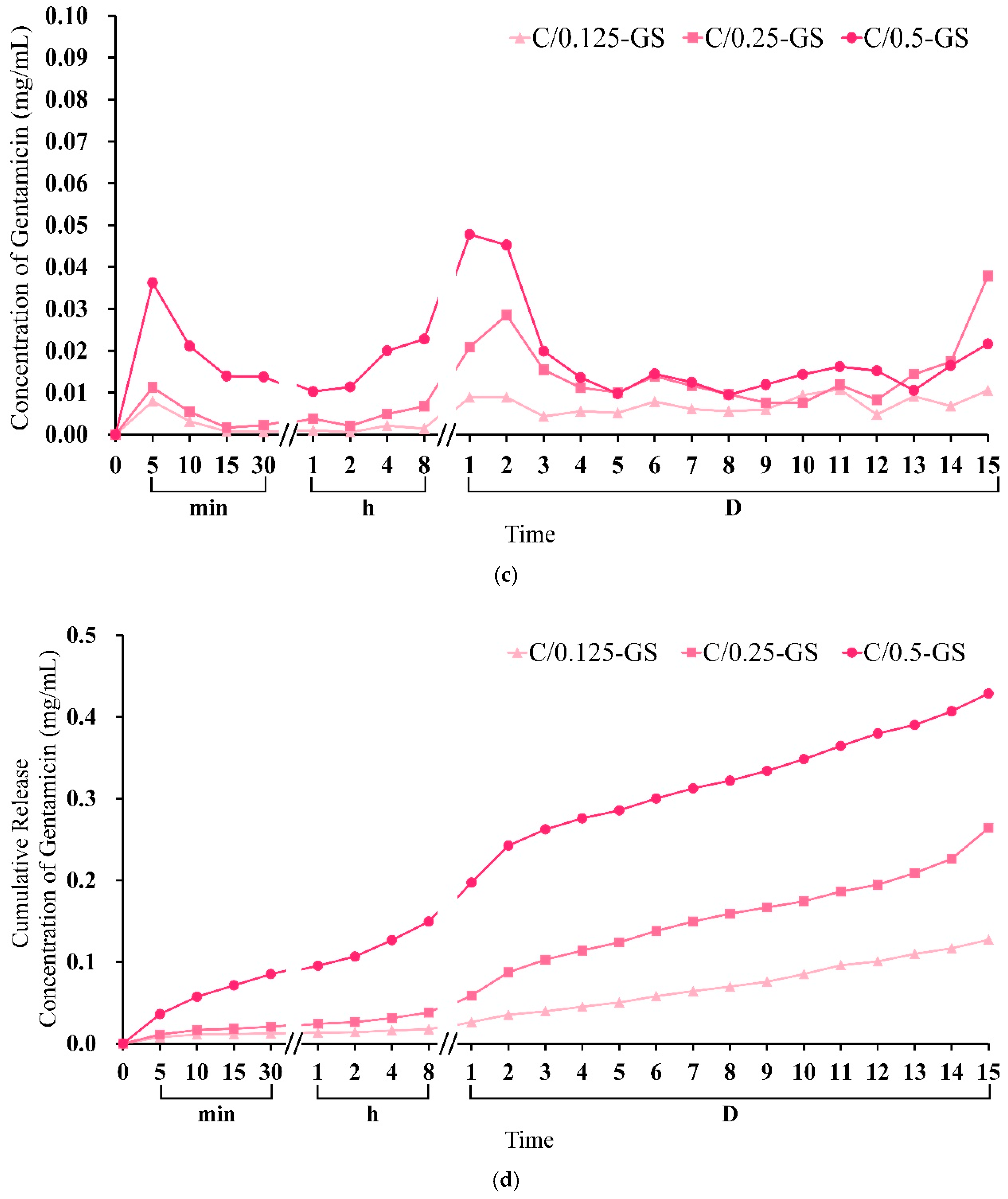
2.1.4. Gentamicin Release from CPC/Hydrogel
2.2. Antibacterial Activity, Biocompatibility, and Mineralization of CPC/Hydrogel
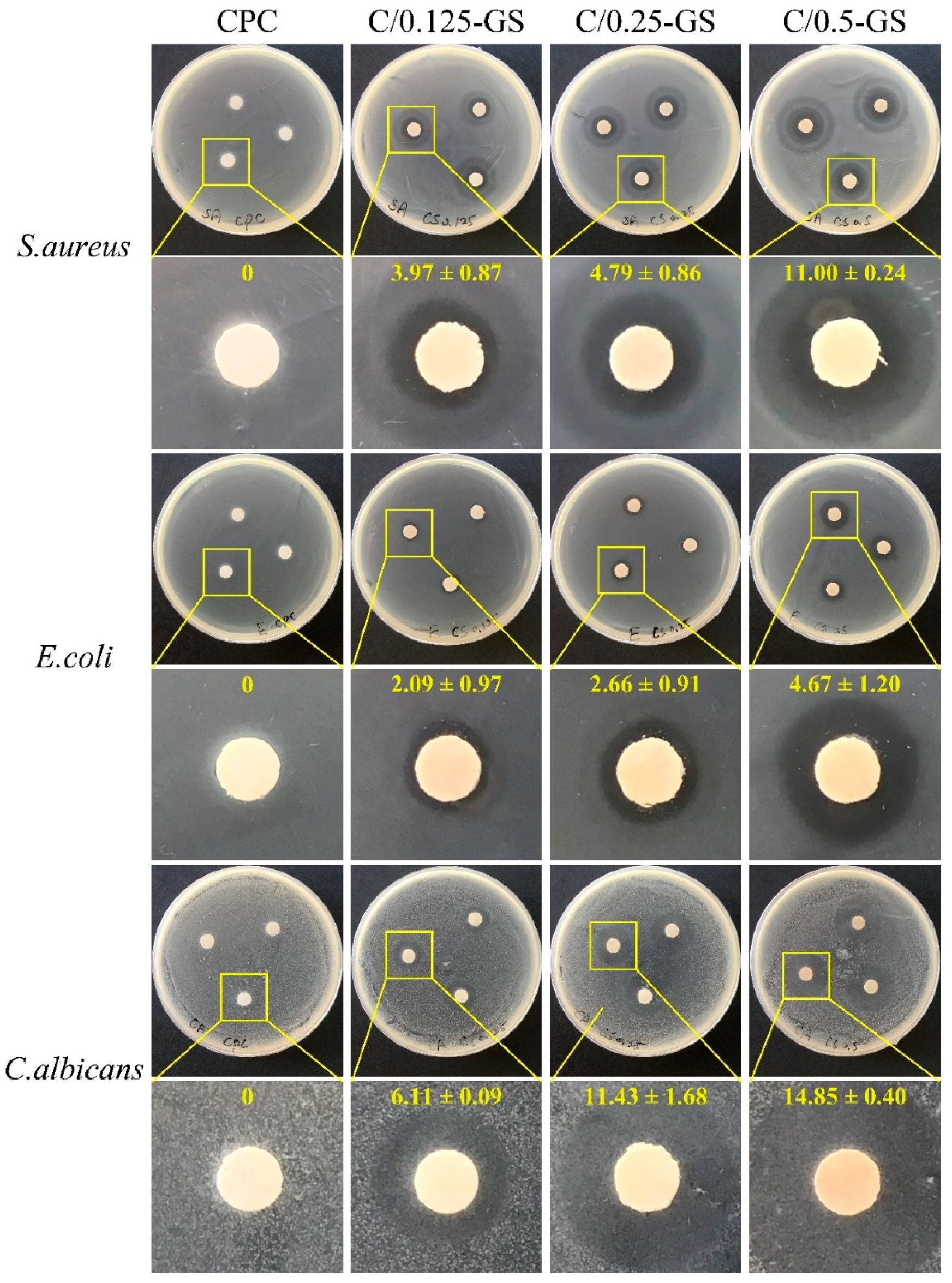
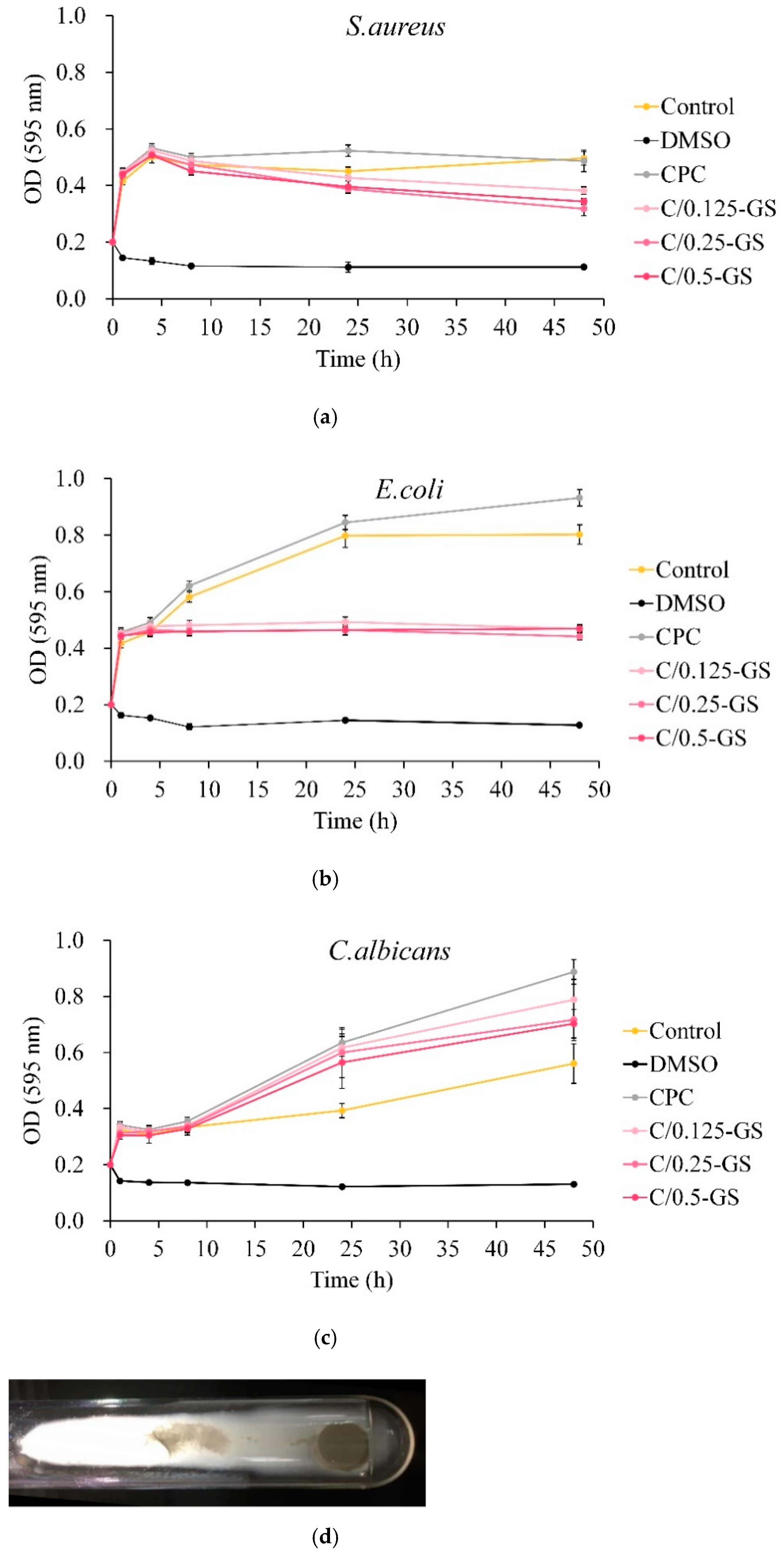
2.2.1. Antibacterial Ability of CPC/Hydrogel with Gentamicin
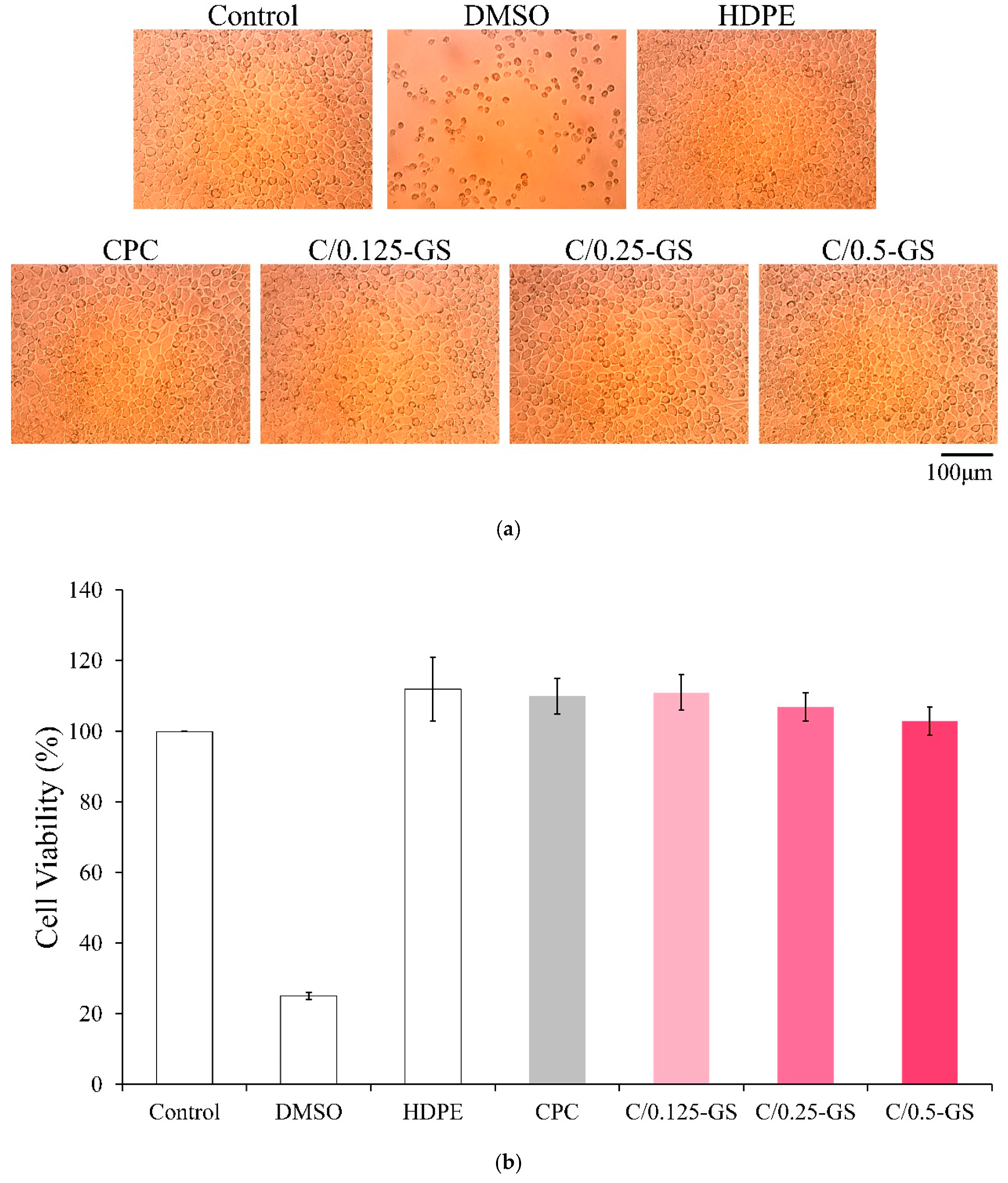
2.2.2. Cytotoxicity of CPC/Hydrogel Composites
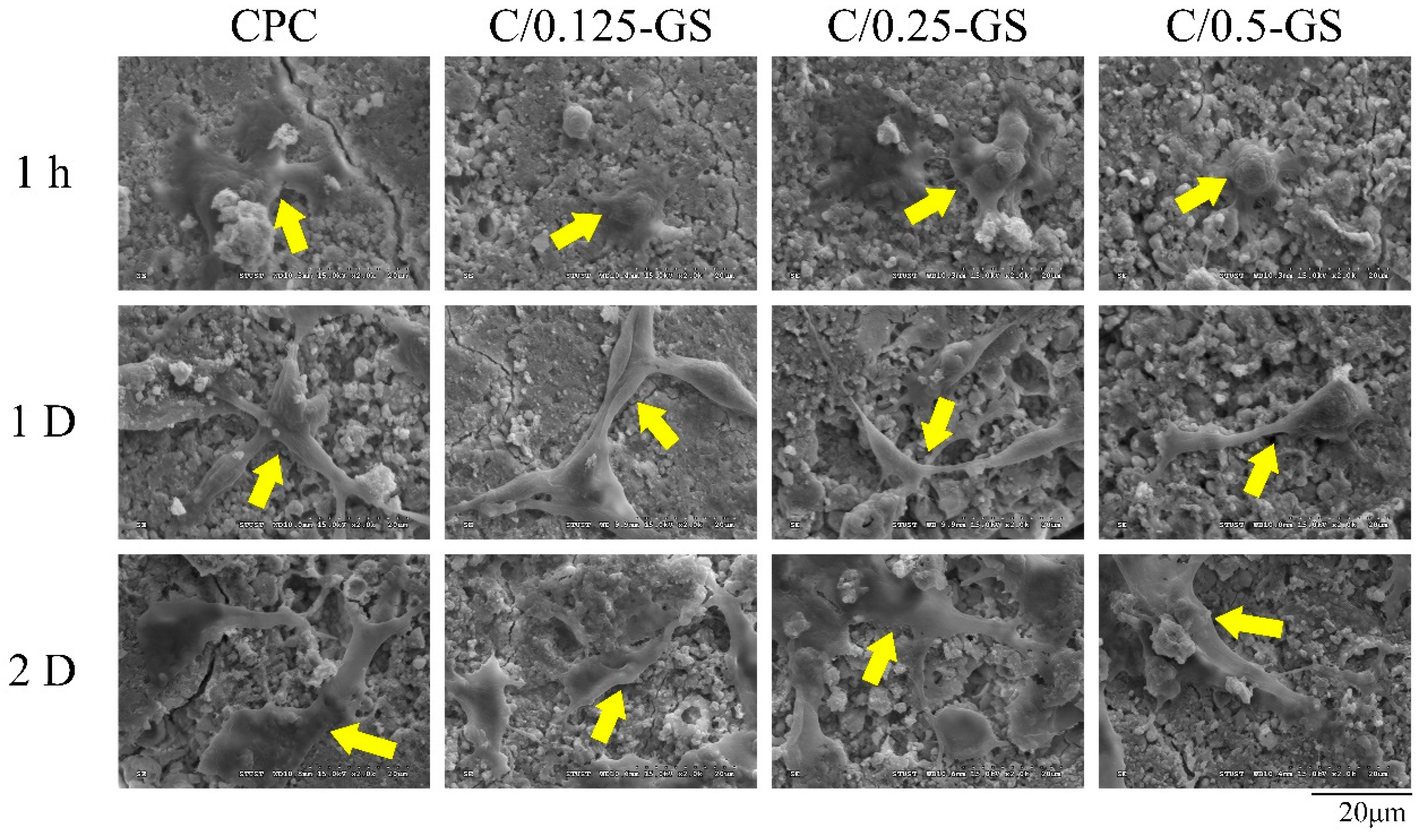
2.2.3. D1 Cell Attachment, Proliferation, and Semi-Quantitative Mineralization
3. Materials and Methods
3.1. Raw Materials
3.2. In Situ Preparation of CPC Composite with Hydrogel Beads
3.2.1. CPC Bone Cement Preparation
3.2.2. Hydrogel Bead Preparation
3.3. Characterization of CPC/Hydrogel Bead Composites
3.3.1. Compressive Strength and Fracture Surface Observation
3.3.2. ATR-FTIR Spectroscopy and XRD Analyses
3.3.3. Working/Setting Time, Injectability, and Dispersibility
3.3.4. Antibiotic Release Testing by Ultraviolet/Visible (UV-Vis) Spectrometry
3.4. Antibacterial Activity, Biocompatibility, and Mineralization of CPC/Hydrogel with Gentamicin after Sterilization
3.4.1. Antibacterial Ability of CPC/Hydrogel with Gentamicin
3.4.2. Cytotoxicity of CPC/Hydrogel Composites
3.4.3. D1 Cell Attachment, Proliferation, and Semi-Quantitative Mineralization
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namba, R.S.; Paxton, L.; Fithian, D.C.; Stone, M.L. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J. Arthroplast. 2005, 20, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, A.D.; Dicks, L.M. Calcium orthophosphate-based bone cements (CPCs): Applications, antibiotic release and alternatives to antibiotics. J. Appl. Biomater. Funct. Mater. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Wininger, D.A.; Fass, R.J. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob. Agents Chemother. 1996, 40, 2675–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornell, C.; Tyndall, D.; Waller, S.; Lane, J.; Brause, B. Treatment of experimental osteomyelitis with antibiotic-impregnated bone graft substitute. J. Orthop. Res. 1993, 11, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Brown, W. A new calcium phosphate settimg cement. J. Dent. Res. 1983, 62, 672. [Google Scholar]
- Hamanishi, C.; Kitamoto, K.; Ohura, K.; Tanaka, S.; Doi, Y. Self-setting, bioactive, and biodegradable TTCP-DCPD apatite cement. J. Biomed. Mater. Res. Part A 1996, 32, 383–389. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Ambrosio, L.; Guarino, V.; Sanginario, V.; Torricelli, P.; Fini, M.; Ginebra, M.; Planell, J.; Giardino, R. Injectable calcium-phosphate-based composites for skeletal bone treatments. Biomed. Mater. 2012, 7, 024113. [Google Scholar] [CrossRef]
- Le Ferrec, M.; Mellier, C.; Lefèvre, F.X.; Boukhechba, F.; Janvier, P.; Montavon, G.; Bouler, J.M.; Gauthier, O.; Bujoli, B. In vivo resorption of injectable apatitic calcium phosphate cements: Critical role of the intergranular microstructure. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 367–376. [Google Scholar] [CrossRef]
- Ko, C.-L.; Chen, J.-C.; Hung, C.-C.; Wang, J.-C.; Tien, Y.-C.; Chen, W.-C. Biphasic products of dicalcium phosphate-rich cement with injectability and nondispersibility. Mater. Sci. Eng. C 2014, 39, 40–46. [Google Scholar] [CrossRef]
- Habraken, W.; Liao, H.; Zhang, Z.; Wolke, J.; Grijpma, D.; Mikos, A.; Feijen, J.; Jansen, J. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010, 6, 2200–2211. [Google Scholar] [CrossRef]
- Ooms, E.; Wolke, J.; Van Der Waerden, J.; Jansen, J. Trabecular bone response to injectable calcium phosphate (Ca-P) cement. J. Biomed. Mater. Res. Part A 2002, 61, 9–18. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. JBJS 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Weir, M.D.; Sun, J.; Zhao, L.; Simon, C.G.; Xu, H.H. A self-setting iPSMSC-alginate-calcium phosphate paste for bone tissue engineering. Dent. Mater. 2016, 32, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Nouri-Felekori, M.; Khakbiz, M.; Nezafati, N.; Mohammadi, J.; Eslaminejad, M.B.; Fani, N. Characterization and multiscale modeling of novel calcium phosphate composites containing hydroxyapatite whiskers and gelatin microspheres. J. Alloys Compd. 2020, 832, 154938. [Google Scholar] [CrossRef]
- Qiao, P.; Wang, J.; Xie, Q.; Li, F.; Dong, L.; Xu, T. Injectable calcium phosphate–alginate–chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater. Sci. Eng. C 2013, 33, 4633–4639. [Google Scholar] [CrossRef]
- Alkhraisat, M.; Rueda, C.; Marino, F.; Torres, J.; Jerez, L.; Gbureck, U.; Cabarcos, E. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, H.; Weir, M.D.; Bao, C.; Xu, H.H. Umbilical cord stem cells released from alginate–fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012, 8, 2297–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Nezafati, N.; Farokhi, M.; Heydari, M.; Hesaraki, S.; Nasab, N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. B Eng. 2019, 175, 107146. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.-H.; Kweon, O.-K. Effect of gelatin on osteogenic cell sheet formation using canine adipose-derived mesenchymal stem cells. Cell Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Yomoda, M.; Sobajima, S.; Kasuya, A.; Neo, M. Calcium phosphate cement–gelatin powder composite testing in canine models: Clinical implications for treatment of bone defects. J. Biomater. Appl. 2015, 29, 1385–1393. [Google Scholar] [CrossRef]
- Amirian, J.; Van, T.T.T.; Bae, S.-H.; Jung, H.-I.; Choi, H.-J.; Cho, H.-D.; Lee, B.-T. Examination of in vitro and in vivo biocompatibility of alginate-hyaluronic acid microbeads as a promising method in cell delivery for kidney regeneration. Int. J. Biol. Macromol. 2017, 105, 143–153. [Google Scholar] [CrossRef]
- Fuji, T.; Anada, T.; Honda, Y.; Shiwaku, Y.; Koike, H.; Kamakura, S.; Sasaki, K.; Suzuki, O. Octacalcium phosphate-precipitated alginate scaffold for bone regeneration. Tissue Eng. Part A 2009, 15, 3525–3535. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Singh, A.; Kipke, D.R. Alginate composition effects on a neural stem cell–seeded scaffold. Tissue Eng. Part C 2009, 15, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.D.; Chen, C.-C.; Shie, M.-Y.; Huang, C.-H.; Ding, S.-J. Controlled release of gentamicin from calcium phosphate/alginate bone cement. Mater. Sci. Eng. C 2011, 31, 334–341. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaître, J.; Van Landuyt, P.; Zambelli, P.-Y.; Merkle, H.P.; Gander, B. Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J. Pharm. Sci. 1997, 86, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Hu, R.; Zhou, J.; Ye, Y.; Xu, Z.; Lin, C. A further insight into the adsorption mechanism of protein on hydroxyapatite by FTIR-ATR spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 527–531. [Google Scholar] [CrossRef]
- Koh, B.T.; Tan, J.; Ramruttun, A.K.; Wang, W. Effect of storage temperature and equilibration time on polymethyl methacrylate (PMMA) bone cement polymerization in joint replacement surgery. J. Orthop. Surg. Res. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Translat. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ince, A.; Schütze, N.; Karl, N.; Löhr, J.F.; Eulert, J. Gentamicin negatively influenced osteogenic function in vitro. Int. Orthop. 2007, 31, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-C.; Chen, W.-C.; Haung, S.-M.; Liu, S.-M.; Lin, C.-L. Effects of hinokitiol and dicalcium phosphate on the osteoconduction and antibacterial activity of gelatin-hyaluronic acid crosslinked hydrogel membrane in vitro. Pharmaceuticals 2021, 14, 802. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of cross-linked chitosan quaternary ammonium salt hydrogel films loading drug of gentamicin sulfate for antibacterial wound dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Takechi, M.; Miyamoto, Y.; Momota, Y.; Yuasa, T.; Tatehara, S.; Nagayama, M.; Ishikawa, K.; Suzuki, K. The in vitro antibiotic release from anti-washout apatite cement using chitosan. J. Mater. Sci. Mater. Med. 2002, 13, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.-S.; Wang, J.-C.; Lai, P.-L.; Liu, S.-M.; Chen, Y.-S.; Chen, W.-C.; Hung, C.-C. Effects of gamma radiation on the sterility assurance, antibacterial ability, and biocompatibility of impregnated hydrogel macrosphere protein and drug release. Polymers 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Named Groups | Working Time/Setting Time (Mean ± Standard Deviation; n = 10; Unit: min) | Adjusted Powder-to-Liquid Ratio (g/mL) |
|---|---|---|
| CPC | 8.49 ± 0.11/16.79 ± 0.14 | 0.8/0.280 |
| C/0.125-S | 8.43 ± 0.07/16.70 ± 0.13 | 0.8/0.285 |
| C/0.125-GS | 8.47 ± 0.09/16.82 ± 0.09 | |
| C/0.25-S | 8.42 ± 0.06/16.72 ± 0.13 | 0.8/0.290 |
| C/0.25-GS | 8.43 ± 0.07/16.80 ± 0.12 | |
| C/0.5-S | 8.39 ± 0.05/16.74 ± 0.16 | 0.8/0.300 |
| C/0.5-GS | 8.44 ± 0.07/16.80 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-M.; Chen, W.-C.; Ko, C.-L.; Chang, H.-T.; Chen, Y.-S.; Haung, S.-M.; Chang, K.-C.; Chen, J.-C. In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals 2021, 14, 1000. https://doi.org/10.3390/ph14101000
Liu S-M, Chen W-C, Ko C-L, Chang H-T, Chen Y-S, Haung S-M, Chang K-C, Chen J-C. In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals. 2021; 14(10):1000. https://doi.org/10.3390/ph14101000
Chicago/Turabian StyleLiu, Shih-Ming, Wen-Cheng Chen, Chia-Ling Ko, Hsu-Ting Chang, Ya-Shun Chen, Ssu-Meng Haung, Kai-Chi Chang, and Jian-Chih Chen. 2021. "In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold" Pharmaceuticals 14, no. 10: 1000. https://doi.org/10.3390/ph14101000
APA StyleLiu, S.-M., Chen, W.-C., Ko, C.-L., Chang, H.-T., Chen, Y.-S., Haung, S.-M., Chang, K.-C., & Chen, J.-C. (2021). In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals, 14(10), 1000. https://doi.org/10.3390/ph14101000