Effects of Hinokitiol and Dicalcium Phosphate on the Osteoconduction and Antibacterial Activity of Gelatin-Hyaluronic Acid Crosslinked Hydrogel Membrane In Vitro
Abstract
1. Introduction
2. Results
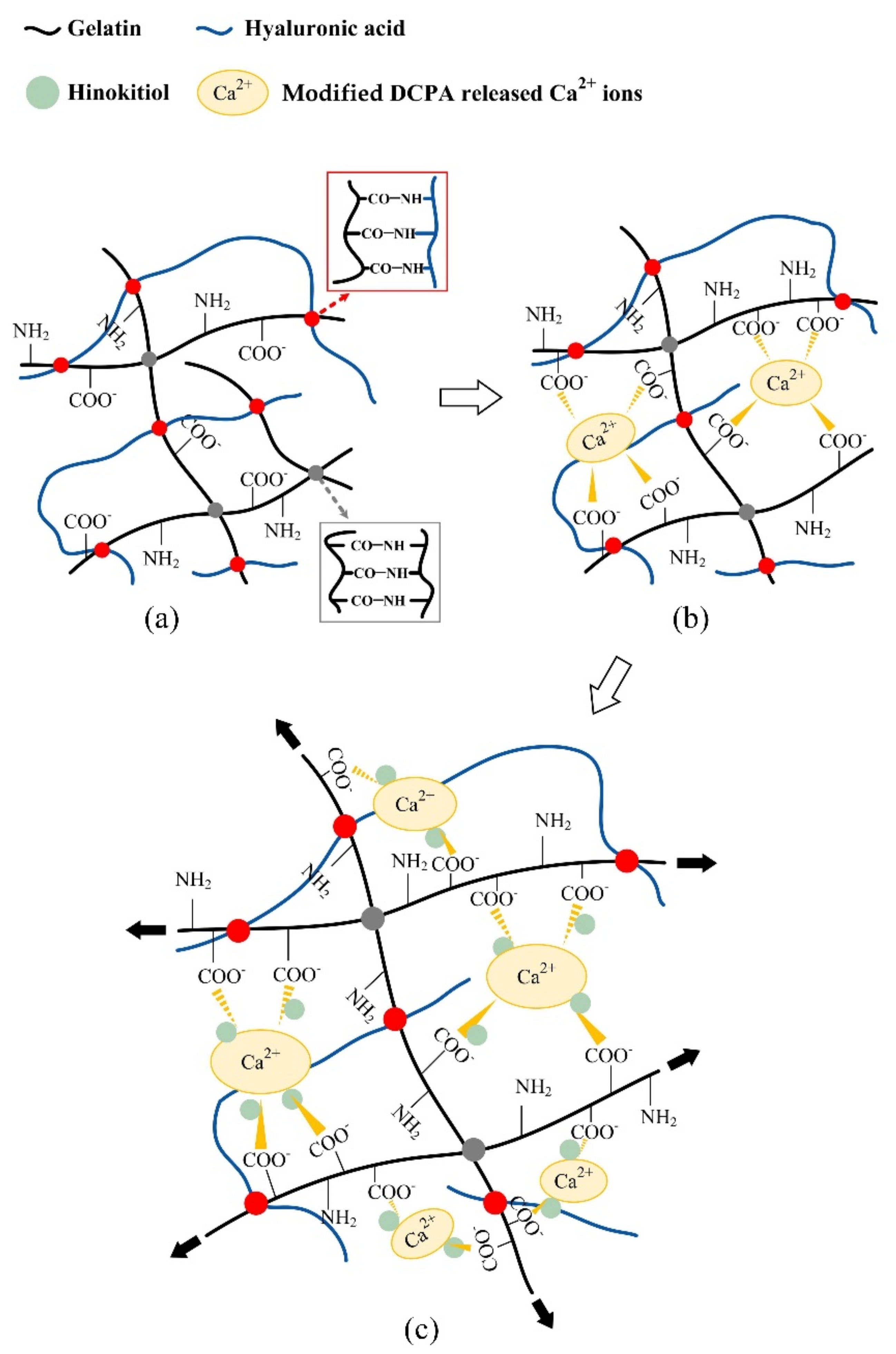
2.1. Reaction Mechanisms between the Hydrogels and Additives
2.2. Physiochemical Properties of Different Hydrogel Membranes
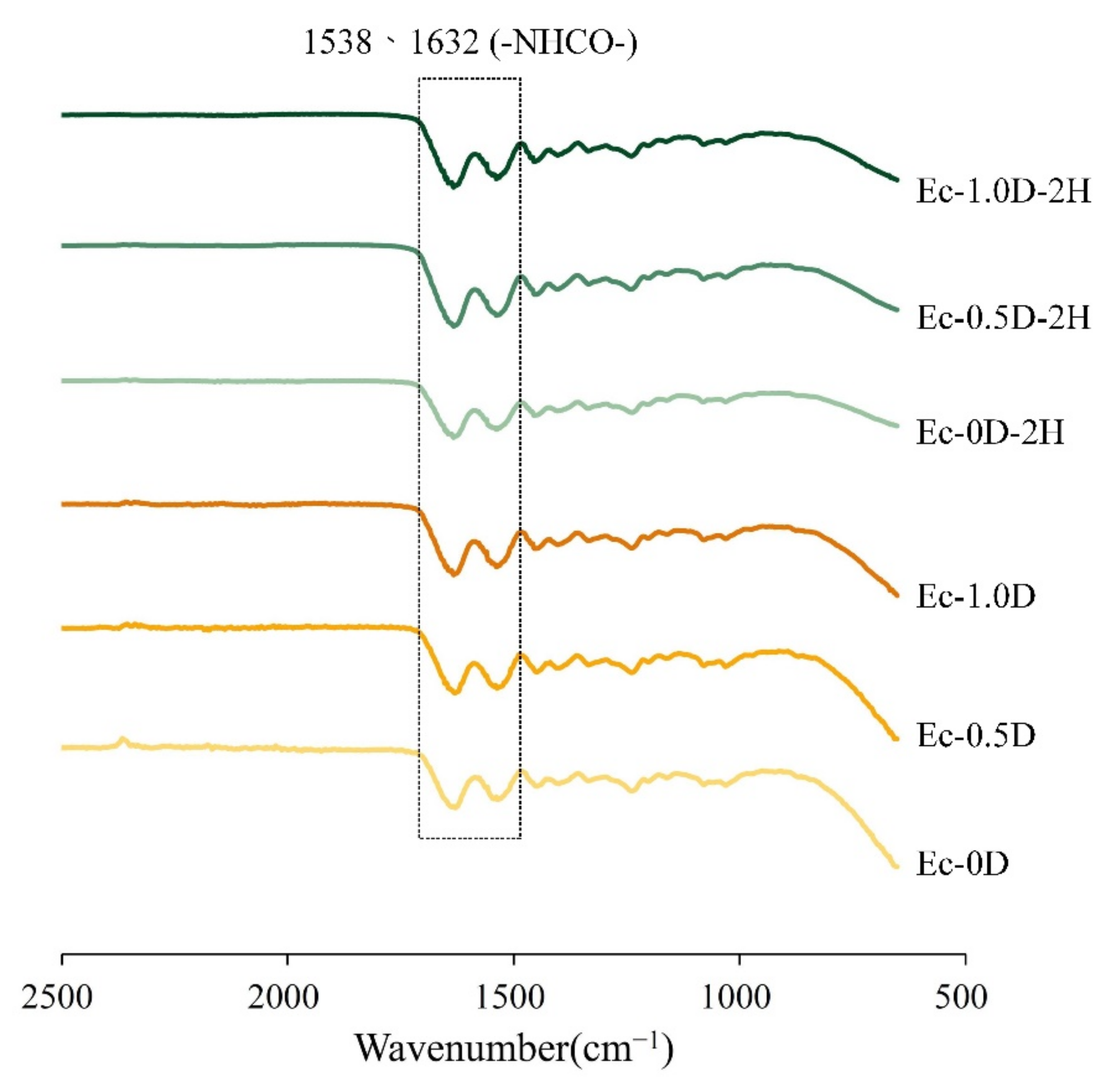
2.2.1. Fourier Transform Infrared (FTIR)
2.2.2. Strength Measurements
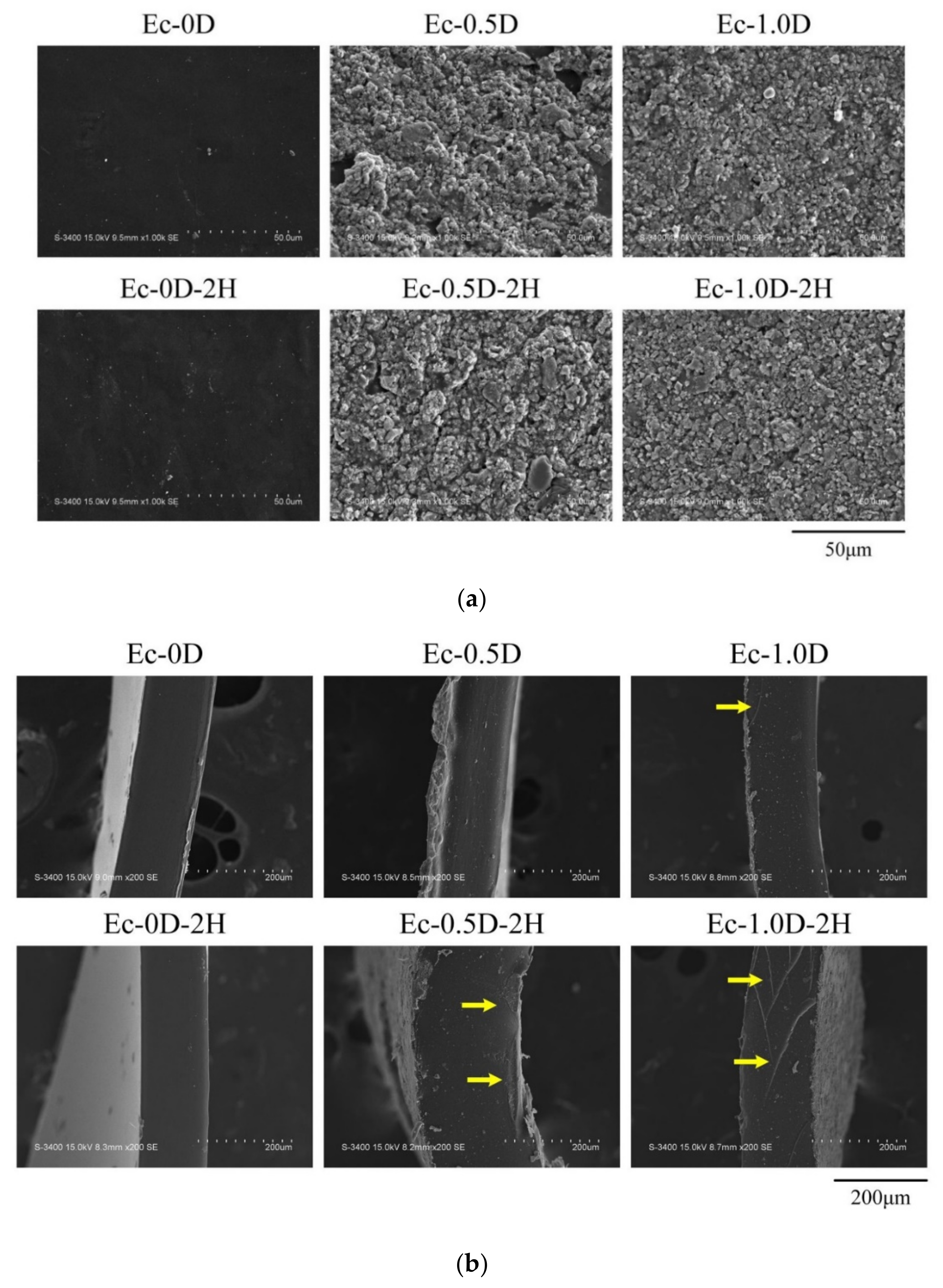
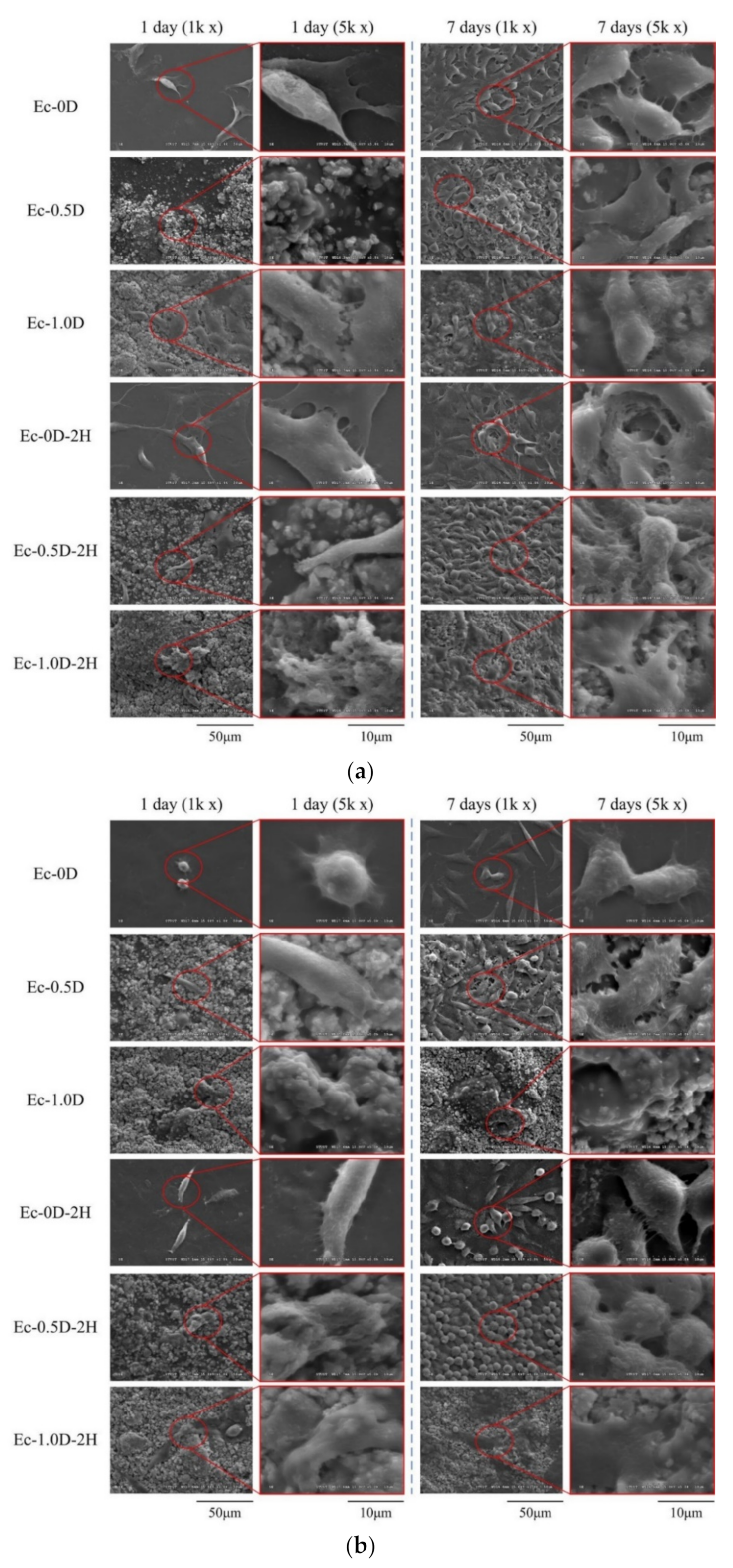
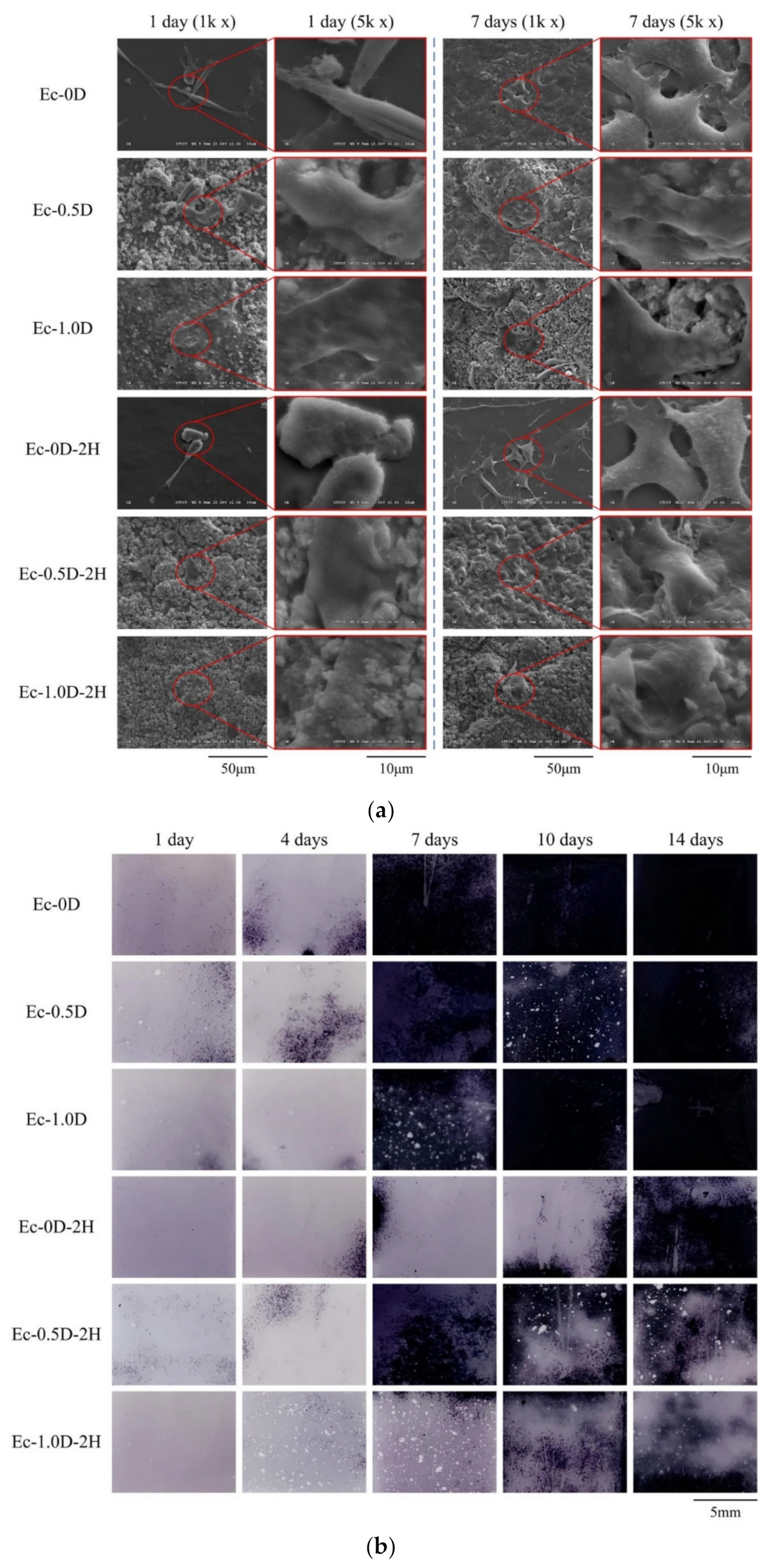
2.2.3. Topographies and Fracture Surfaces
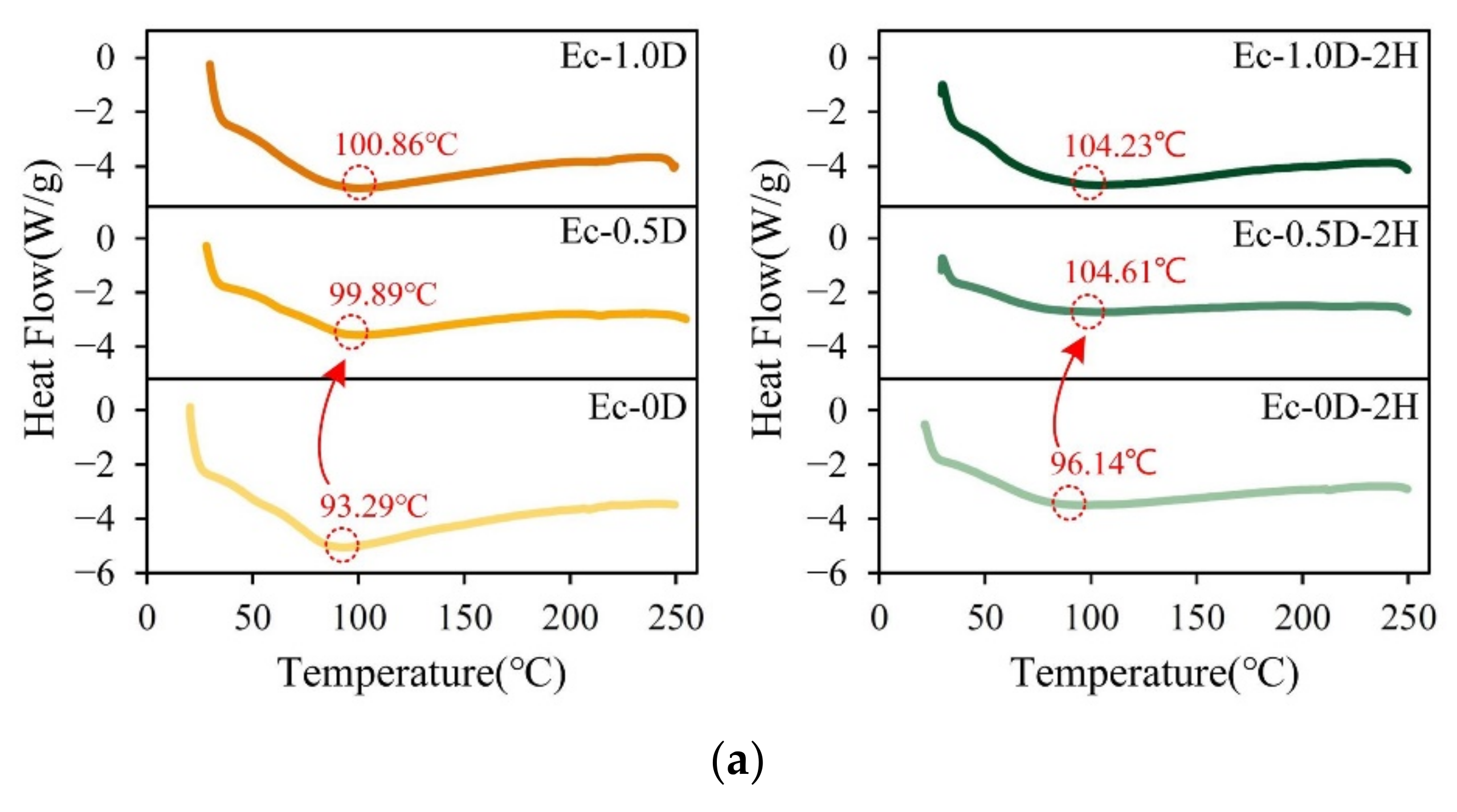
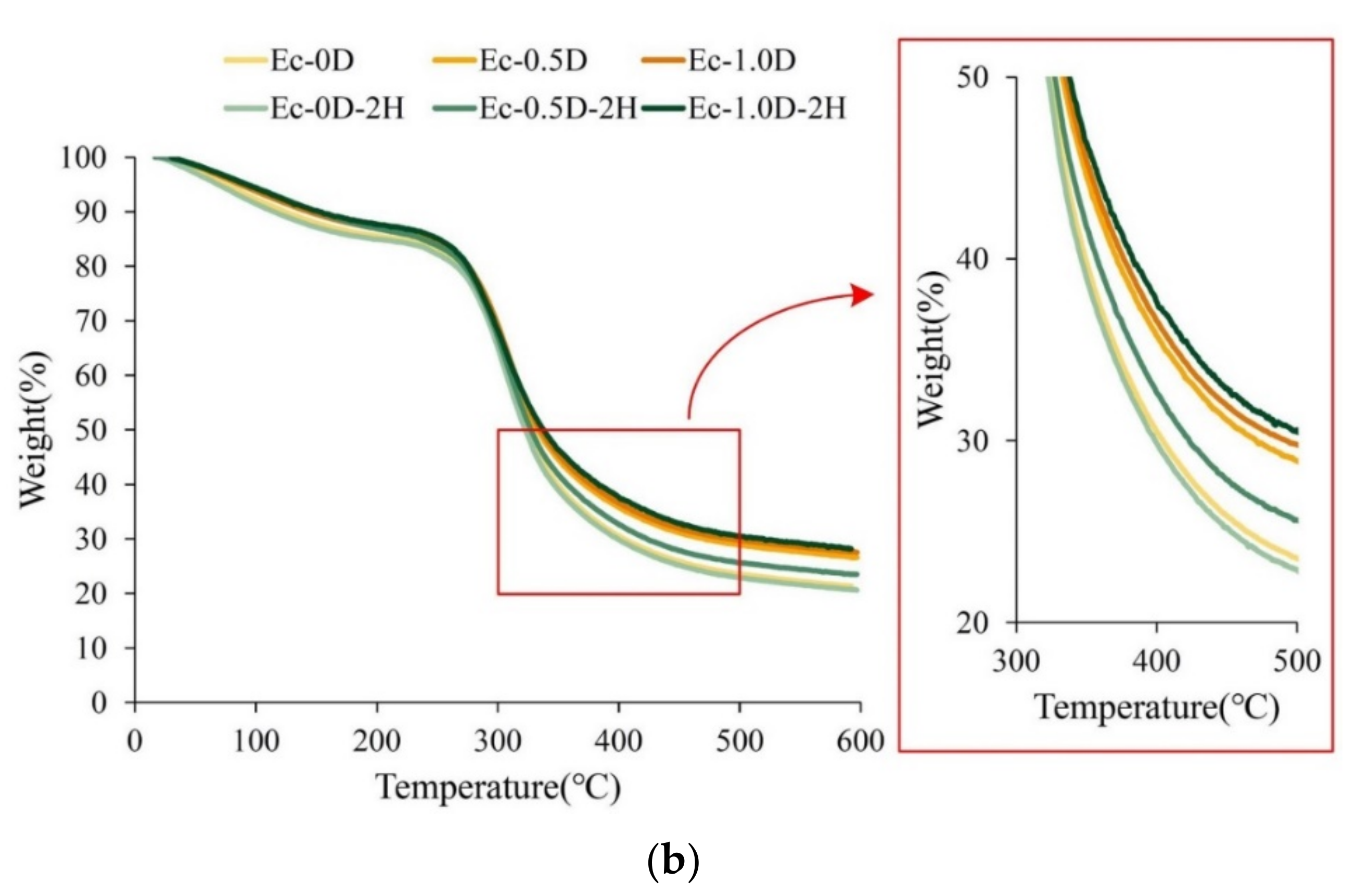
2.2.4. DSC and TGA Analysis
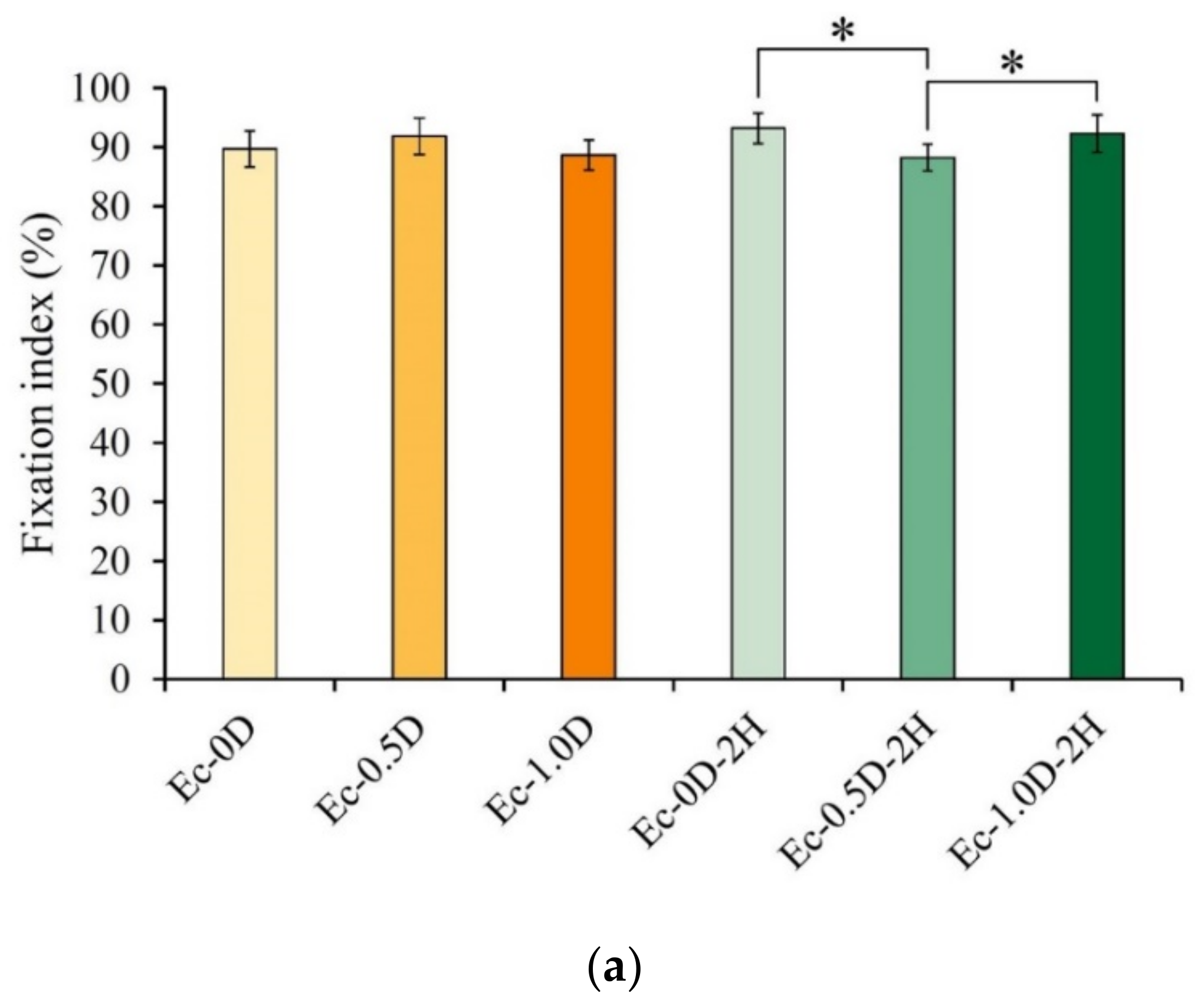
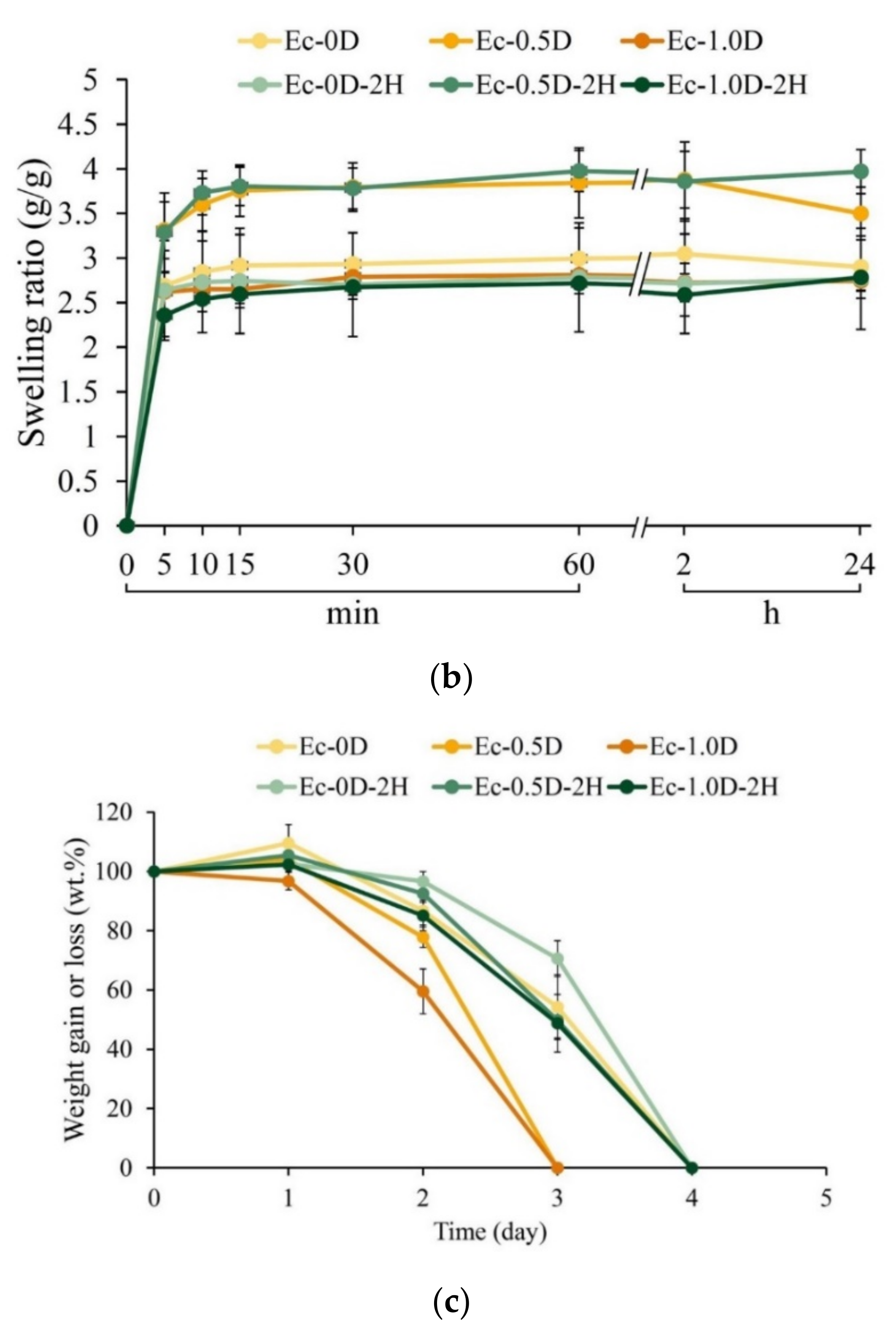
2.2.5. Evaluations of the Fixation Index, Swelling Ratio, and Degradation
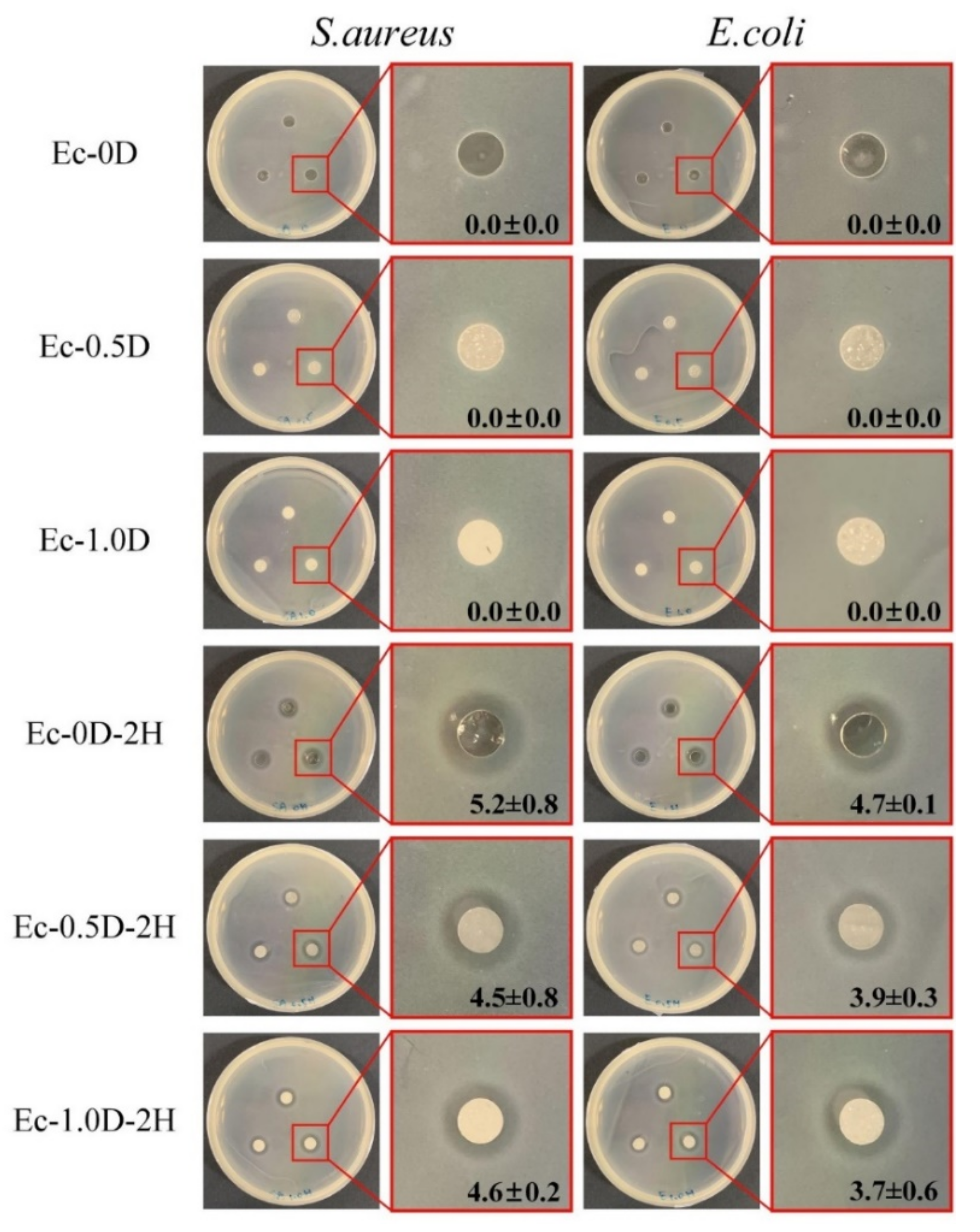
2.2.6. Antibacterial Properties of Hydrogel Membranes with Hinokitiol
2.3. Viability of Fibroblasts and Mineralization of Osteoprogenitor Cell
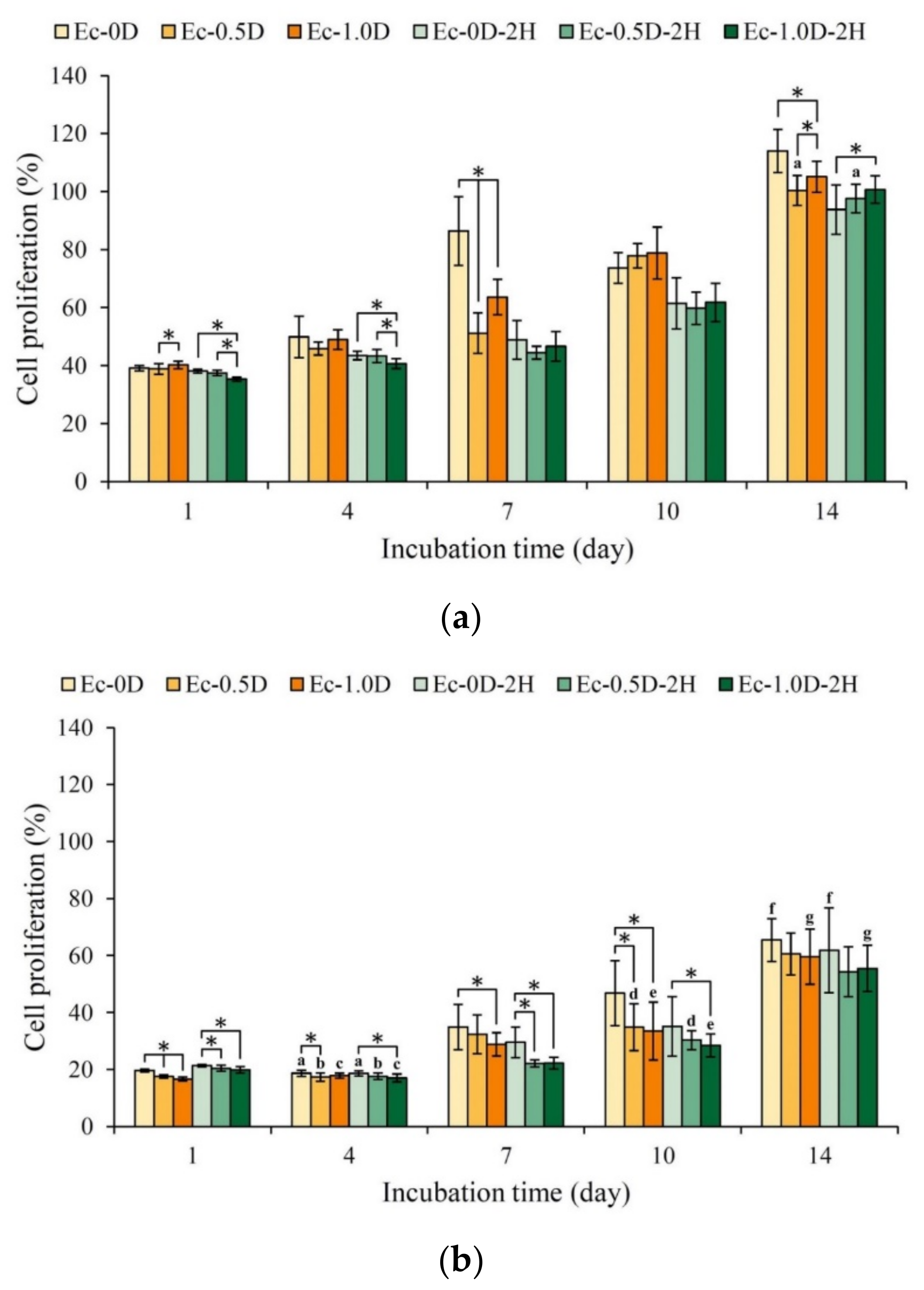
2.3.1. Quantitative and Qualitative Evaluations of Cytotoxicity
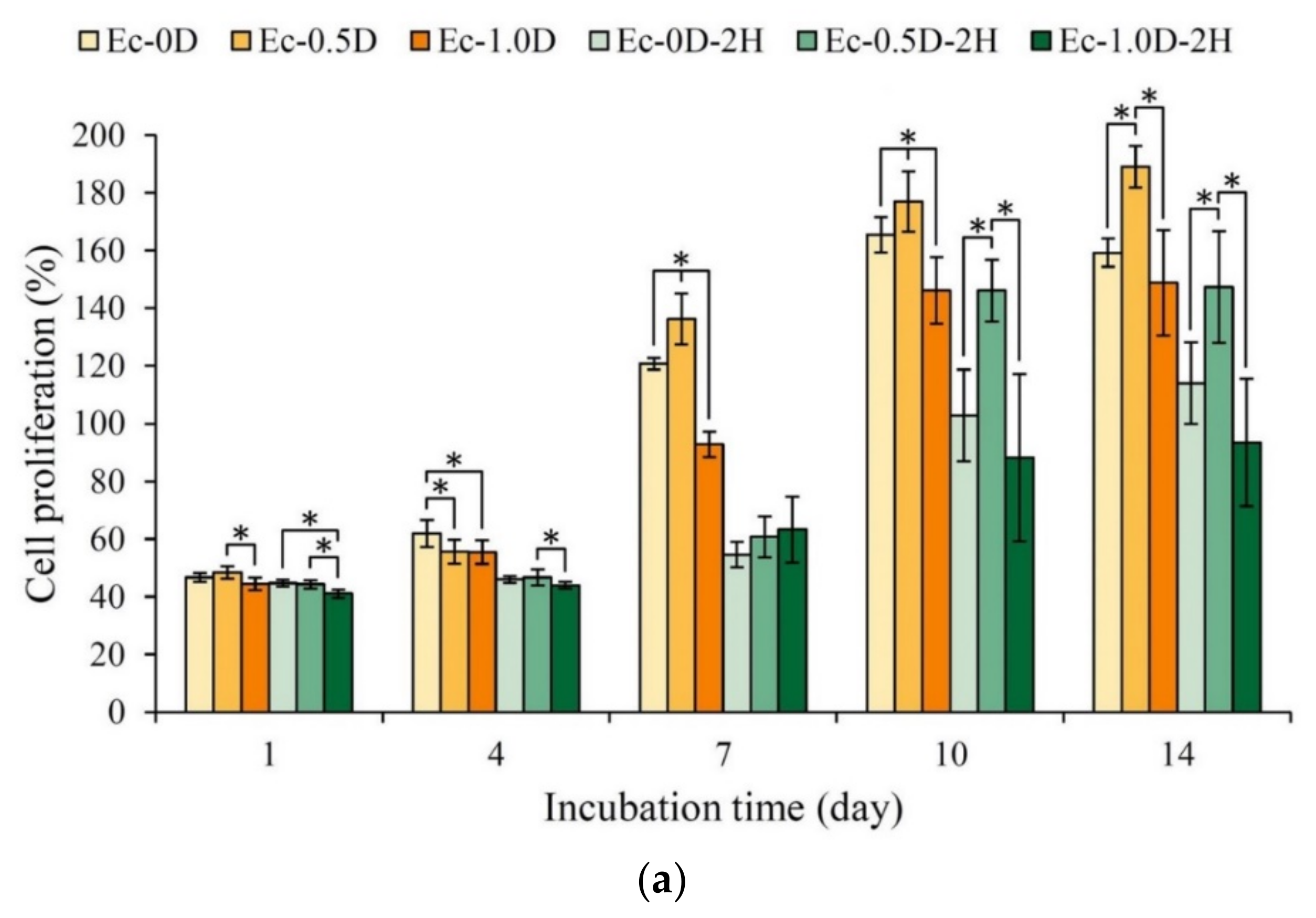
2.3.2. Proliferation of the Hydrogels in Contact with Cells
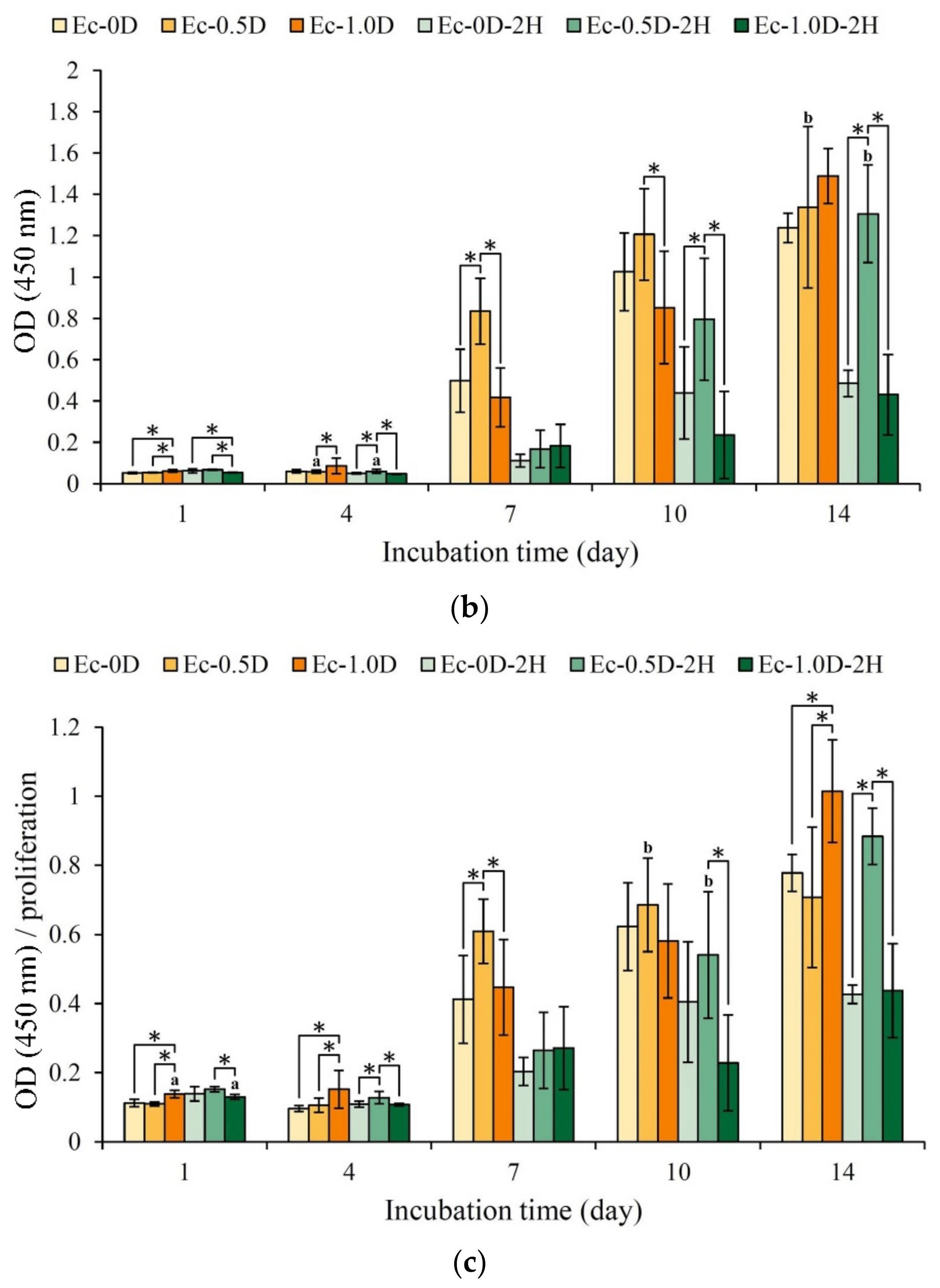
2.3.3. Mineralization of the Hydrogels in Contact with D1 Cell
3. Discussion
4. Materials and Methods
4.1. Raw Materials
4.2. Preparing Hydrogel Specimens
4.3. Functional Groups from Infrared Spectrum
4.4. Tensile Measurements
4.5. Characterization of the Hydrogels’ Thermo-Physical Properties
4.6. Hydrogel Fixation Indices of the Crosslinking
4.7. Solution Absorption of Hydrogels
4.8. Degradation Rate of Hydrogels
4.9. Evaluation of the Hydrogel Membrane with Antibacterial Properties
4.10. Viability of NIH-3T3 and L929 Fibroblasts Cultured in Sample Extracts
4.11. Proliferation and Attachment of NIH3T3, L929 and D1 Cells on Hydrogels
4.12. Alkaline Phosphatase Activities and Staning
4.13. Statistical Analysis
5. Conclusions
- (1)
- Adding modified DCPA powder combined with hinokitiol does not affect the crosslinking index of the hydrogels, and the further impregnation of the hydrogel membranes with hinokitiol can delay immersion degradation;
- (2)
- Adding modified DCPA powder to the hydrogel group significantly reduces its modulus. However, because the cation Ca2+ forms a physical ion–dipole bond on the gelatin molecular chain, a small amount of addition during stretching cannot significantly reduce the tensile strength. When a large amount of modified DCPA is added, the tensile strength decreases significantly;
- (3)
- In the contact culture with D1 cells, the group without hinokitiol exhibited obvious ALP production on the 7th day of culture, and the Ec-0.5D group with a small amount of modified DCPA had the best ALP production capability;
- (4)
- The hydrogel membrane impregnated with hinokitiol (Ec-0.5D-2H) showed significant antibacterial activity against S. aureus and E. coli. In the cell viability test, the addition of modified DCPA powder and impregnation with hinokitiol had no adverse effects on NIH-3T3 and L929 cells. The Ec-0.5D-2H group may be the best hydrogel membrane candidate for guiding tissue regeneration in in vitro tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwach Abdellaoui, K.; Vivien Castioni, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Sundararaj, S.C.; Thomas, M.V.; Peyyala, R.; Dziubla, T.D.; Puleo, D.A. Design of a multiple drug delivery system directed at periodontitis. Biomaterials 2013, 34, 8835–8842. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Faruq, O.; Kim, B.; Padalhin, A.R.; Lee, G.H.; Lee, B.T. A hybrid composite system of biphasic calcium phosphate granules loaded with hyaluronic acid–gelatin hydrogel for bone regeneration. J. Biomater. Appl. 2017, 32, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Shu, Z.X.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar] [CrossRef]
- Falcone, S.J.; Palmeri, D.; Berg, R.A. Biomedical applications of hyaluronic acid. In Polysaccharides for Drug Delivery and Pharmaceutical Applications; American Chemical Society: Washington, DC, USA, 2006; Volume 934, pp. 155–174. [Google Scholar]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolář, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Ko, C.L.; Chen, J.C.; Hung, C.C.; Wang, J.C.; Tien, Y.C.; Chen, W.C. Biphasic products of dicalcium phosphate-rich cement with injectability and nondispersibility. Mater. Sci. Eng. C 2014, 39, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.L.; Chen, J.C.; Tien, Y.C.; Hung, C.C.; Wang, J.C.; Chen, W.C. Osteoregenerative capacities of dicalcium phosphate-rich calcium phosphate bone cement. J. Biomed. Mater. Res. Part A 2015, 103, 203–210. [Google Scholar] [CrossRef]
- Saeki, Y.; Ito, Y.; Shibata, M.; Sato, Y.; Okuda, K.; Takazoe, I. Antimicrobial action of natural substances on oral bacteria. Bull. Tokyo Dent. Coll. 1989, 30, 129–135. [Google Scholar]
- Arima, Y.; Nakai, Y.; Hayakawa, R.; Nishino, T. Antibacterial effect of β-thujaplicin on staphylococci isolated from atopic dermatitis: Relationship between changes in the number of viable bacterial cells and clinical improvement in an eczematous lesion of atopic dermatitis. J. Antimicrob. Chemother. 2003, 51, 113–122. [Google Scholar] [CrossRef]
- Imai, N.; Doi, Y.; Nabae, K.; Tamano, S.; Hagiwara, A.; Kawabe, M.; Ichihara, T.; Ogawa, K.; Shirai, T. Lack of hinokitiol (beta-thujaplicin) carcinogenicity in F344/DuCrj rats. J. Toxicol. Sci. 2006, 31, 357–370. [Google Scholar] [CrossRef][Green Version]
- Higashi, Y.; Sakata, M.; Fujii, Y. High-performance liquid chromatography with dual-wavelength ultraviolet detection for measurement of hinokitiol in personal care products. Int. J. Cosmet. Sci. 2010, 32, 237. [Google Scholar] [CrossRef]
- Nagao, Y.; Sata, M. Effect of oral care gel on the quality of life for oral lichen planus in patients with chronic HCV infection. J. Virol. 2011, 8, 348. [Google Scholar] [CrossRef]
- Iha, K.; Suzuki, N.; Yoneda, M.; Takeshita, T.; Hirofuji, T. Effect of mouth cleaning with hinokitiol-containing gel on oral malodor: A randomized, open-label pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 433–439. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chang, K.W.; Hsia, S.M.; Yu, C.C.; Fuh, L.J.; Chi, T.Y.; Shieh, T.M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef]
- Shih, Y.H.; Lin, D.J.; Chang, K.W.; Hsia, S.M.; Ko, S.Y.; Lee, S.Y.; Hsue, S.S.; Wang, T.H.; Chen, Y.L.; Shieh, T.M. Evaluation physical characteristics and comparison antimicrobial and anti-inflammation potentials of dental root canal sealers containing hinokitiol in vitro. PLoS ONE 2014, 9, e94941. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J. Biomed. Mater. Res. 1997, 37, 243–251. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Chang, K.C.; Lin, D.J.; Wu, Y.R.; Chang, C.W.; Chen, C.H.; Ko, C.L.; Chen, W.C. Characterization of genipin-crosslinked gelatin/hyaluronic acid-based hydrogel membranes and loaded with hinokitiol: In vitro evaluation of antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2019, 105, 110074. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Chen, W.C.; Chen, C.H.; Ko, C.L.; Liu, S.M.; Chen, J.C. Chemical cross-linking on gelatin-hyaluronan loaded with hinokitiol for the preparation of guided tissue regeneration hydrogel membranes with antibacterial and biocompatible properties. Mater. Sci. Eng. C 2021, 119, 111576. [Google Scholar] [CrossRef]
- Ko, C.L.; Tien, Y.C.; Wang, J.C.; Chen, W.C. Characterization of controlled highly porous hyaluronan/gelatin cross-linking sponges for tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 14, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Qiu, L.; Zhou, J.J.; Guo, H.Y.; Hu, Y.H.; Li, Z.C.; Wang, Q.; Chen, Q.X.; Liu, B. Inhibitory effects of hinokitiol on tyrosinase activity and melanin biosynthesis and its antimicrobial activities. J. Enzym. Inhib. Med. Chem. 2010, 25, 798–803. [Google Scholar] [CrossRef]
- Hu, M.H.; Lee, P.Y.; Chen, W.C.; Hu, J.J. Incorporation of Collagen in Calcium Phosphate Cements for Controlling Osseointegration. Materials 2017, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a prominent biomolecule with numerous applications in medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xu, Y.F.; Lou, L.X.; Jin, K.; Miao, Q.; Ye, X.; Xi, Y. Anti-inflammatory effects of hinokitiol on human corneal epithelial cells: An in vitro study. Eye 2015, 29, 964–971. [Google Scholar] [CrossRef]
- Chang, Y.L.; Hsieh, C.Y.; Yeh, C.Y.; Lin, F.H. The development of gelatin/hyaluronate copolymer mixed with calcium sulfate, hydroxyapatite, and stromal-cell-derived factor-1 for bone regeneration enhancement. Polymers 2019, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Chang, Y.; Chiu, Y.T.; Hsu, H.L.; Shih, C.C.; Lu, J.H.; Yang, P.C. Evaluation of an epoxy-fixed biological patch with ionically bound heparin as a pericardial substitute. Biomaterials 1996, 17, 1693–1701. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-C.; Chen, W.-C.; Haung, S.-M.; Liu, S.-M.; Lin, C.-L. Effects of Hinokitiol and Dicalcium Phosphate on the Osteoconduction and Antibacterial Activity of Gelatin-Hyaluronic Acid Crosslinked Hydrogel Membrane In Vitro. Pharmaceuticals 2021, 14, 802. https://doi.org/10.3390/ph14080802
Chang K-C, Chen W-C, Haung S-M, Liu S-M, Lin C-L. Effects of Hinokitiol and Dicalcium Phosphate on the Osteoconduction and Antibacterial Activity of Gelatin-Hyaluronic Acid Crosslinked Hydrogel Membrane In Vitro. Pharmaceuticals. 2021; 14(8):802. https://doi.org/10.3390/ph14080802
Chicago/Turabian StyleChang, Kai-Chi, Wen-Cheng Chen, Ssu-Meng Haung, Shih-Ming Liu, and Chih-Lung Lin. 2021. "Effects of Hinokitiol and Dicalcium Phosphate on the Osteoconduction and Antibacterial Activity of Gelatin-Hyaluronic Acid Crosslinked Hydrogel Membrane In Vitro" Pharmaceuticals 14, no. 8: 802. https://doi.org/10.3390/ph14080802
APA StyleChang, K.-C., Chen, W.-C., Haung, S.-M., Liu, S.-M., & Lin, C.-L. (2021). Effects of Hinokitiol and Dicalcium Phosphate on the Osteoconduction and Antibacterial Activity of Gelatin-Hyaluronic Acid Crosslinked Hydrogel Membrane In Vitro. Pharmaceuticals, 14(8), 802. https://doi.org/10.3390/ph14080802






