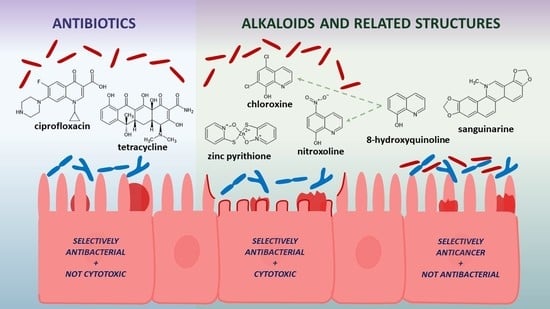
In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells
Abstract
1. Introduction
2. Results
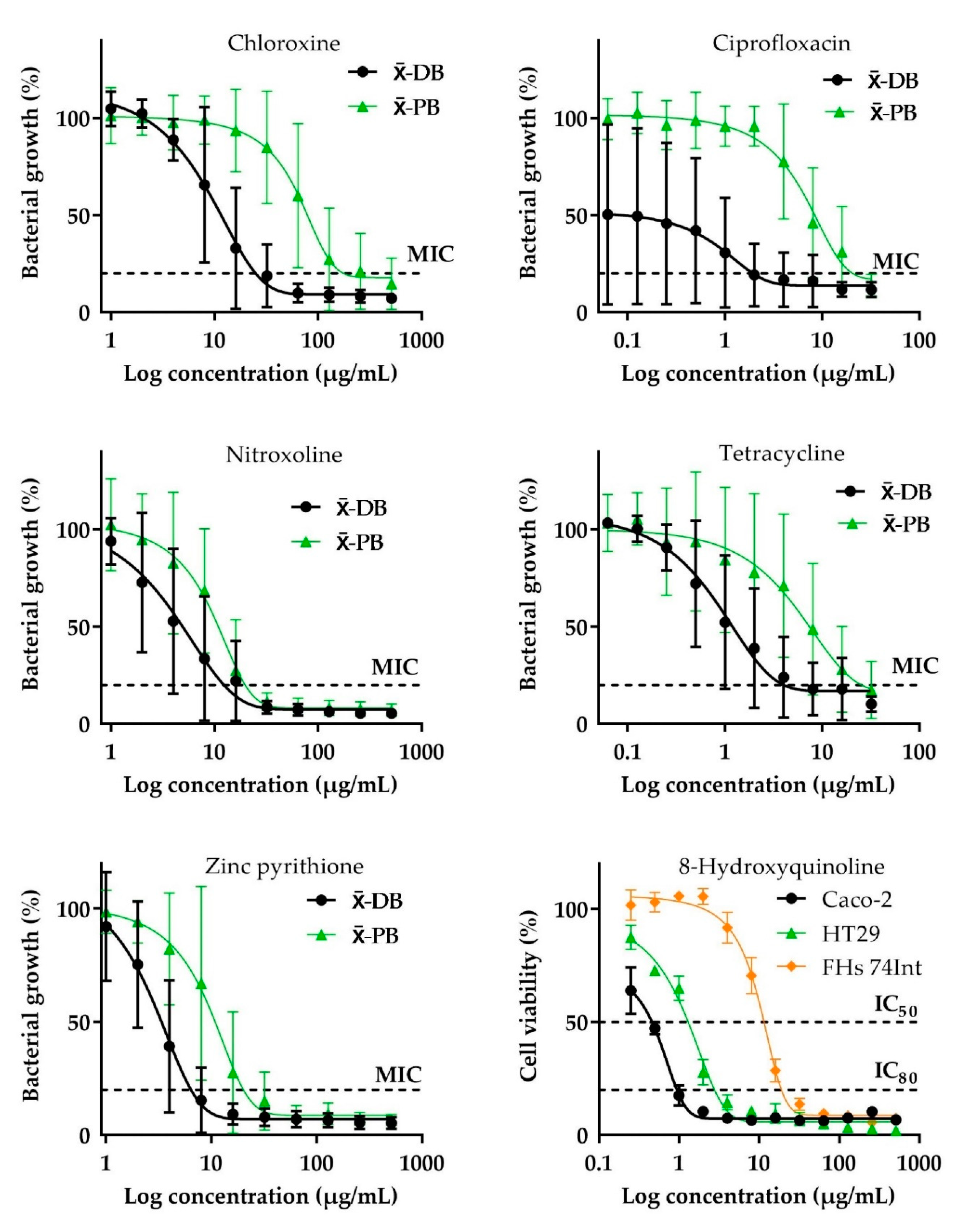
2.1. Antibacterial Activity
2.2. Cytotoxic Effect
2.3. Selective Toxicity
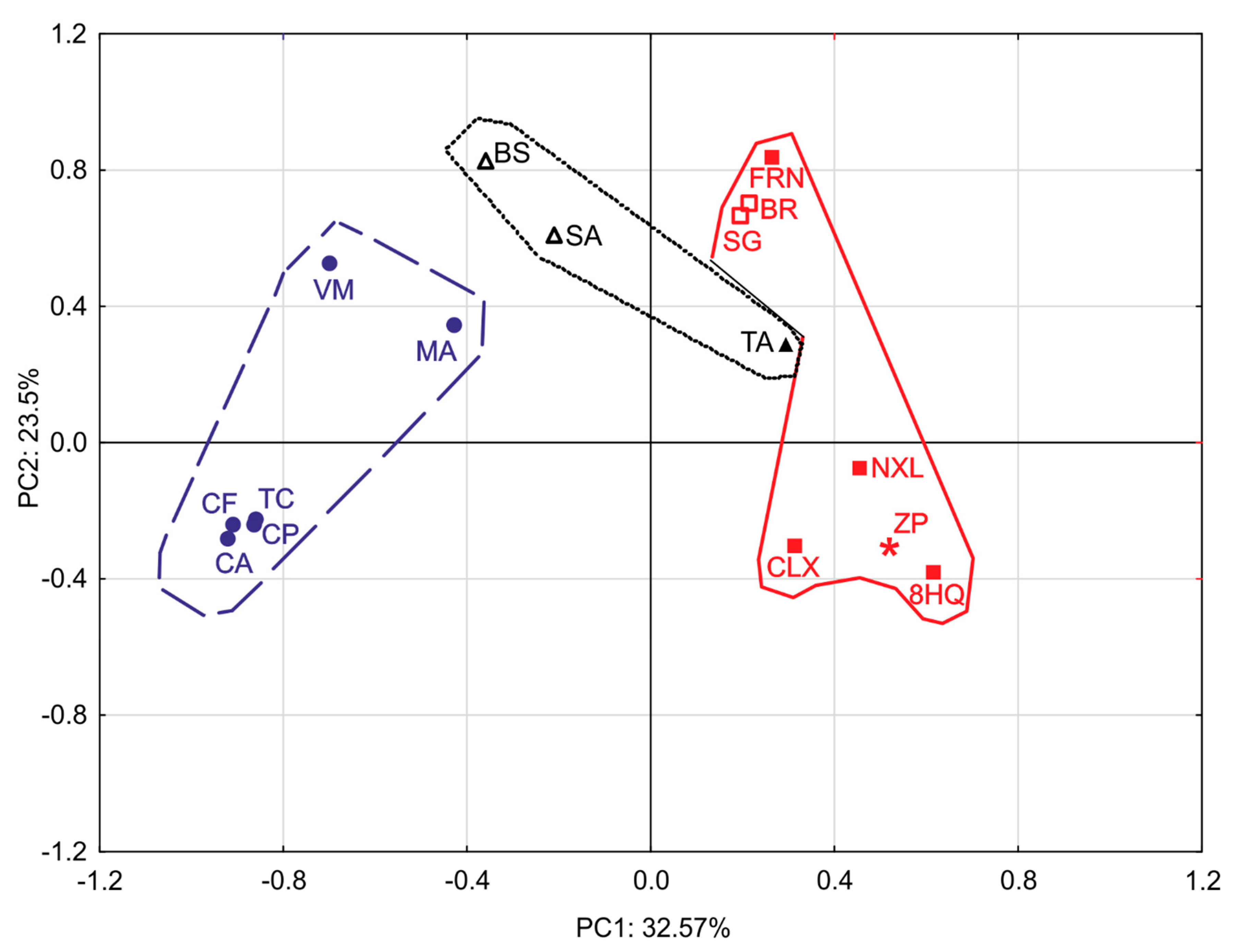
2.4. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains and Growth Media
4.3. Cell Cultures
4.4. Antibacterial Assay
4.5. Cytotoxicity Assay
4.6. Calculations and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Diarrhoeal Disease. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 19 March 2020).
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Santos, D.R.; Silva, L.R.; Silva, N. Antibiotics for the empirical treatment of acute infectious diarrhea in children. Braz. J. Infect. Dis. 2006, 10, 217–227. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Qinghaosu (Artemisinin): The Price of Success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Formiga, R.D.O.; Quirino, Z.G.M.; Diniz, M.D.F.F.M.; Marinho, A.F.; Tavares, J.F.; Batista, L. Maytenus Erythroxylon Reissek (Celastraceae) ethanol extract presents antidiarrheal activity via antimotility and antisecretory mechanisms. World J. Gastroenterol. 2017, 23, 4381–4389. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.C.; Xiao, P.G. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, Q.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res. Int. 2019, 126, 108590. [Google Scholar] [CrossRef]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, Examples, and Specific Applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef]
- Novakova, J.; Vlkova, E.; Bonusova, B.; Rada, V.; Kokoska, L. In vitro selective inhibitory effect of 8-hydroxyquinoline against bifidoba cteria and clostridia. Anaerobe 2013, 22, 134–136. [Google Scholar] [CrossRef]
- WikiZero: Chloroxine. Available online: https:// wikizero.com/en/Chloroxine (accessed on 10 August 2020).
- Bruneau, A.; Baylatry, M.T.; Joly, A.C.; Sokol, H. Gut microbiota: What impact on colorectal carcinogenesis and treatment? Bull. Cancer 2018, 105, 70–80. [Google Scholar] [CrossRef]
- Sambruy, Y.; Ferruzza, S.; Ranaldi, G.; De Angelis, I. Intestinal cell culture models: Applications in toxicology and pharmacology. Cell Biol. Toxicol. 2001, 17, 301–317. [Google Scholar] [CrossRef]
- Elsea, S.H.; Osheroff, N.; Nitiss, J.L. Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase-II as the primary cellular target for the quinolone CP-115,953 in yeast. J. Biol. Chem. 1992, 267, 13150–13153. [Google Scholar] [PubMed]
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar] [PubMed]
- Bourikas, L.A.; Kolios, G.; Valatas, V.; Notas, G.; Drygiannakis, I.; Pelagiadis, I.; Manousou, P.; Klironomos, S.; A Mouzas, I.; Kouroumalis, E. Ciprofloxacin decreases survival in HT-29 cells via the induction of TGF-β1 secretion and enhances the anti-proliferative effect of 5-fluorouracil. Br. J. Pharmacol. 2009, 157, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.H.; Li, Y.H.; Wu, S.S.; Hao, R.L.; Li, H.; Ni, H.; Han, H.B.; Lin, L. New and Highly Efficient Column Chromatographic Extraction and Simple Purification of Camptothecin from Camptotheca acuminata and Nothapodytes pittosporoides. Phytochem. Anal. 2013, 24, 623–630. [Google Scholar] [CrossRef]
- Arafa, R.K.; Hegazy, G.H.; Piazza, G.; Abadi, A.H. Synthesis and in vitro antiproliferative effect of novel quinoline-based potential anticancer agents. Eur. J. Med. Chem. 2013, 63, 826–832. [Google Scholar] [CrossRef]
- Slaninova, I.; Pencikova, K.; Urbanova, J.; Slanina, J.; Taborska, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 2013, 13, 51–68. [Google Scholar] [CrossRef]
- Khalifa, N.; Eweas, A.; Al-Omar, M.A.; Hozzein, W.N. Synthesis and antimicrobial activity of some novel 8-hydroxy-7-iodoquinoline-5-sulfonamide derivatives. J. Pure Appl. Microbiol. 2014, 8, 629–637. [Google Scholar]
- Kos, J.; Mitrovic, A. Nitroxoline: Repurposing its antimicrobial to antitumor application. Acta Biochim. Pol. 2019, 521–531. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Yang, C.; Zang, D.; Lan, X.; Liao, S.; Zhang, P.; Wu, J.; Li, X.; Liu, N.; et al. Repurposing an antidandruff agent to treating cancer: Zinc pyrithione inhibits tumor growth via targeting proteasome-associated deubiquitinases. Oncotarget 2017, 8, 13942–13956. [Google Scholar] [CrossRef]
- Merriam, C.V.; Citron, D.M.; Tyrrell, K.L.; Warren, Y.A.; Goldstein, E.J. In vitro activity of azithromycin and nine comparator agents against 296 strains of oral anaerobes and 31 strains of Eikenella corrodens. Int. J. Antimicrob. Agents 2006, 28, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kheadr, E.; Bernoussi, N.; Lacroix, C.; Fliss, I. Comparison of the sensitivity of commercial strains and infant isolates of bifidobacteria to antibiotics and bacteriocins. Int. Dairy J. 2004, 14, 1041–1053. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Gavini, F.; Vaugien, L.; Butel, M.J.; Doucet-Populaire, F. Antimicrobial susceptibility of bifidobacteria. J. Antimicrob. Chemother. 2005, 55, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Park, T.E.; Moy, S. Ceftazidime-Avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms. Clin. Ther. 2016, 38, 431–444. [Google Scholar] [CrossRef]
- Novakova, J.; Dzunkova, M.; Musilova, S.; Vlkova, E.; Kokoska, L.; Moya, A.; D’Auria, G.; Unkova, M.D. Selective growth-inhibitory effect of 8-hydroxyquinoline towards Clostridium difficile and Bifidobacterium longum subsp. longum in co-culture analysed by flow cytometry. J. Med. Microbiol. 2014, 63, 1663–1669. [Google Scholar] [CrossRef]
- Novakova, J.; Vlkova, E.; Salmonova, H.; Pechar, R.; Rada, V.; Kokoska, L. Anticlostridial agent 8-hydroxyquinoline improves the isolation of faecal bifidobacteria on modified Wilkins-Chalgren agar with mupirocin. Lett. Appl. Microbiol. 2016, 62, 330–335. [Google Scholar] [CrossRef]
- Skrivanova, E.; Van Immerseel, F.; Hovorkova, P.; Kokoska, L. In Vitro Selective Growth-Inhibitory Effect of 8-Hydroxyquinoline on Clostridium perfringens versus Bifidobacteria in a Medium Containing Chicken Ileal Digesta. PLoS ONE 2016, 11, e0167638. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, E.Y.; Lim, J.H.; Lee, H.S. Antimicrobial effects of 8-quinolinol. J. Food Sci. Biotechnol. 2006, 15, 817–819. [Google Scholar]
- Prapasarakul, N.; Tummaruk, P.; Niyomtum, W.; Tripipat, T.; Serichantalergs, O. Virulence Genes and Antimicrobial Susceptibilities of Hemolytic and Nonhemolytic Escherichia coli Isolated from Post-Weaning Piglets in Central Thailand. J. Vet. Med. Sci. 2010, 72, 1603–1608. [Google Scholar] [CrossRef]
- Tranter, R.W. The in vitro activity of halquinol against Vibrio cholerae. J. Trop. Med. Hyg. 1968, 71, 146–149. [Google Scholar]
- Sobke, A.; Makarewicz, O.; Baier, M.; Bar, C.; Pfister, W.; Gatermann, S.; Pletz, M.; Forstner, C. Empirical treatment of lower urinary tract infections in the face of spreading multidrug resistance: In vitro study on the effectiveness of nitroxoline. Int. J. Antimicrob. Agents 2018, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.J.; Segel, I.H. Mechanism of the Antimicrobial Action of Pyrithione: Effects on Membrane Transport, ATP Levels, and Protein Synthesis. Antimicrob. Agents Chemother. 1978, 14, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.C.; Dutta, N.K. Berberine and chloramphenicol in the treatment of cholera and severe diarrhoea. J. Indian Med. Assoc. 1967, 48, 1–11. [Google Scholar]
- Sack, R.B.; Froehlich, J.L. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect. Immun. 1982, 35, 471–475. [Google Scholar] [CrossRef]
- Hamoud, R.; Reichling, J.; Wink, M.R. Synergistic antibacterial activity of the combination of the alkaloid sanguinarine with EDTA and the antibiotic streptomycin against multidrug resistant bacteria. J. Pharm. Pharmacol. 2014, 67, 264–273. [Google Scholar] [CrossRef]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Han, Z.; Li, C.; Lv, L.; Cheng, Z.; Liu, S. Florfenicol induces more severe hemotoxicity and immunotoxicity than equal doses of chloramphenicol and thiamphenicol in Kunming mice. Immunopharmacol. Immunotoxicol. 2016, 38, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Nagasue, N. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int. J. Cancer 2005, 118, 1309–1315. [Google Scholar] [CrossRef]
- Dewdney, J.M. Effects of beta-lactam antibiotics on eukaryotic cells. Cell Boil. Toxicol. 1986, 2, 509–511. [Google Scholar] [CrossRef]
- Eisenstein, B.I.; Schaechter, M. DNA and Chromosome Mechanics. In Schaechter’s Mechanisms of Microbial Disease; Schaechter, M., Engleberg, N.C., DiRita, V.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; p. 28. [Google Scholar]
- Hanaki, H.; Kuwahara-Arai, K.; Boyle-Vavra, S.; Daum, R.S.; Labischinski, H.; Hiramatsu, K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 1998, 42, 199–209. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.B.D.O.; Borgati, T.F.; Gil, R.F.; Ruiz, A.; Marchetti, G.M.; De Carvalho, J.E.; Da Cunha, E.F.; Ramalho, T.D.C.; Alves, R.B. Synthesis and antiproliferative activity of 8-hydroxyquinoline derivatives containing a 1,2,3-triazole moiety. Eur. J. Med. Chem. 2014, 84, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, A.; Jovanovic, J.; Monteiro, S.; Decleer, M.; Andjelkovic, M.; Foubert, A.; Beloglazova, N.; Tsilla, V.; Sas, B.; Madder, A.; et al. Detection of toxins involved in foodborne diseases caused by Gram-positive bacteria. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1605–1657. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Hecht, D.W. Antimicrobial agents and susceptibility testing: Susceptibility testing of anaerobic bacteria. In Manual of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 1555–1562. [Google Scholar]
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56–62. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Houdkova, M.; Urbanova, K.; Doskocil, I.; Rondevaldova, J.; Novy, P.; Nguon, S.; Chrun, R.; Kokoska, L. In vitro growth-inhibitory effect of Cambodian essential oils against pneumonia causing bacteria in liquid and vapour phase and their toxicity to lung fibroblasts. S. Afr. J. Bot. 2018, 118, 85–97. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica, Data Analysis Software System, Version 13.0. Available online: http://statistica.io (accessed on 30 September 2017).

| Cultures Tested | Alkaloids | Phenolic Compounds | Antibiotics | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | SG | 8HQ | CLX | NXL | FRN | ZP | SA | BS | TA | CF | CP | MA | VM | CA | TC | |||
| Bacterial strain/MIC (μg/mL) | BC | - a | 128 | 512 | 8 | 4 | 512 | 4 | - | 512 | 512 | 128 | 1 | 128 | 256 | 8 | 0.5 | |
| CD | - | 64 | 128 | 8 | 2 | 64 | 8 | 256 | 128 | - | 64 | 16 | 0.5 | 2 | 4 | 0.5 | ||
| CP | 256 | 128 | 128 | 16 | 4 | 64 | 8 | - | 512 | 512 | 4 | 1 | 8 | 1 | 4 | 32 | ||
| EF | - | 32 | 4 | 16 | 32 | - | 8 | - | - | - | 512 | 2 | - | - | 128 | 4 | ||
| EC | - | 256 | 128 | 16 | 4 | - | 4 | - | - | - | 0.062 | 0.062 | 0.062 | - | 4 | 1 | ||
| ECS | - | 128 | 256 | 64 | 32 | - | 8 | - | - | 512 | 0.5 | 0.016 | - | 512 | 8 | 4 | ||
| LM | - | 16 | 1 | 32 | 16 | 512 | 8 | - | 256 | - | 32 | 4 | 512 | 8 | 8 | 2 | ||
| SF | - | 64 | 128 | 16 | 2 | - | 1 | - | - | - | 0.5 | 0.016 | - | - | 4 | 2 | ||
| SE | - | 256 | 256 | 64 | 16 | - | 8 | - | - | - | 0.25 | 0.031 | - | 512 | 4 | 4 | ||
| ST | 512 | 512 | 512 | 16 | 16 | - | 8 | - | 512 | - | 0.25 | 0.031 | - | 512 | 8 | 4 | ||
| VP | 512 | 32 | 128 | 16 | 8 | 256 | 4 | - | - | 128 | 0.125 | 0.062 | - | 256 | 2 | 1 | ||
| YE | - | 256 | 512 | 16 | 8 | - | 16 | - | - | 512 | 0.25 | 0.125 | - | - | 16 | 2 | ||
| -DB ± SD | 874.7 ± 256 | 156 ± 137 | 224.4 ± 181 | 24 ± 19 | 12 ± 10 | 714.7 ± 388 | 7.1 ± 4 | 960 ± 212 | 757.3 ± 332 | 778.7 ± 307 | 61.8 ± 141 | 2 ± 4 | 651.4 ± 459 | 512.9 ± 404 | 16.5 ± 34 | 4.8 ± 8 | ||
| BF | - | 32 | 32 | 32 | 4 | 128 | 8 | 128 | 32 | - | 128 | 8 | 0.5 | 32 | 4 | 0.5 | ||
| BA | 128 | 16 | 512 | 128 | 16 | 256 | 16 | - | 32 | - | 1 | 8 | 64 | 2 | 4 | 64 | ||
| BLC | 32 | 32 | - | 512 | 32 | 512 | 16 | 128 | 64 | - | 2 | 32 | 32 | 2 | 4 | 32 | ||
| BBF | 64 | 32 | 512 | - | 16 | 512 | 8 | - | 64 | - | 4 | 16 | - | 4 | 4 | 16 | ||
| BB | 64 | 32 | - | 64 | 32 | 512 | 16 | - | 64 | 256 | 32 | 64 | 32 | 4 | 4 | 16 | ||
| BL | 32 | 64 | 512 | 64 | 16 | 256 | 16 | 512 | 64 | 128 | 8 | 16 | 8 | 2 | 4 | 2 | ||
| LC | 64 | 32 | - | 128 | 32 | 256 | 16 | 512 | 128 | 512 | 32 | 32 | - | 256 | 16 | 8 | ||
| LR | - | 32 | - | 128 | 16 | 512 | 64 | 512 | 64 | 512 | 0.5 | 32 | 256 | 64 | 8 | 32 | ||
| LRM | 64 | 64 | - | 128 | 16 | 512 | 32 | 512 | 128 | - | 32 | 4 | - | 64 | 8 | 8 | ||
| -PB ± SD | 277.3 ± 400 | 37.3 ± 15 | 743.1 ± 343 | 245.3 ± 306 | 20 ± 9 | 384 ± 148 | 21.3 ± 16 | 597.3 ± 336 | 71.1 ± 33 | 725.3 ± 352 | 26.6 ± 38 | 23.6 ± 18 | 384.9 ± 458 | 47.8 ± 78 | 6.2 ± 4 | 19.8 ± 19 | ||
| Cell line (μg/mL) | IC50 ± SD | HT29 | 5 ± 1 | 0.9 ± 0.2 | 1.3 ± 0.3 | 3.7 ± 0.3 | 2.6 ± 0.3 | 86.3 ± 12 | 0.6 ± 0.01 | - | 461.9 ± 17 | 35.9 ± 4.9 | - | 130.3 ± 13 | - | - | 271.1 ± 1 | 392.9± 20 |
| Caco-2 | 19.4 ± 2.9 | 0.8 ± 0.1 | 0.3 ± 0.1 | 1.3 ± 0.04 | 1.1 ± 0.03 | 55.2 ± 4.8 | 0.7 ± 0.2 | - | 45.3 ± 5 | 27.6 ± 1.5 | - | 69.9 ± 4.9 | - | - | 439.3 ± 4 | 70.4 ± 15 | ||
| -CC ± SD | 12.2 ± 7 | 0.8 ± 0.05 | 0.8 ± 0.5 | 2.5 ± 1.2 | 1.8 ± 0.8 | 70.8 ± 16 | 0.6 ± 0.05 | - | 253.6 ± 208 | 31.7 ± 4 | - | 100.1 ± 30 | - | - | 355.2 ± 84 | 231.6± 161 | ||
| FHs 74 Int | 1 ± 0.1 | 1 ± 0.1 | 10.7 ± 0.2 | 0.5 ± 0.02 | 0.4 ± 0.05 | 22.6 ± 3.3 | 0.3 ± 0.1 | 73.2 ± 4.6 | 8.7 ± 1.3 | 5.9 ± 1.2 | - | 51.8 ± 27 | - | - | 30.7 ± 5.6 | 14.7 ± 2.3 | ||
| IC80 ± SD | HT29 | 42.1 ± 7.3 | 1.8 ± 0.4 | 4.8 ± 2.8 | 5 ± 0.7 | 3.7 ± 0.4 | 149.4 ± 6 | 0.7 ± 0.01 | - | - | 43 ± 2.2 | - | - | - | - | - | - | |
| Caco-2 | 78.9 ± 0.3 | 1.5 ± 0.1 | 0.9 ± 0.1 | 5.9 ± 0.7 | 5 ± 1.5 | 139.9 ± 26 | 0.7 ± 0.01 | - | 454.9 ± 43 | - | - | - | - | - | - | - | ||
| -CC ± SD | 60.5 ± 18.4 | 1.6 ± 0.2 | 2.9 ± 2 | 5.4 ± 0.5 | 4.4 ± 0.7 | 144.7 ± 4.8 | 0.7 ± 0 | - | 740 ± 285 | 534 ± 491 | - | - | - | - | - | - | ||
| FHs 74 Int | 26.4 ± 0.8 | 1.9 ± 0.2 | 20.3 ± 2.4 | 2 ± 0.1 | 0.8 ± 0.05 | 46 ± 2.2 | 0.5 ± 0.03 | 206.9 ± 69 | 31.4 ± 6.6 | 10.2 ± 2.4 | - | 129.5 ± 24 | - | - | - | 108.2 ± 5 | ||
| SI | (a) | −1.5 | −1.9 | −1 | −1.1 | −1.2 | −1.2 | −1.1 | −0.7 | −1.4 | −1.9 | 1.2 | 1.8 | 0.2 | 0.3 | 1.8 | 1.4 | |
| (b) | −0.5 | −0.6 | 0.5 | 1 | 0.2 | −0.3 | 0.5 | −0.2 | −1 | −0.03 | −0.4 | 1.1 | −0.2 | −1 | −0.4 | 0.6 | ||
| (c) | −1.1 | 0.1 | 1.1 | −0.7 | −0.6 | −0.5 | −0.4 | −1.1 | −1.5 | −0.7 | 0 | −0.3 | 0 | 0 | −1.1 | −1.2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudera, T.; Doskocil, I.; Salmonova, H.; Petrtyl, M.; Skrivanova, E.; Kokoska, L. In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells. Pharmaceuticals 2020, 13, 233. https://doi.org/10.3390/ph13090233
Kudera T, Doskocil I, Salmonova H, Petrtyl M, Skrivanova E, Kokoska L. In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells. Pharmaceuticals. 2020; 13(9):233. https://doi.org/10.3390/ph13090233
Chicago/Turabian StyleKudera, Tomas, Ivo Doskocil, Hana Salmonova, Miloslav Petrtyl, Eva Skrivanova, and Ladislav Kokoska. 2020. "In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells" Pharmaceuticals 13, no. 9: 233. https://doi.org/10.3390/ph13090233
APA StyleKudera, T., Doskocil, I., Salmonova, H., Petrtyl, M., Skrivanova, E., & Kokoska, L. (2020). In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells. Pharmaceuticals, 13(9), 233. https://doi.org/10.3390/ph13090233