Metformin: A Potential Therapeutic Tool for Rheumatologists
Abstract
1. Introduction
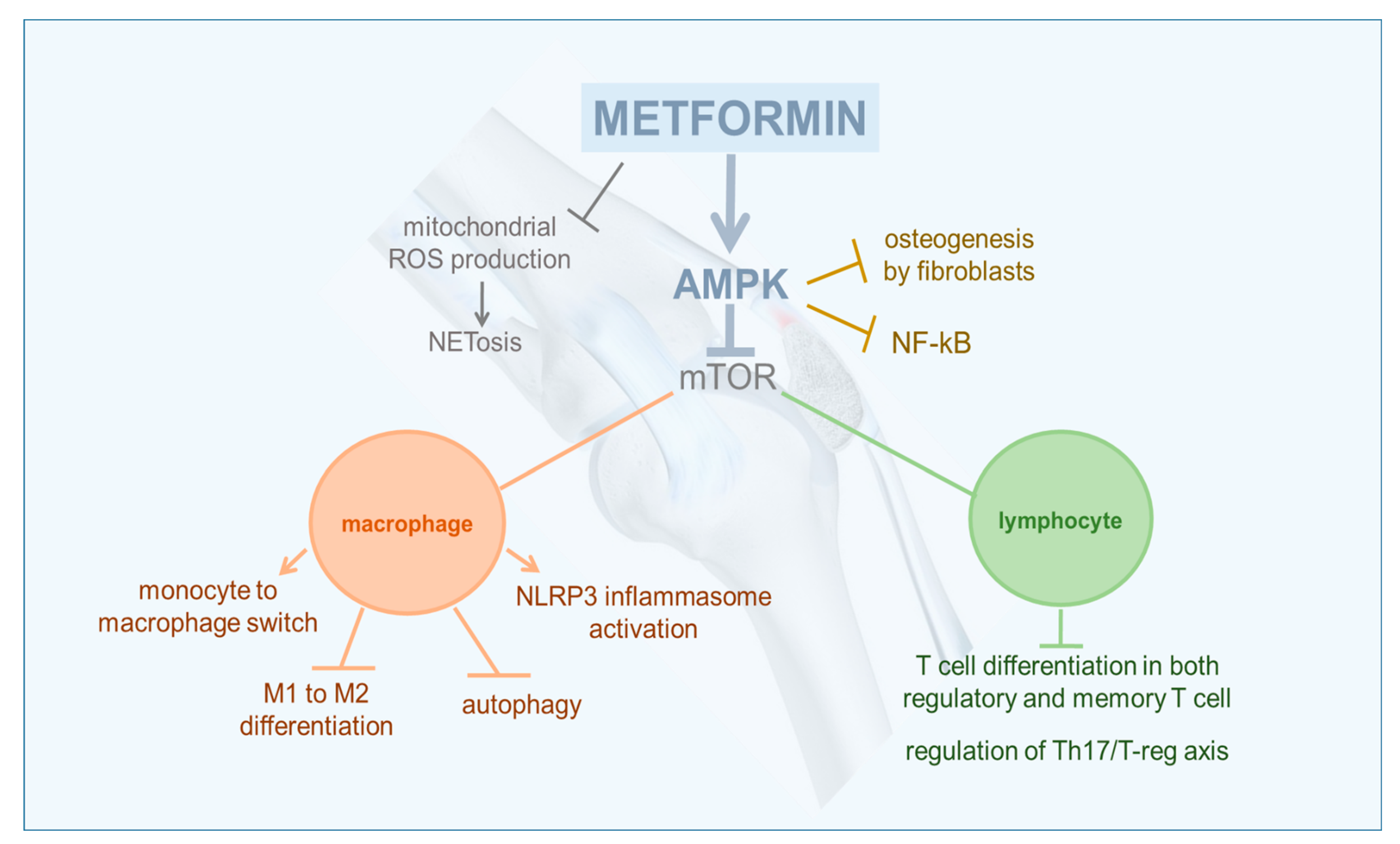
2. Anti-Inflammatory and Immuno-Modulating Effects of AMPK
3. Rheumatoid Arthritis
4. Osteoarthritis
5. Gout
6. Systemic Lupus Erythematosus
7. Sjögren Syndrome
8. Ankylosing Spondylitis
9. Conclusions
Funding
Conflicts of Interest
References
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Lockwood, T.D. The lysosome among targets of metformin: New anti-inflammatory uses for an old drug? Expert Opin. Ther. Targets 2010, 14, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef]
- Salt, I.P.; Palmer, T.M. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin. Investig. Drugs 2012, 21, 1155–1167. [Google Scholar] [CrossRef]
- Gejjalagere Honnappa, C.; Mazhuvancherry Kesavan, U. A concise review on advances in development of small molecule anti-inflammatory therapeutics emphasising AMPK: An emerging target. Int. J. Immunopathol. Pharmacol. 2016, 29, 562–571. [Google Scholar] [CrossRef]
- Speirs, C.; Williams, J.J.L.; Riches, K.; Salt, I.P.; Palmer, T.M. Linking energy sensing to suppression of JAK-STAT signalling: A potential route for repurposing AMPK activators? Pharmacol Res. 2018, 128, 88–100. [Google Scholar] [CrossRef]
- Ma, C.S.; Avery, D.T.; Chan, A.; Batten, M.; Bustamante, J.; Boisson-Dupuis, S.; Arkwright, P.D.; Kreins, A.Y.; Averbuch, D.; Engelhard, D.; et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012, 119, 3997–4008. [Google Scholar] [CrossRef]
- Ray, J.P.; Marshall, H.D.; Laidlaw, B.J.; Staron, M.M.; Kaech, S.M.; Craft, J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity 2014, 40, 367–377. [Google Scholar] [CrossRef]
- Deenick, E.K.; Avery, D.T.; Chan, A.; Berglund, L.J.; Ives, M.L.; Moens, L.; Stoddard, J.L.; Bustamante, J.; Boisson-Dupuis, S.; Tsumura, M.; et al. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J. Exp. Med. 2013, 210, 2739–2753. [Google Scholar] [CrossRef] [PubMed]
- Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 2016, 12, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease [published correction appears in Cell. 2017 Apr 6;169, 361-371]. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Ma, L.; Ren, Y.; Liu, H. Modulation of the immune response in rheumatoid arthritis with strategically released rapamycin. Mol. Med. Rep. 2017, 16, 5257–5262. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Perl, A. Blockade of Treg Cell Differentiation and Function by the Interleukin-21-Mechanistic Target of Rapamycin Axis Via Suppression of Autophagy in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018, 70, 427–438. [Google Scholar] [CrossRef]
- Dowling, R.J.; Goodwin, P.J.; Stambolic, V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011, 9, 33. [Google Scholar] [CrossRef]
- Antonioli, L.; Colucci, R.; Pellegrini, C.; Giustarini, G.; Sacco, D.; Tirotta, E.; Caputi, V.; Marsilio, I.; Giron, M.C.; Németh, Z.H.; et al. The AMPK enzyme-complex: From the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin. Ther. Targets 2016, 20, 179–191. [Google Scholar] [CrossRef]
- Kang, K.Y.; Kim, Y.K.; Yi, H.; Kim, J.; Jung, H.R.; Kim, I.J.; Cho, J.H.; Park, S.H.; Kim, H.Y.; Ju, J.H. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 2013, 16, 85–92. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Park, M.J.; Kim, K.W.; Park, S.H.; Cho, M.L. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediat. Inflamm. 2014, 2014, 973986. [Google Scholar] [CrossRef] [PubMed]
- Indo, Y.; Takeshita, S.; Ishii, K.A.; Hoshii, T.; Aburatani, H.; Hirao, A.; Ikeda, K. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013, 28, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, H.J. The K/BxN mouse: A model of human inflammatory arthritis. Trends Mol. Med. 2004, 10, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.; Kim, S.Y.; Na, H.S.; Kim, E.K.; Kim, J.K.; Jeong, J.H.; Park, S.H.; Cho, M.L. Combination therapy with metformin and coenzyme Q10 in murine experimental autoimmune arthritis. Immunopharmacol. Immunotoxicol. 2016, 38, 103–112. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.H.; Lee, S.Y.; Kim, J.K.; Jhun, J.Y.; Na, H.S.; Kim, S.Y.; Choi, J.Y.; Yang, C.W.; Park, S.H.; et al. Metformin ameliorates experimental-obesity-associated autoimmune arthritis by inducing FGF21 expression and brown adipocyte differentiation. Exp. Mol. Med. 2018, 50, e432. [Google Scholar] [CrossRef]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Chen, K.; Lin, Z.W.; He, S.M.; Wang, C.Q.; Yang, J.C.; Lu, Y.; Xie, X.B.; Li, Q. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed. Pharmacother. 2019, 115, 108875. [Google Scholar] [CrossRef]
- Kim, E.K.; Min, H.K.; Lee, S.Y.; Kim, D.S.; Ryu, J.G.; Na, H.S.; Jung, K.A.; Choi, J.W.; Park, S.H.; Cho, M.L. Metformin rescues rapamycin-induced mitochondrial dysfunction and attenuates rheumatoid arthritis with metabolic syndrome. Arthritis Res. Ther. 2020, 22, 77. [Google Scholar] [CrossRef]
- Gallagher, L.; Cregan, S.; Biniecka, M.; Cunningham, C.; Veale, D.J.; Kane, D.J.; Fearon, U.; Mullan, R.H. Insulin-Resistant Pathways Are Associated with Disease Activity in Rheumatoid Arthritis and Are Subject to Disease Modification through Metabolic Reprogramming: A Potential Novel Therapeutic Approach. Arthritis Rheumatol. 2020, 72, 896–902. [Google Scholar] [CrossRef]
- Naffaa, M.E.; Rosenberg, V.; Watad, A.; Tiosano, S.; Yavne, Y.; Chodick, G.; Amital, H.; Shalev, V. Adherence to metformin and the onset of rheumatoid arthritis: A population-based cohort study. Scand. J. Rheumatol. 2020, 49, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chung, C.H.; Lee, C.H.; Su, S.C.; Liu, J.S.; Lin, F.H.; Tsao, C.H.; Hsieh, P.S.; Hung, Y.J.; Hsieh, C.H.; et al. Combination of COX-2 inhibitor and metformin attenuates rate of admission in patients with rheumatoid arthritis and diabetes in Taiwan. Medicine 2019, 98, e17371. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Liu, W.X.; Lu, K.; Pan, H.; Wang, T.; Oh, C.D.; Yi, D.; Huang, J.; Zhao, L.; et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020, 79, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hussain, S.M.; Wluka, A.E.; Lim, Y.Z.; Abram, F.; Pelletier, J.P.; Martel-Pelletier, J.; Cicuttini, F.M. Association between metformin use and disease progression in obese people with knee osteoarthritis: Data from the Osteoarthritis Initiative-a prospective cohort study. Arthritis Res. Ther. 2019, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chung, C.H.; Lee, C.H.; Hsieh, C.H.; Hung, Y.J.; Lin, F.H.; Tsao, C.H.; Hsieh, P.S.; Chien, W.C. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS ONE 2018, 13, e0191242. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, X.; Terkeltaub, R.; Lin, H.; Zhang, Y.; Zhou, B.; He, K.; Li, K.; Liu, Z.; Wei, J.; et al. Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020, 22, 34. [Google Scholar] [CrossRef]
- Park, M.J.; Moon, S.J.; Baek, J.A.; Lee, E.J.; Jung, K.A.; Kim, E.K.; Kim, D.S.; Lee, J.H.; Kwok, S.K.; Min, J.K.; et al. Metformin Augments Anti-Inflammatory and Chondroprotective Properties of Mesenchymal Stem Cells in Experimental Osteoarthritis. J. Immunol. 2019, 203, 127–136. [Google Scholar] [CrossRef]
- Vazirpanah, N.; Ottria, A.; van der Linden, M.; Wichers, C.G.K.; Schuiveling, M.; van Lochem, E.; Phipps-Green, A.; Merriman, T.; Zimmermann, M.; Jansen, M.; et al. mTOR inhibition by metformin impacts monosodium urate crystal-induced inflammation and cell death in gout: A prelude to a new add-on therapy? Ann. Rheum. Dis. 2019, 78, 663–671. [Google Scholar] [CrossRef]
- Barskova, V.G.; Eliseev, M.S.; Nasonov, E.L.; Volkov, A.V.; Tsapina, T.N.; Zilov, A.V.; Iakunina, I.A.; Il’inykh, E.V.; Kudaeva, F.M. [Use of metformin (siofor) in patients with gout and insulin resistance (pilot 6-month results)]. Ter. Arkh. 2005, 77, 44–49. (In Russian) [Google Scholar]
- Wang, H.; Li, T.; Chen, S.; Gu, Y.; Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015, 67, 3190–3200. [Google Scholar] [CrossRef]
- Yin, Y.; Choi, S.C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra18. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.M.; Park, J.S.; Hwang, S.H.; Choi, J.; Jung, K.A.; Ryu, J.G.; Lee, S.Y.; Kwok, S.K.; Cho, M.L.; et al. Metformin improves salivary gland inflammation and hypofunction in murine Sjögren’s syndrome. Arthritis Res. Ther. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, T.; Liu, S.; Tan, J.; Wu, H.; Zheng, L.; Zhao, J. Effect of metformin on ossification and inflammation of fibroblasts in ankylosing spondylitis: An in vitro study. J. Cell Biochem. 2018, 119, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Fearnley, G.R.; Chakrabarti, R. Fibrinolytic treatment of rheumatoid arthritis with phenformin plus ethyloestrenol. Lancet 1966, 2, 757–761. [Google Scholar] [CrossRef]
- Guma, M.; Wang, Y.; Viollet, B.; Liu-Bryan, R. AMPK Activation by A-769662 Controls IL-6 Expression in Inflammatory Arthritis. PLoS ONE 2015, 10, e0140452. [Google Scholar] [CrossRef] [PubMed]
- Crispín, J.C.; Tsokos, G.C. IL-17 in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 2010, 943254. [Google Scholar] [CrossRef]
- Li, X.; Yuan, F.L.; Lu, W.G.; Zhao, Y.Q.; Li, C.W.; Li, J.P.; Xu, R.S. The role of interleukin-17 in mediating joint destruction in rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2010, 397, 131–135. [Google Scholar] [CrossRef]
- Van den Berg, W.B.; Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 549–553. [Google Scholar] [CrossRef]
- Van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81. [Google Scholar] [CrossRef]
- Münz, C. Enhancing immunity through autophagy. Annu. Rev. Immunol. 2009, 27, 423–449. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Jones, S.A.; Mills, K.H.; Harris, J. Autophagy and inflammatory diseases. Immunol. Cell Biol. 2013, 91, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhou, H.F.; Hu, Y.; Pham, C.T. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J. Rheum. Dis. Treat. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Piel, S.; Ehinger, J.K.; Elmér, E.; Hansson, M.J. Metformin induces lactate production in peripheral blood mononuclear cells and platelets through specific mitochondrial complex I inhibition. Acta Physiol. (Oxf.) 2015, 213, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef]
- Muraki, A.; Miyashita, K.; Mitsuishi, M.; Tamaki, M.; Tanaka, K.; Itoh, H. Coenzyme Q10 reverses mitochondrial dysfunction in atorvastatin-treated mice and increases exercise endurance. J. Appl. Physiol. 2012, 113, 479–486. [Google Scholar] [CrossRef]
- Jhun, J.; Lee, S.H.; Byun, J.K.; Jeong, J.H.; Kim, E.K.; Lee, J.; Jung, Y.O.; Shin, D.; Park, S.H.; Cho, M.L. Coenzyme Q10 suppresses Th17 cells and osteoclast differentiation and ameliorates experimental autoimmune arthritis mice. Immunol. Lett. 2015, 166, 92–102. [Google Scholar] [CrossRef]
- Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007, 9, 203. [Google Scholar] [CrossRef]
- Manning, P.J.; Sutherland, W.H.; McGrath, M.M.; de Jong, S.A.; Walker, R.J.; Williams, M.J. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity 2008, 16, 2046–2052. [Google Scholar] [CrossRef]
- Laria, A.; Lurati, A.; Marrazza, M.; Mazzocchi, D.; Re, K.A.; Scarpellini, M. The macrophages in rheumatic diseases. J. Inflamm. Res. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Hayer, S.; Niederreiter, B.; Sieghart, W.; Fuereder, T.; Zwerina, J.; Schett, G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G.A.; Tate, G.; Caeiro, F.; Maldonado-Cocco, J.; Westhovens, R.; Tannenbaum, H.; Bell, M.; Forre, O.; Bjorneboe, O.; Tak, P.P. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: A 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann. Rheum Dis. 2008, 67, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Jabs, A.; Göbel, S.; Wenzel, P.; Kleschyov, A.L.; Hortmann, M.; Oelze, M.; Daiber, A.; Münzel, T. Sirolimus-induced vascular dysfunction. Increased mitochondrial and nicotinamide adenosine dinucleotide phosphate oxidase-dependent superoxide production and decreased vascular nitric oxide formation. J. Am. Coll. Cardiol. 2008, 51, 2130–2138. [Google Scholar] [CrossRef]
- Lombardi, A.; Gambardella, J.; Du, X.L.; Sorriento, D.; Mauro, M.; Iaccarino, G.; Trimarco, B.; Santulli, G. Sirolimus induces depletion of intracellular calcium stores and mitochondrial dysfunction in pancreatic beta cells. Sci. Rep. 2017, 7, 15823. [Google Scholar] [CrossRef]
- Valcárcel-Ares, M.N.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Loureiro, J.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction promotes and aggravates the inflammatory response in normal human synoviocytes. Rheumatology 2014, 53, 1332–1343. [Google Scholar] [CrossRef]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; Ciccia, F.; Liakouli, V.; Carubbi, F.; Guggino, G.; Berardicurti, O.; Grembiale, R.; Triolo, G. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: Results from a cross-sectional study [published correction appears in Medicine (Baltimore). 2017 Sep 15;96, e8132]. Medicine 2017, 96, e7896. [Google Scholar] [CrossRef]
- Dessein, P.H.; Joffe, B.I. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2765–2775. [Google Scholar] [CrossRef]
- Rajaei, E.; Haybar, H.; Mowla, K.; Zayeri, Z.D. Metformin one in a Million Efficient Medicines for Rheumatoid Arthritis Complications: Inflammation, Osteoblastogenesis, Cardiovascular Disease, Malignancies. Curr. Rheumatol. Rev. 2019, 15, 116–122. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lu, W.; Chen, L.; Ge, Q.; Chen, D.; Xu, Z.; Shi, D.; Dai, J.; Li, J.; Ju, H.; et al. AMPK deficiency in chondrocytes accelerated the progression of instability-induced and ageing-associated osteoarthritis in adult mice. Sci. Rep. 2017, 7, 43245. [Google Scholar] [CrossRef] [PubMed]
- Petursson, F.; Husa, M.; June, R.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res. Ther. 2013, 15, R77. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Yang, B.; Lotz, M.; Liu-Bryan, R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011, 63, 1928–1937. [Google Scholar] [CrossRef]
- Murphy, L.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008, 59, 1207–1213. [Google Scholar] [CrossRef]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef]
- Dawson, L.P.; Fairley, J.L.; Papandony, M.C.; Hussain, S.M.; Cicuttini, F.M.; Wluka, A.E. Is abnormal glucose tolerance or diabetes a risk factor for knee, hip, or hand osteoarthritis? A systematic review. Semin. Arthritis Rheum. 2018, 48, 176–189. [Google Scholar] [CrossRef]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef]
- Dickson, B.M.; Roelofs, A.J.; Rochford, J.J.; Wilson, H.M.; De Bari, C. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res. Ther. 2019, 21, 289. [Google Scholar] [CrossRef]
- Nieves-Plaza, M.; Castro-Santana, L.E.; Font, Y.M.; Mayor, A.M.; Vilá, L.M. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J. Clin. Rheumatol. 2013, 19, 1–6. [Google Scholar] [CrossRef]
- Louati, K.; Vidal, C.; Berenbaum, F.; Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 2015, 1, e000077. [Google Scholar] [CrossRef]
- Rahman, M.M.; Cibere, J.; Anis, A.H.; Goldsmith, C.H.; Kopec, J.A. Risk of Type 2 Diabetes among Osteoarthritis Patients in a Prospective Longitudinal Study. Int. J. Rheumatol. 2014, 2014, 620920. [Google Scholar] [CrossRef] [PubMed]
- Barnett, L.A.; Jordan, K.P.; Edwards, J.J.; van der Windt, D.A. Does metformin protect against osteoarthritis? An electronic health record cohort study. Prim. Health Care Res. Dev. 2017, 18, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Das, V.; Dussor, G. Adenosine Monophosphate-activated Protein Kinase (AMPK) Activators for the Prevention, Treatment and Potential Reversal of Pathological Pain. Curr. Drug Targets 2016, 17, 908–920. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Crişan, T.O.; Cleophas, M.C.P.; Novakovic, B.; Erler, K.; van de Veerdonk, F.L.; Stunnenberg, H.G.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5485–5490. [Google Scholar] [CrossRef]
- Harris, J.; Hartman, M.; Roche, C.; Zeng, S.G.; O’Shea, A.; Sharp, F.A.; Lambe, E.M.; Creagh, E.M.; Golenbock, D.T.; Tschopp, J.; et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem. 2011, 286, 9587–9597. [Google Scholar] [CrossRef]
- Barskova, V.G.; Eliseev, M.S.; Kudaeva, F.M.; Aleksandrova, E.N.; Volkov, A.V.; Nasonova, V.A.; Nasonov, E.L. Effect of metformin on the clinical course of gout and insulin resistance. Klin. Med. 2009, 87, 41–46. (In Russian) [Google Scholar]
- Bruderer, S.G.; Bodmer, M.; Jick, S.S.; Meier, C.R. Poorly controlled type 2 diabetes mellitus is associated with a decreased risk of incident gout: A population-based case-control study. Ann. Rheum. Dis. 2015, 74, 1651–1658. [Google Scholar] [CrossRef]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Pontarini, E.; Lucchesi, D.; Bombardieri, M. Current views on the pathogenesis of Sjögren’s syndrome. Curr. Opin. Rheumatol. 2018, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.L.; Valencia-Pacheco, G.; Alcocer-Varela, J. Constitutive STAT3 activation in peripheral CD3(+) cells from patients with primary Sjögren’s syndrome. Scand. J. Rheumatol. 2008, 37, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Edman, M.C.; Janga, S.R.; Shi, P.; Dhandhukia, J.; Liu, S.; Louie, S.G.; Rodgers, K.; Mackay, J.A.; Hamm-Alvarez, S.F. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren’s syndrome. J. Control. Release. 2013, 171, 269–279. [Google Scholar] [CrossRef]
- Wendling, D.; Claudepierre, P. New bone formation in axial spondyloarthritis. Joint Bone Spine 2013, 80, 454–458. [Google Scholar] [CrossRef]
- Yu, F.; Cui, Y.; Zhou, X.; Zhang, X.; Han, J. Osteogenic differentiation of human ligament fibroblasts induced by conditioned medium of osteoclast-like cells. Biosci. Trends 2011, 5, 46–51. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Xue, J.; Jia, Y.; Hu, J. Effect of the anti-diabetic drug metformin on bone mass in ovariectomized rats. Eur. J. Pharmacol. 2010, 635, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mai, Q.G.; Zhang, Z.M.; Xu, S.; Lu, M.; Zhou, R.P.; Zhao, L.; Jia, C.H.; Wen, Z.H.; Jin, D.D.; Bai, X.C. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J. Cell Biochem. 2011, 112, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, J.; Li, R.; Qin, X.; Wang, F.; Mao, Y.; Liang, W.; Sheng, X.; Guo, M.; Song, Y.; et al. Metformin alleviates vascular calcification induced by vitamin D3 plus nicotine in rats via the AMPK pathway. Vascul. Pharmacol. 2016, 81, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Monaco, L.; Ricozzi, C.; Imbriani, S.; Nevola, R.; Adinolfi, L.E.; et al. Metformin lactic acidosis: Should we still be afraid? Diabetes Res. Clin. Pract. 2019, 157, 107879. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Marfella, R.; Monaco, L.; Rinaldi, L.; Adinolfi, L.E.; et al. Metformin: An old drug against old age and associated morbidities. Diabetes Res. Clin. Pract. 2020, 160, 108025. [Google Scholar] [CrossRef]
- Del Rincón, I.D.; Williams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Kuo, C.F.; See, L.C.; Luo, S.F.; Ko, Y.S.; Lin, Y.S.; Hwang, J.S.; Lin, C.M.; Chen, H.W.; Yu, K.H. Gout: An independent risk factor for all-cause and cardiovascular mortality. Rheumatology 2010, 49, 141–146. [Google Scholar] [CrossRef]
- Perez-Ruiz, F.; Martínez-Indart, L.; Carmona, L.; Herrero-Beites, A.M.; Pijoan, J.I.; Krishnan, E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann. Rheum. Dis. 2014, 73, 177–182. [Google Scholar] [CrossRef]
| Rheumatoid arthritis (RA) | Lower increase in Th17 cells with reduction of proinflammatory cytokines and improved arthritis score in a CAIA mouse model [20,21] |
| Suppression of Th17 cells and enhancement of Treg cells in a CIA murine model [22] | |
| Suppression of osteoclast differentiation [23] | |
| Impaired autophagy correction with suppression of inflammatory cytokines and clinical arthritis in a murine model of immune arthritis [24] | |
| Higher reduction of Th17 cells, induction of Treg cells, and inhibition of osteoclastogenesis with higher arthritis improvement by metformin combined with CoQ10 vs. metformin or CoQ10 alone in a CIA murine model [25] | |
| Restoration of reciprocal Th17/Treg balance with dampened CIA development in a murine model of diet-induced obesity [26] | |
| Modulation of macrophage polarization toward M2 phenotype in a model of high-fat diet-fed obese C57/6J male mice [27] | |
| Inhibition of RA-FLS proliferation on synovial tissue from patients with RA [28] | |
| Mitochondrial dysfunction reduction by rapamycin combined with metformin vs. rapamycin alone in a CIA obese mouse model [29] | |
| Reduction of GLUT-1 expression in synovial tissue from patients with RA [30] | |
| Inverse association between risk of RA and exposure to metformin inT2DM patients [31] | |
| Lower admission rate of T2DM patients with RA treated with metformin and Cyclooxygenase (COX)-2 inhibitor vs. COX-2 inhibitors alone [32] | |
| Osteoarthritis (OA) | Osteoarthritis limited development and delayed progression in a DMM murine model, not in an AMPK/α1 knockout DMM mice [33] |
| Chondroprotection in a partial medial meniscectomy model of non-human primates [33] | |
| Reduced knee osteoarthritis progression in obese patients [34] | |
| Decreased risk of joint replacement surgery by 25% over 10 years [35] | |
| Improvement of osteoarthritis-related pain on a DMM OA mouse model [36] | |
| Chondroprotective and antinociceptive effect of intravenous [i.v.] administration of metformin-stimulated Ad-hMSCs [37] | |
| Gout | Reduction in release of cell death and inflammatory mediators from monocytes encountering MSU crystals [38] |
| Decrease of incident gout in T2DM patients and of gout attacks in gouty non-diabetic patients [39] | |
| Systemic lupus erythematous | Reduction of NET DNA release in cultured neutrophils and inhibition of Interferon (INF)-α generation from stimulated PDCs [40] |
| 51% reduction of flares frequency in patients with mild or moderate disease [40] | |
| Restoration of Cluster of Differentiation (CD)4+ T function and reversion of disease phenotypes in a lupus-prone mouse model [41] | |
| Sjögren syndrome | Suppression of effector T cells and induction of regulatory T cells in a murine model of Sjögren syndrome [42] |
| Ankylosing spondylitis | Potent antiosteogenic effect on human fibroblasts [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, T.; Pafundi, P.C.; Galiero, R.; Gjeloshi, K.; Masini, F.; Acierno, C.; Di Martino, A.; Albanese, G.; Alfano, M.; Rinaldi, L.; et al. Metformin: A Potential Therapeutic Tool for Rheumatologists. Pharmaceuticals 2020, 13, 234. https://doi.org/10.3390/ph13090234
Salvatore T, Pafundi PC, Galiero R, Gjeloshi K, Masini F, Acierno C, Di Martino A, Albanese G, Alfano M, Rinaldi L, et al. Metformin: A Potential Therapeutic Tool for Rheumatologists. Pharmaceuticals. 2020; 13(9):234. https://doi.org/10.3390/ph13090234
Chicago/Turabian StyleSalvatore, Teresa, Pia Clara Pafundi, Raffaele Galiero, Klodian Gjeloshi, Francesco Masini, Carlo Acierno, Anna Di Martino, Gaetana Albanese, Maria Alfano, Luca Rinaldi, and et al. 2020. "Metformin: A Potential Therapeutic Tool for Rheumatologists" Pharmaceuticals 13, no. 9: 234. https://doi.org/10.3390/ph13090234
APA StyleSalvatore, T., Pafundi, P. C., Galiero, R., Gjeloshi, K., Masini, F., Acierno, C., Di Martino, A., Albanese, G., Alfano, M., Rinaldi, L., & Sasso, F. C. (2020). Metformin: A Potential Therapeutic Tool for Rheumatologists. Pharmaceuticals, 13(9), 234. https://doi.org/10.3390/ph13090234





