Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative Phytochemical Analysis with Acute Toxicity Study
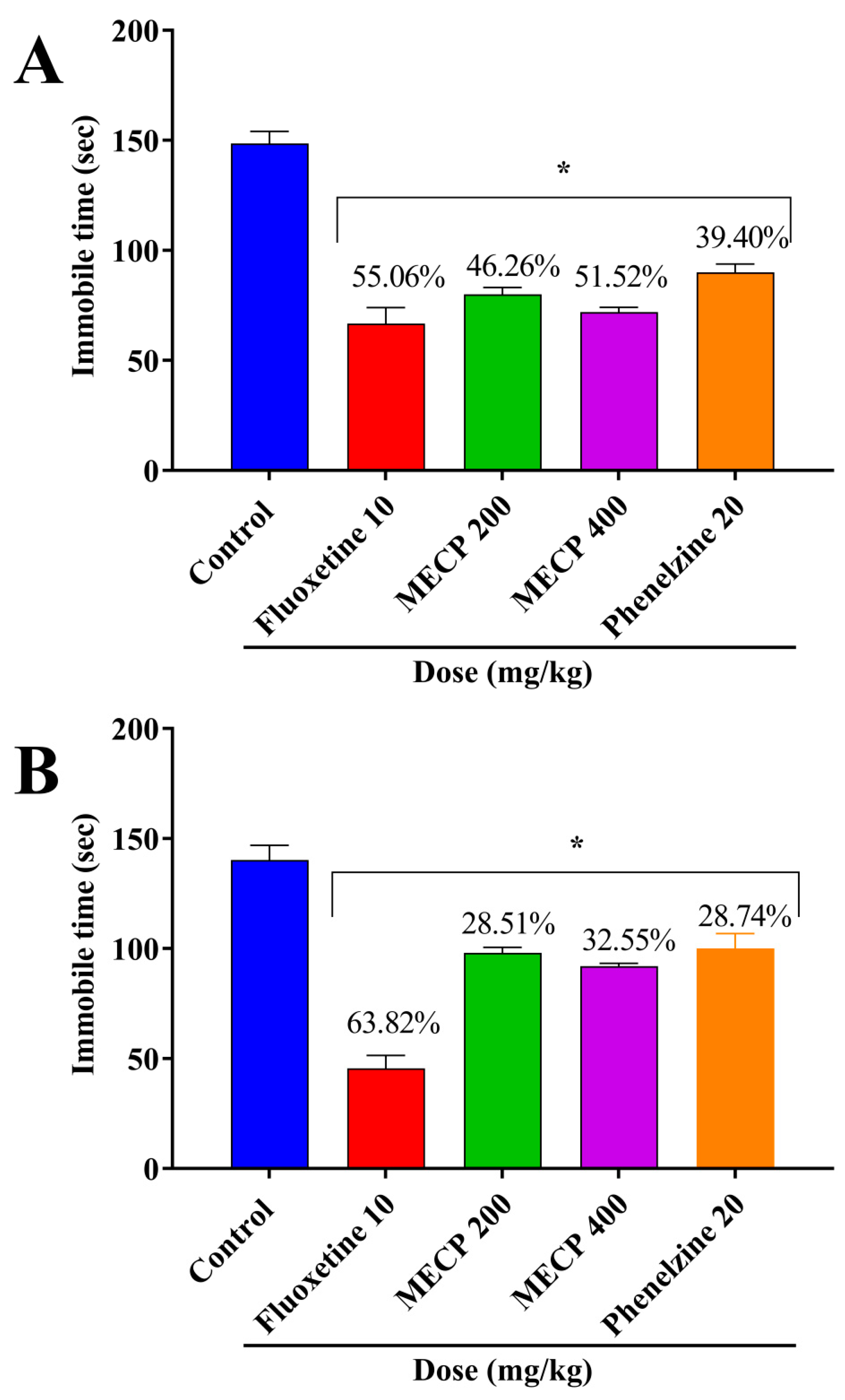
2.2. Antidepressant Activity
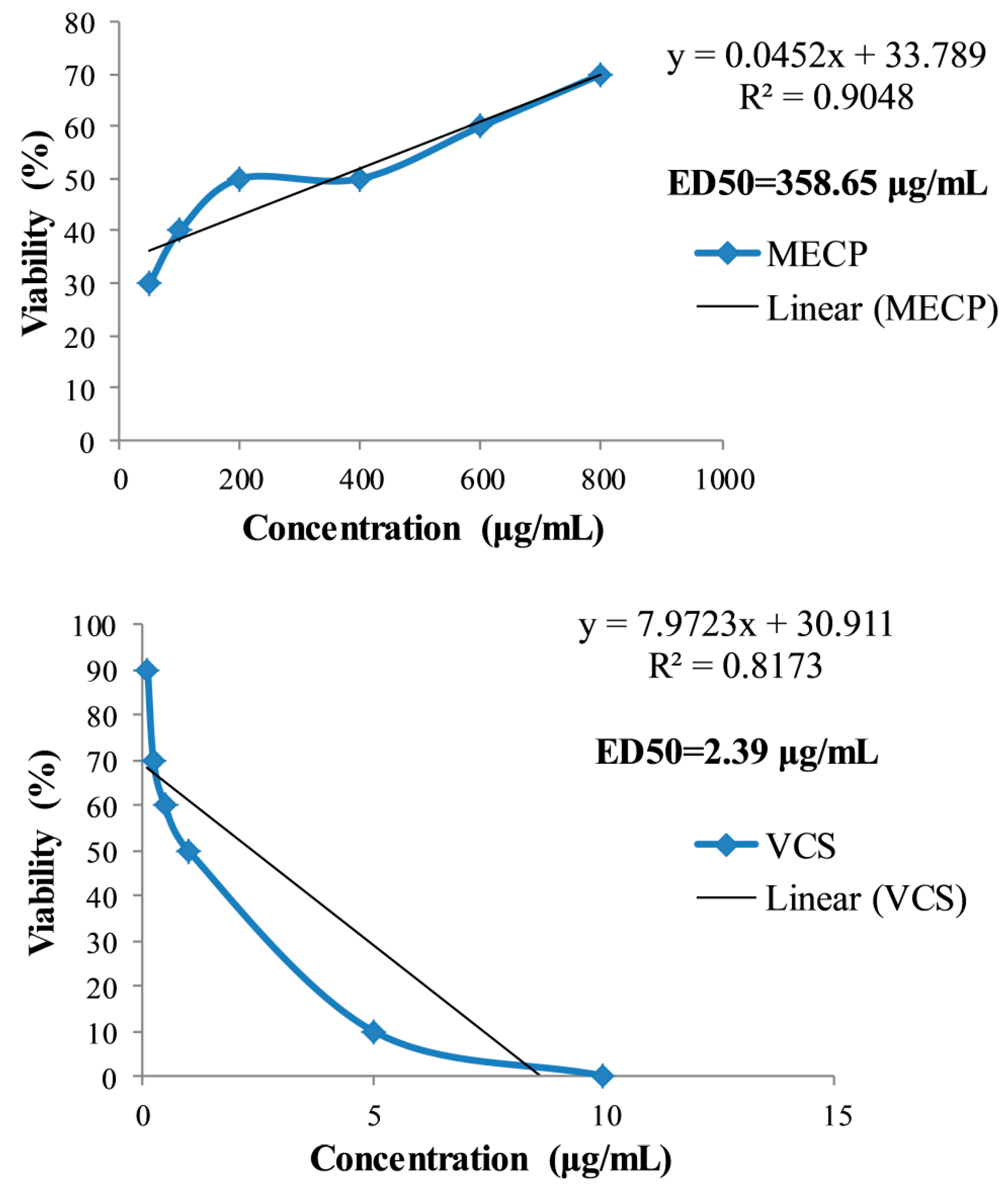
2.3. Cytotoxicity Activity
2.4. In Silico Study
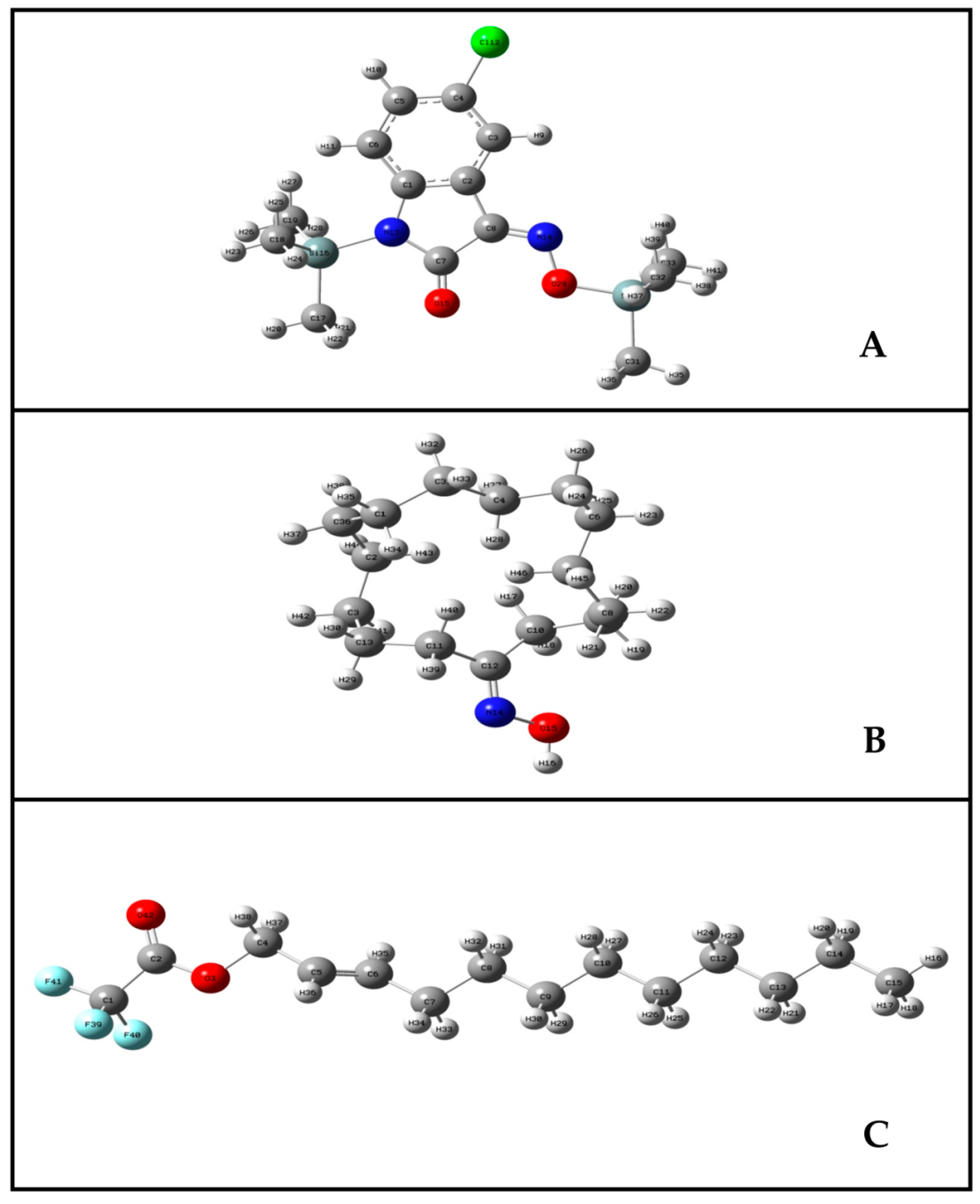
2.4.1. Molecular Geometry
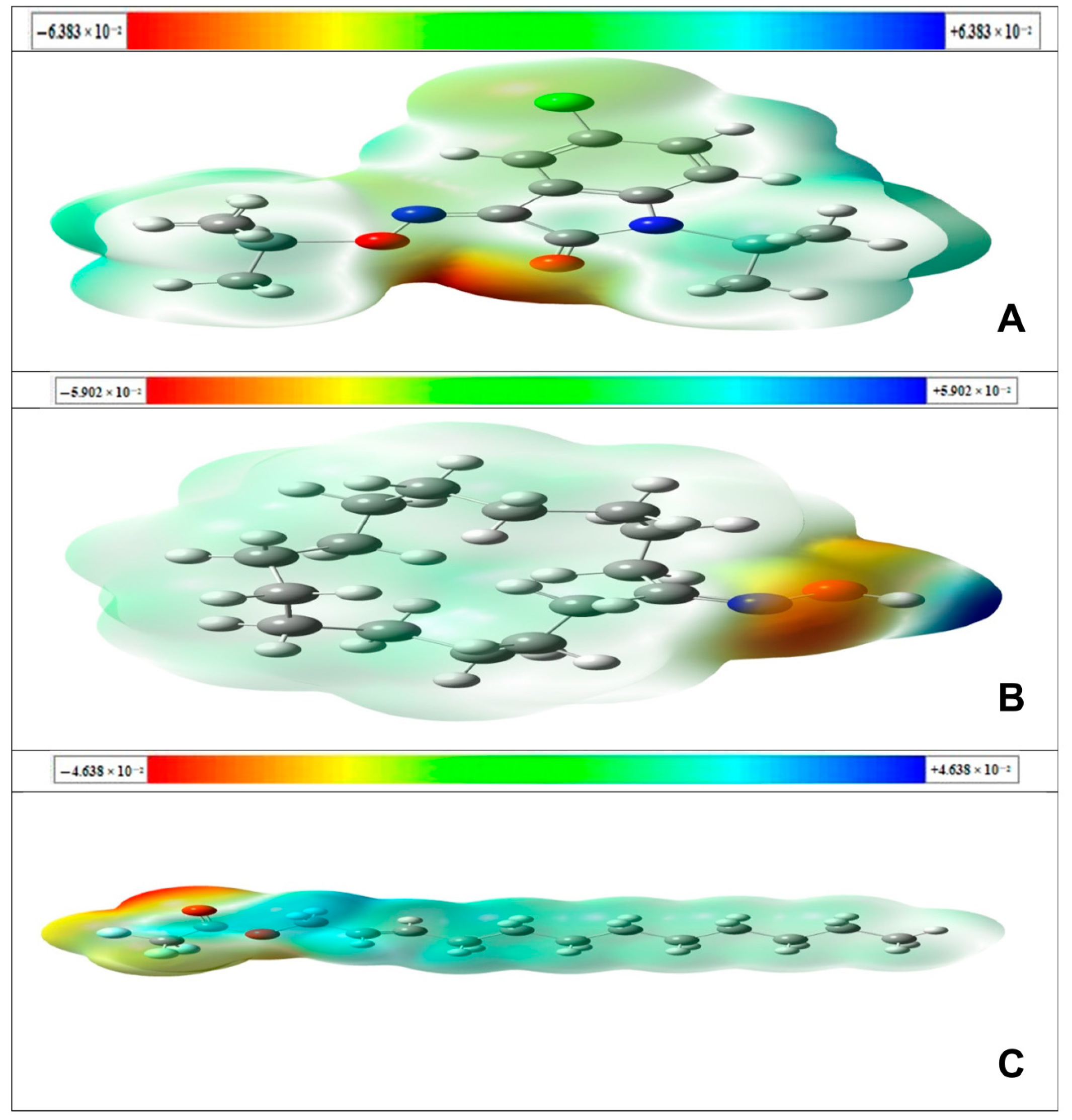
2.4.2. Charges and MESP Calculations
2.4.3. FMOs and Global Descriptors
2.4.4. Vibrational Spectral Analysis
2.4.5. Hydroxyl (O–H) Group Vibrations
2.4.6. C-H Vibrations
2.4.7. Methylene (H-C-H) group Vibrations
2.4.8. C-N Vibrations
2.4.9. C=C Vibrations
2.4.10. Carbonyl (C=O) Group Vibration
2.4.11. NMR Analysis
2.4.12. Molecular Docking Study
2.4.13. ADME/T and Toxicological Properties Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials and Preparation of Crude Extract
3.3. Experimental Animals
3.4. GC-MS (Gas Chromatography-Mass Spectroscopy) Analysis of MECP
3.5. Acute Toxicity Study
3.6. Phytochemical Screening
3.7. Antidepressant Activity
3.7.1. Experimental Design for Anti-Depressant Activity
- Group I: Negative control received 1% Tween-80 (10 mL/kg, b.w.) orally
- Group II: Positive control phenelzine received 20 mg/kg b.w. I.P.
- Group III: Positive control fluoxetine received 10 mg/kg b.w. I.P.
- Group IV: Received MECP 200 mg/kg b.w. orally
- Group V: Received MECP 400 mg/kg b.w. orally
3.7.2. Tail Suspension Test (TST)
3.7.3. Forced Swimming Test (FST)
3.8. Brine Shrimp Lethality Bioassay
3.9. In Silico Study
3.9.1. Quantum Chemical Analysis
3.9.2. Molecular Docking Study
3.9.3. ADME/T and Toxicological Properties Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MECP | methanol extract of C. pectinata leaves |
| GC-MS | Gas Chromatography-Mass Spectroscopy |
| IP | intraperitoneal |
| b.w. | body weight |
| MAO | monoamine oxidase |
| NMR | nuclear magnetic resonance |
| DFT | density functional theory |
| ADME/T | absorption, distribution, metabolism, excretion, and toxicity |
| PDB | protein data bank |
| SEM | standard error mean |
| ANOVA | one-way analysis of variance |
| FMOs | frontier molecular orbitals |
| HOMO | highest occupied molecular orbital |
| LUMO | lowest unoccupied molecular orbital |
| MESP | molecular electrostatic potential |
| NBO | natural bond orbital |
References
- Rosenbaum, D.; Hagen, K.; Deppermann, S.; Kroczek, A.M.; Haeussinger, F.B.; Heinzel, S.; Berg, D.; Fallgatter, A.J.; Metzger, F.G.; Ehlis, A.-C. State-dependent altered connectivity in late-life depression: A functional near-infrared spectroscopy study. Neurobiol. Aging 2016, 39, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Calvó-Perxas, L.; Vilalta-Franch, J.; Turró-Garriga, O.; López-Pousa, S.; Garre-Olmo, J. Gender differences in depression and pain: A two year follow-up study of the Survey of Health, Ageing and Retirement in Europe. J. Affect. Disord. 2016, 193, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gadassi, R.; Mor, N. Confusing acceptance and mere politeness: Depression and sensitivity to Duchenne smiles. J. Behav. Ther. Exp. Psychiatry 2016, 50, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2016, 191, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Perviz, S.; Khan, H.; Pervaiz, A. Plant Alkaloids as an Emerging Therapeutic Alternative for the Treatment of Depression. Front. Pharmacol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Gold, P.W.; Goodwin, F.K.; Chrousos, G.P. Clinical and Biochemical Manifestations of Depression. N. Engl. J. Med. 1988, 319, 413–420. [Google Scholar] [CrossRef]
- Tondo, L.; Isacsson, G.; Baldessarini, R.J. Suicidal behaviour in bipolar disorder: Risk and prevention. CNS Drugs 2003, 17, 491–511. [Google Scholar] [CrossRef]
- Alexander, R.C.; Preskorn, S. Clinical pharmacology in the development of new antidepressants: The challenges. Curr. Opin. Pharmacol. 2014, 14, 6–10. [Google Scholar] [CrossRef]
- García-Ríos, R.I.; Mora-Pérez, A.; Ramos-Molina, A.R.; Soria-Fregozo, C. Neuropharmacology of Secondary Metabolites from Plants with Anxiolytic and Antidepressant Properties. In Behavioral Pharmacology-From Basic to Clinical Research; IntechOpen: London, UK, 2020. [Google Scholar]
- Sørensen, M.; Neilson, E.H.J.; Møller, B.L. Oximes: Unrecognized Chameleons in General and Specialized Plant Metabolism. Mol. Plant 2018, 11, 95–117. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef]
- Wei, K.; Wang, G.-Q.; Bai, X.; Niu, Y.-F.; Chen, H.-P.; Wen, C.-N.; Li, Z.-H.; Dong, Z.-J.; Zuo, Z.-L.; Xiong, W.-Y.; et al. Structure-Based Optimization and Biological Evaluation of Pancreatic Lipase Inhibitors as Novel Potential Antiobesity Agents. Nat. Prod. Bioprospect. 2015, 5, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, A.; Dalkara, S.; Özalp, M.; Özbey, S.; Kendi, E.; Stables, J.P. Synthesis of some 1-(2-naphthyl)-2-(imidazole-1-yl) ethanone oxime and oxime ether derivatives and their anticonvulsant and antimicrobial activities. Eur. J. Med. Chem. 2001, 36, 421–433. [Google Scholar] [CrossRef]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Filippelli, W.; Falcone, G.; Rinaldi, B. O-[2-Hydroxy-3-(dialkylamino) propyl]ethers of (+)-1,7,7-trimethyl bicyclo[2.2.1]heptan-2-one oxime (camphor oxime) with analgesic and antiarrhythmic activities. IL Farmaco 2000, 55, 495–498. [Google Scholar] [CrossRef]
- Sivaraman, D.; Vignesh, G.; Selvaraj, R.; Dare, B.J. Identification of potential monoamine oxidase inhibitor from herbal source for the treatment of major depressive disorder: An in-silico screening approach. Der. Pharma. Chem. 2015, 7, 224–234. [Google Scholar]
- Rudorfer, M.V.; Potter, W.Z. Antidepressants. Drugs 1989, 37, 713–738. [Google Scholar] [CrossRef]
- Bozdağ, O.; Gümüşel, B.; Demirdamar, R.; Büyükbingöl, E.; Rolland, Y.; Ertan, R. Synthesis of some novel oxime ether derivatives and their activity in the ‘behavioral despair test’. Eur. J. Med. Chem. 1998, 33, 133–141. [Google Scholar] [CrossRef]
- Davrinche, C.; Nguyen-Tri-Xuong, E.; El Hamad, Y.; Reynaud, P.; Rinjard, P.; Tran, G. Amide-oximes et hydroximates benzodioxaniques: Synthèse de nouveaux composés et étude en neuropsycho-pharmacologie. Eur. J. Med. Chem. 1992, 27, 765–778. [Google Scholar] [CrossRef]
- Ertan, R.; BozdaĞ, O.Y.A.; KesİCİ, B.; Palaska, E.; Ertan, M. Studies on the synthesis and antidepressant activity of some new oxime-ether derivatives. Acta Pharm. Sci. 1998, 40, 131–135. [Google Scholar]
- Oh, J.M.; Rangarajan, T.M.; Chaudhary, R.; Singh, R.P.; Singh, M.; Singh, R.P.; Tondo, A.R.; Gambacorta, N.; Nicolotti, O.; Mathew, B. Novel Class of Chalcone Oxime Ethers as Potent Monoamine Oxidase-B and Acetylcholinesterase Inhibitors. Molecules 2020, 25, 2356. [Google Scholar] [CrossRef]
- Islam, M.; Rahman, M.; Hossain, G. Floristic composition and phytodiversity status of Sitakunda Ecopark, Chittagong, Bangladesh. Jahangirnagar Univ. J. Biol. Sci. 2016, 5, 29. [Google Scholar] [CrossRef]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Uddin, M.M.N.; Paul, A.; Chy, M.N.U.; Emran, T.B.; Seidel, V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytotherapy Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tareq, A.M.; Farhad, S.; Neshar Uddin, A.B.M.; Hoque, M.; Nasrin, M.S.; Uddin, M.M.R.; Hasan, M.; Sultana, A.; Munira, M.S.; Lyzu, C.; et al. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas Pectinata. Heliyon 2020, 6, e04061. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.; Hetta, M.; Zjawiony, J.K.; Jacob, M.R.; Hifnawy, M.; Marais, J.P.J.; Ferreira, D. Phytochemical investigation of Cycas circinalis and Cycas revoluta leaflets: Moderately active antibacterial biflavonoids. Planta Med. 2010, 76, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Isolation and quantification of the toxic methylazoxymethanol glycoside macrozamin in selected South African cycad species. S. Afr. J. Bot. 2012, 82, 108–112. [Google Scholar] [CrossRef]
- Negm, W.; Ibrahim, A.R.; Aboelsauod, K.; Ragab, A.; Attia, G.I. GC-MS Analysis of Petroleum Ether Extract and Volatiles of Cycas revoluta Thunb Growing in Egypt. Inventi Rapid Planta Act. 2016, 2016, 1–5. [Google Scholar]
- Kumar, S.B.; Kumar, V.J. GC-MS Analysis of Bioactive Constituents from Cycas circinalis L. and Ionidium suffruticosum Ging. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 197–201. [Google Scholar]
- Ben, I.O.; Woode, E.; Abotsi, W.K.M.; Boakye-Gyasi, E. Preliminary phytochemical screening and in vitro antioxidant prop-erties of Trichilia monadelpha (Thonn.) JJ De Wilde (Meliaceae). J. Med. Biomed. Sci. 2013, 2, 6–15. [Google Scholar]
- Bell, W.R. Evaluation of Thrombolytic Agents. Drugs 1997, 54, 11–17. [Google Scholar] [CrossRef]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Filippelli, W.; Falcone, G.; Rinaldi, B. Treating Depression and Anxiety in Primary Care. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 145–152. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, M.N.; Kamal, A.T.M.M.; Chowdhury, K.A.; Rahman, M.A.; Reza, A.S.M.A.; Moniruzzaman, M.; Rony, S.R.; Nasrin, M.S.; Azad, M.O.; et al. Intervention in Neuropsychiatric Disorders by Suppressing Inflammatory and Oxidative Stress Signal and Exploration of In Silico Studies for Potential Lead Compounds from Holigarna caustica (Dennst.) Oken leaves. Biomolecules 2020, 10, 561. [Google Scholar] [CrossRef]
- Benneh, C.K.; Biney, R.P.; Adongo, D.W.; Mante, P.K.; Ampadu, F.A.; Tandoh, A.; Jato, J.; Woode, E. Anxiolytic and Antidepressant Effects of Maerua angolensis DC. Stem Bark Extract in Mice. Depress. Res. Treat. 2018, 2018, 1537371. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, F.; Khazaei, M.; Hosseini, M. Neuropharmacological effects of Nigella sativa. Avicenna J. Phytomed. 2016, 6, 104–116. [Google Scholar] [PubMed]
- Baldwin, D.S.; Polkinghorn, C. Evidence-based pharmacotherapy of Generalized Anxiety Disorder. Int. J. Neuropsychopharmacol. 2005, 8, 293–302. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- El Refaey, H. Fluoxetine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–9. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002, 4, 7–20. [Google Scholar]
- Abdulrasheed, M.; Ibrahim, I.H.; Mubarak, M.A.; Umar, F.A. Comparison of antimicrobial activity of seed oil of garlic and Moringa oleifera against some food-borne microorganisms. Bayero J. Pure Appl. Sci. 2015, 8, 196–201. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.J.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Parvez, M.; Mosaddik, A. Evaluation of Brine Shrimp Cytotoxicity of Mango Peel and Flesh after Formalin Treatment. Int. J. Innov. Pharm. Sci. Res. 2016, 4, 900–908. [Google Scholar] [CrossRef]
- Soga, S.; Sharma, S.V.; Shiotsu, Y.; Shimizu, M.; Tahara, H.; Yamaguchi, K.; Ikuina, Y.; Murakata, C.; Tamaoki, T.; Kurebayashi, J.; et al. Stereospecific antitumor activity of radicicol oxime derivatives. Cancer Chemoth. Pharm. 2001, 48, 435–445. [Google Scholar] [CrossRef]
- Minkin, V.I.; Osipov, O.A.; Zhdanov, Y.A. Dipole Moments in Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Baldin, A.M. Polarizability of nucleons. Nucl. Phys. 1960, 18, 310–317. [Google Scholar] [CrossRef]
- Gómez-Jeria, J.S. An empirical way to correct some drawbacks of mulliken population analysis. J. Chil. Chem. Soc. 2009, 54, 482–485. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Sheikhi, M.; Sheikh, D. Quantum chemical investigations on phenyl-7, 8-dihydro-[1,3]-dioxolo [4,5-g] quinolin-6 (5h)-one. Rev. Roum. Chim. 2014, 59, 761–767. [Google Scholar]
- Choi, C.H.; Kertesz, M. Bond length alternation and aromaticity in large annulenes. J. Chem. Phys. 1998, 108, 6681–6688. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Koopmans, T. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Phillips, J.C. Generalized Koopmans’ Theorem. Phys. Rev. 1961, 123, 420–424. [Google Scholar] [CrossRef]
- Flippin, L.A.; Gallagher, D.W.; Jalali-Araghi, K. A convenient method for the reduction of ozonides to alcohols with borane-dimethyl sulfide complex. J. Org. Chem. 1989, 54, 1430–1432. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S. Stability, Reactivity, and Aromaticity of Compounds of a Multivalent Superatom. J. Phys. Chem. A 2007, 111, 11116–11121. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Parthasarathi, R.; Subramanian, V.; Chattaraj, P.K. Electrophilicity-Based Charge Transfer Descriptor. J. Phys. Chem. A 2007, 111, 1358–1361. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G. Variational Principles for Describing Chemical Reactions: The Fukui Function and Chemical Hardness Revisited. J. Am. Chem. Soc. 2000, 122, 2010–2018. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B.; El-Khaiary, M.I.; Umoren, S.A.; Ebenso, E.E. Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some phenanthroline derivatives on mild steel surface. Int. J. Electrochem. Sci. 2011, 6, e5675. [Google Scholar]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Pulay, P.; Fogarasi, G.; Pang, F.; Boggs, J.E. Systematic ab initio gradient calculation of molecular geometries, force constants, and dipole moment derivatives. J. Am. Chem. Soc. 1979, 101, 2550–2560. [Google Scholar] [CrossRef]
- Rauhut, G.; Pulay, P. Transferable Scaling Factors for Density Functional Derived Vibrational Force Fields. J. Phys. Chem. 1995, 99, 3093–3100. [Google Scholar] [CrossRef]
- Rajesh, P.; Gunasekaran, S.; Gnanasambandan, T.; Seshadri, S. Experimental and theoretical study of ornidazole. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 496–504. [Google Scholar] [CrossRef]
- Lampert, H.; Mikenda, W.; Karpfen, A. Molecular Geometries and Vibrational Spectra of Phenol, Benzaldehyde, and Salicylaldehyde: Experimental versus Quantum Chemical Data. J. Phys. Chem. A 1997, 101, 2254–2263. [Google Scholar] [CrossRef]
- Puviarasan, N.; Arjunan, V.; Mohan, S. FT-IR and FT-Raman studies on 3-aminophthalhydrazide and N-aminophthalimide. Turk. J. Chem. 2002, 26, 323–334. [Google Scholar]
- Govindarajan, M.; Ganasan, K.; Periandy, S.; Karabacak, M. Experimental (FT-IR and FT-Raman), electronic structure and DFT studies on 1-methoxynaphthalene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 646–653. [Google Scholar] [CrossRef]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Roeges, N.P.G.; Baas, J.M.A. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Laszlo, V.; Endre, K.; Jozsef, B.; Boris, D.; Antal, S. Diethylamino and Pyrrolidino Lower Alkyl Esters of 3,5-dimethoxy-4-butoxy and Amyloxy Benzoic Acids. US3228961A, 11 January 1966. [Google Scholar]
- Silverstein, R.; Bassler, G.; Morrill, T. Spectrometric Identification of Organic Compounds; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Krishnakumar, V.; Manohar, S.; Nagalakshmi, R. Crystal growth and characterization of N-hydroxyphthalimide (C8H5NO3) crystal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 71, 110–115. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar] [CrossRef]
- Abbas, A.; Gökce, H.; Bahçeli, S. Spectroscopic (vibrational, NMR and UV–vis.) and quantum chemical investigations on 4-hexyloxy-3-methoxybenzaldehyde. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Roy, S.; Ashraf, J.M.; Adil, M.; Siddiqui, M.H.; Khan, S.; Kamal, M.A.; Provazník, I.; Choi, I. Computer Aided Drug Design: Success and Limitations. Curr. Pharm. Des. 2016, 22, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sahakian, D.C.; de Morais, S.M.; Xu, J.J.; Polzer, R.J.; Winter, S.M. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 2003, 3, 1125–1154. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Mielke, H.; Strickland, J.; Jacobs, M.N.; Mehta, J.M. Biometrical evaluation of the performance of the revised OECD Test Guideline 402 for assessing acute dermal toxicity. Regul. Toxicol. Pharmacol. 2017, 89, 26–39. [Google Scholar] [CrossRef]
- Hossain, M.S.; Reza, A.; Rahaman, M.M.; Nasrin, M.S.; Rahat, M.R.U.; Islam, M.R.; Uddin, M.J.; Rahman, M.A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 291–299. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans Pharmacognosy, International Edition E-Book; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09; Revision d. 01; Gaussian Inc.: Wallingford CT, USA, 2009; p. 201. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee, KS, USA, 2016; p. 16. [Google Scholar]
- Fekete, Z.A.; Hoffmann, E.A.; Körtvélyesi, T.; Penke, B. Harmonic vibrational frequency scaling factors for the new NDDO Hamiltonians: RM1 and PM6. Mol. Phys. 2007, 105, 2597–2605. [Google Scholar] [CrossRef]
- Ramazani, A.; Sheikhi, M.; Yahyaei, H. Molecular Structure, NMR, FMO, MEP and NBO Analysis of Ethyl-(Z)-3-phenyl-2-(5-phenyl-2H-1,2,3,4-tetraazol-2-yl)-2-propenoate Based on HF and DFT Calculations. Chem. Methodol. 2017, 1, 28–48. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, M.N.; Kamal, A.T.M.M.; Chowdhury, M.R.; Islam, M.S.; Hossain, M.A.; Tareq, A.M.; Bhuiyan, M.I.; Uddin, M.N.; Tahamina, A.; et al. Unveiling Pharmacological Responses and Potential Targets Insights of Identified Bioactive Constituents of Cuscuta reflexa Roxb. Leaves through In Vivo and In Silico Approaches. Pharmaceuticals 2020, 13, 50. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular docking studies of (4Z,12Z)-cyclopentadeca-4, 12-dienone from Grewia hirsuta with some targets related to type 2 diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef]

| Sl. No. | RT | Compound Name | m/z | Area | PA (%) | Molecular Formula | MW (g/mol) | Class |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.881 | 1H-Indole-2,3-dione, 5-chloro-1-(trimethylsilyl)-, 3-[O-(trimethylsilyl)oxime] | 73.00 | 851549 | 3.80 | C14H21ClN2O2Si2 | 340.95 | Oxime |
| 2 | 11.640 | 3-Octyn-2-ol | 44.00 | 25927 | 0.12 | C8H14O | 126.2 | Fatty alcohol |
| 3 | 11.640 | 2-Cyclohexen-1-one, 3-(3-hydroxybutyl)-2,4,4-trimethyl- | 44.00 | 25927 | 0.12 | C13H22O2 | 210.31 | Ketone |
| 4 | 11.640 | Bioallethrin | 44.00 | 25927 | 0.12 | C19H26O3 | 302.4 | Pyrethroid |
| 5 | 11.640 | 3-Nonyn-2-ol | 44.00 | 25927 | 0.12 | C9H16O | 140.22 | Secondary alcohol |
| 6 | 12.516 | 1-Octadecyne | 43.00 | 209536 | 0.94 | C18H34 | 250.5 | Hydrocarbon |
| 7 | 12.516 | Z-2-Dodecenol | 43.00 | 209536 | 0.94 | C12H24O | 184.32 | Fatty alcohol |
| 8 | 12.516 | Phytol, acetate | 43.00 | 209536 | 0.94 | C22H42O2 | 338.6 | Diterpene |
| 9 | 12.515 | 5-Nonadecen-1-ol | 81.00 | 122608 | 0.55 | C19H38O | 282.5 | Alcohols |
| 10 | 12.515 | 2-Tridecyne | 81.00 | 122608 | 0.55 | C13H24 | 180.33 | Alkyne |
| 11 | 12.516 | 9-Eicosyne | 43.00 | 187141 | 0.84 | C20H38 | 278.5 | Alkyne |
| 12 | 12.516 | Dodecanal | 43.00 | 187141 | 0.84 | CH3(CH2)10CHO | 184.32 | Aldehyde |
| 13 | 12.516 | trans-2-Dodecen-1-ol, trifluoroacetate | 43.00 | 187141 | 0.84 | C14H23F3O2 | 280.33 | Ester |
| 14 | 13.450 | Tridecanoic acid, 12-methyl-, methyl ester | 74.00 | 417474 | 1.86 | C15H30O2 | 242.4 | Fatty acid |
| 15 | 13.450 | Eicosanoic acid, methyl ester | 74.00 | 417474 | 1.86 | C21H42O2 | 326.6 | FAME |
| 16 | 13.450 | Octadecanoic acid, 17-methyl-, methyl ester | 74.00 | 417474 | 1.86 | C20H40O2 | 312.5 | FAME |
| 17 | 15.170 | 13-Tetradece-11-yn-1-ol | 67.00 | 47905 | 0.21 | C14H24O | 208.34 | Alcohol |
| 18 | 15.170 | 9,12-Octadecadienoic acid, methyl ester, (E,E)- | 67.00 | 47905 | 0.21 | C19H34O2 | 294.5 | FAME |
| 19 | 15.339 | Cyclopropaneoctanoic acid, 2-[[2-[(2-ethyl- cyclopropyl)methyl]cyclopropyl]methyl]-, methyl ester | 55.00 | 70317 | 0.31 | C22H38O2 | 334.5 | Fatty acid |
| 20 | 15.339 | 3-Tetradecyn-1-ol | 55.00 | 70317 | 0.31 | C14H26O | 210.36 | Alkyne |
| 21 | 15.339 | 7-Hexadecenoic acid, methyl ester, (Z)- | 55.00 | 70317 | 0.31 | C17H32O2 | 268.4 | Fatty acid |
| 22 | 15.339 | Ethyl iso-allocholate | 55.00 | 70317 | 0.31 | C26H44O5 | 436.6 | Steroid |
| 23 | 15.337 | Isophytol, acetate | 71.00 | 172950 | 0.77 | C22H42O2 | 338.6 | Diterpene |
| 24 | 15.337 | E-2-Tetradecen-1-ol | 71.00 | 172950 | 0.77 | C14H28O | 212.37 | Alkyne |
| 25 | 15.478 | Tetradecanoic acid, 12-methyl-, methyl ester, (S)- | 74.00 | 107333 | 0.96 | C16H32O2 | 316.5 | FAME |
| 26 | 15.478 | Heptacosanoic acid, methyl ester | 74.00 | 107333 | 0.48 | C28H56O2 | 424.7 | Fatty acid |
| 27 | 15.478 | Cyclopentanetridecanoic acid, methyl ester | 74.00 | 107333 | 0.48 | C19H36O2 | 296.5 | Fatty acid |
| 28 | 16.199 | Dodecanoic acid, 2-(acetyloxy)-1-[(acetyloxy)methyl]ethyl ester | 73.00 | 111054 | 0.49 | C19H34O6 | 338.5 | Ester |
| 29 | 16.199 and 5.881 | Phloroglucitol | 73.00 | 111054 and 851549 | 0.49 and 3.80 | C6H12O3 | 132.16 | Alcohol |
| 30 | 16.604 | Octadecanal, 2-bromo- | 44.00 | 11355 | 0.05 | C18H35BrO | 347.4 | Aldehyde |
| 31 | 16.604 and 12.515 | Undecanal | 81.00 and 44.00 | 122608 and 11355 | 0.05 and 0.55 | C10H21CHO | 170.29 | Aldehyde |
| 32 | 17.623 | Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- | 73.00 | 77448 | 0.35 | C16H50O7Si8 | 577.2 | Volatile organic compound |
| 33 | 17.623 | Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | 73.00 | 77448 | 0.35 | C19H34O6 | 358.5 | Ester |
| 34 | 19.440 | D-Mannitol, 1-O-(16-hydroxyhexadecyl)- | 73.00 | 77215 | 0.34 | C22H46O7 | 422.6 | Alcohol |
| 35 | 19.440 | Cyclopentadecanone, oxime | 73.00 | 77215 | 0.34 | C15H29NO | 239.4 | Oxime |
| 36 | 19.440 | Docosanoic acid, docosyl ester | 73.00 | 77215 | 0.34 | C44H88O2 | 649.2 | Emollient |
| 37 | 20.009 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 44.00 | 49605 | 0.22 | C19H38O4 | 330.5 | Fatty acid glycerol ester |
| 38 | 20.009 | Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester | 44.00 | 49605 | 0.22 | C39H76O5 | 625.0 | Monoalkyl ester |
| 39 | 20.009 | Glycerol 1-palmitate | 44.00 | 49605 | 0.22 | C19H38O4 | 330.5 | Fatty acid |
| 40 | 20.360 | Chloroacetic acid, 4-pentadecyl ester | 44.00 | 24894 | 0.11 | C17H33ClO2 | 304.9 | Ester |
| 41 | 20.360 | 2-Decen-1-ol, (E)- | 44.00 | 24894 | 0.11 | C10H20O | 156.26 | Fatty acid |
| Compounds | Energy (a.u) | Dipole Moment (Debye) | Polarizability (a.u) |
|---|---|---|---|
| 5-Chloro-1-(trimethylsilyl)-1H-indole-2,3-dione 3-[O-(trimethylsilyl)oxime] | −1845.68068 | 1.367 | 261.403 |
| Cyclopentadecanone oxime | −718.77081 | 0.712 | 162.046 |
| trans-2-Dodecen-1-ol trifluoroacetate | −997.04879 | 4.968 | 159.682 |
| Global Reactivity Descriptors | 5-Chloro-1-(trimethylsilyl)-1H-indole-2,3-dione 3-[O-(trimethyl-silyl)oxime] | Cyclopentadecanone Oxime | trans-2-Dodecen-1-ol Trifluoroacetate |
|---|---|---|---|
| Ionisation potential (I) eV | 6.17671 | 6.59740 | 7.39524 |
| Electron affinity (A) eV | 2.40902 | 0.27320 | 1.55567 |
| Chemical hardness (η) | 1.88385 | 3.16210 | 2.91979 |
| Softness (S) | 0.53083 | 0.31625 | 0.34249 |
| Chemical potential (μ) | −4.29287 | −3.43530 | −4.47546 |
| Electronegativity (χ) | 4.29287 | 3.43530 | 4.47546 |
| Electrophilicity index (ώ) | 9.21434 | 5.90064 | 10.01485 |
| MD | 5-Chloro-1-(trimethyl-silyl)-1H-indole-2,3-dione 3-[O-(trimethylsilyl)oxime] | MD | Cyclopentadecanone Oxime | MD | trans-2-Dodecen-1-ol Trifluoroacetate |
|---|---|---|---|---|---|
| - | - | υ(O15-H16) | 3884 | - | - |
| υ(C- H) | 3081~3099 | υ(C- H) | 3100~3200 | υ(C- H) | 3023~3069 |
| υAsy(H-C-H) | 2927~3017 | υAsy(H-C-H) | 3051~3093 | υAsy(H-C-H) | 2920~3001 |
| υSy(H-C-H) | 2916~2919 | υSy(H-C-H) | 3007~3019 | υSy(H-C-H) | 2805~2917 |
| - | - | δS(H-C-H) | 3023 ~ 3037 | - | - |
| υ(C=C)Aro | 1555~1579 | - | - | υ(C=C) | 1680~1689 |
| υ(C4=Cl12) | 695 | - | - | υ(C-C) | 1283 |
| - | - | δS(H-C-H) | 1459~1499 | δS(H-C-H) | 1407~1506 |
| - | - | δW(H-C-H) | 1346~1373 | δW(H-C-H) | 1324~1373 |
| - | - | δT(H-C-H) | 1246~1287 | δT(H-C-H) | 1287~1312 |
| δW(C-H)Aro | 916 | - | - | δ(C-H) | 989 |
| δ(C-H)Aro | 1137 | - | - | - | - |
| υ(C7=O15) | 1717 | - | - | - | - |
| υ(C18=N14) | 1593 | υ(C12=N14) | 1759 | - | - |
| υ(O29-N14) | 1035/979 | υ(O15-N14) | 891 | - | - |
| - | - | δS(C12-N14-O15) | 524 | - | - |
| - | - | - | - | υ(C-F) | 1123~1169 |
| Compound (Chemical Shift-ppm) | |||||
|---|---|---|---|---|---|
| Proton No. | 5-Chloro-1-(trimethylsilyl)- 1H-indole-2,3-dione 3-[O- (trimethylsilyl)oxime] | Proton No. | Cyclopentadecanone Oxime | Proton No. | trans-2-Dodecen-1-ol, Trifluoroacetate |
| 9-H | 6.364 | 16-H | 4.643 | 35-H | 5.567 |
| 10-H | 6.129 | 18-H | 2.074 | 36-H | 5.183 |
| 11-H | 5.943 | 40-H | 1.809 | 38-H | 4.266 |
| 22-H | 0.097 | 17-H | 1.221 | 37-H | 3.952 |
| 21-H | 0.078 | 39-H | 1.126 | 33-H | 1.459 |
| 27-H | −0.276 | 28-H | 1.119 | 34-H | 1.319 |
| 25-H | −0.281 | 19-H | 1.068 | 31-H | 0.699 |
| 39-H | −0.364 | 43-H | 0.971 | 30-H | 0.645 |
| 34-H | −0.387 | 46-H | 0.736 | 19-H | 0.611 |
| 40-H | −0.412 | 30-H | 0.705 | 20-H | 0.607 |
| 36-H | −0.420 | 24-H | 0.673 | 28-H | 0.600 |
| 24-H | −0.621 | 27-H | 0.637 | 24-H | 0.596 |
| 28-H | −0.630 | 20-H | 0.587 | 26-H | 0.594 |
| 42-H | −0.743 | 25-H | 0.574 | 23-H | 0.593 |
| 37-H | −0.759 | 34-H | 0.570 | 27-H | 0.592 |
| 23-H | −0.848 | 29-H | 0.524 | 22-H | 0.582 |
| 26-H | −0.851 | 37-H | 0.520 | 29-H | 0.579 |
| 35-H | −0.904 | 41-H | 0.508 | 21-H | 0.579 |
| 20-H | −0.918 | 33-H | 0.505 | 25-H | 0.579 |
| 41-H | −0.948 | 38-H | 0.505 | 32-H | 0.503 |
| 38-H | −0.956 | 21-H | 0.478 | 16-H | 0.245 |
| 35-H | 0.395 | 18-H | 0.131 | ||
| 22-H | 0.354 | 17-H | 0.124 | ||
| 44-H | 0.316 | ||||
| 42-H | 0.298 | ||||
| 23-H | 0.256 | ||||
| 32-H | 0.252 | ||||
| 45-H | 0.248 | ||||
| 26-H | 0.181 | ||||
| Compound (Chemical Shift-ppm) | |||||
|---|---|---|---|---|---|
| Carbon No. | 5-Chloro-1-(trimethylsilyl)-1H-indole-2,3-dione 3-[O-(trimethylsilyl)oxime] | Carbon No. | Cyclopentadecanone Oxime | Carbon No. | trans-2-Dodecen-1-ol Trifluoroacetate |
| 7-C | 142.311 | 12-C | 146.455 | 2-C | 151.741 |
| 8-C | 133.869 | 11-C | 21.760 | 6-C | 129.323 |
| 1-C | 128.589 | 10-C | 16.876 | 1-C | 121.591 |
| 4-C | 120.975 | 8-C | 15.603 | 5-C | 105.659 |
| 5-C | 113.284 | 36-C | 15.499 | 4-C | 63.818 |
| 2-C | 108.036 | 1-C | 15.387 | 7-C | 24.997 |
| 3-C | 103.793 | 13-C | 15.320 | 13-C | 23.385 |
| 6-C | 96.637 | 3-C | 14.511 | 12-C | 22.139 |
| 17-C | −12.527 | 4-C | 14.094 | 11-C | 22.046 |
| 33-C | −13.724 | 31-C | 13.771 | 10-C | 21.930 |
| 19-C | −13.895 | 5-C | 13.444 | 9-C | 21.861 |
| 18-C | −13.914 | 6-C | 13.024 | 8-C | 21.102 |
| 32-C | −13.918 | 7-C | 12.407 | 14-C | 14.600 |
| 31-C | −14.110 | 2-C | 11.635 | 15-C | 3.962 |
| 9-C | 11.045 | ||||
| Compounds | Docking Score (kcal/mol) | |||
|---|---|---|---|---|
| 2Z5X | 5I6X | 1ERR | 1M17 | |
| 5-Chloro-1-(trimethylsilyl)-1H-indole-2,3-dione 3-[O-(trimethyl- silyl)oxime] | – | – | – | – |
| Cyclopentadecanone oxime | −4.333 | −6.537 | −7.685 | −4.59 |
| trans-2-Dodecen-1-ol trifluoroacetate | −3.155 | −2.387 | −1.857 | −2.674 |
| Standard drugs (Phenelzine/Fluoxetine/Vincristine sulfate) | −5.324 | −9.07 | −3.896 | −3.85 |
| Compounds | Lipinski Rules | Lipinski Violations | Veber Rules | ||||
|---|---|---|---|---|---|---|---|
| MW | HBA | HBD | Log P | nRB | TPSA | ||
| 5-Chloro-1-(trimethylsilyl)-1H-indole-2,3-dione 3-[O-(trimethylsilyl)oxime] | 340.95 | 3 | 0 | 2.38 | 0 | 3 | 41.90 |
| Cyclopentadecanone oxime | 239.40 | 2 | 1 | 3.55 | 0 | 0 | 32.59 |
| trans-2-Dodecen-1-ol trifluoroacetate | 280.33 | 5 | 0 | 3.96 | 0 | 12 | 26.30 |
| Parameters | Compounds | ||
|---|---|---|---|
| 5-Chloro-1-(trimethylsilyl)-1H-Indole- 2,3-dione 3-[O-(trimethylsilyl)oxime] | Cyclopentadecanone Oxime | trans-2-Dodecen-1-ol Trifluoroacetate | |
| Ames toxicity | NAT | AT | NAT |
| Carcinogens | NC | NC | C |
| Acute oral toxicity | III | III | III |
| Rat acute toxicity | 2.6849 | 2.1203 | 2.6831 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, J.; Tareq, A.M.; Hossain, M.M.; Sakib, S.A.; Islam, M.N.; Ali, M.H.; Uddin, A.B.M.N.; Hoque, M.; Nasrin, M.S.; Emran, T.B.; et al. Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals 2020, 13, 232. https://doi.org/10.3390/ph13090232
Rahman J, Tareq AM, Hossain MM, Sakib SA, Islam MN, Ali MH, Uddin ABMN, Hoque M, Nasrin MS, Emran TB, et al. Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals. 2020; 13(9):232. https://doi.org/10.3390/ph13090232
Chicago/Turabian StyleRahman, Jinnat, Abu Montakim Tareq, Md. Mohotasin Hossain, Shahenur Alam Sakib, Mohammad Nazmul Islam, Md. Hazrat Ali, A. B. M. Neshar Uddin, Muminul Hoque, Mst. Samima Nasrin, Talha Bin Emran, and et al. 2020. "Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds" Pharmaceuticals 13, no. 9: 232. https://doi.org/10.3390/ph13090232
APA StyleRahman, J., Tareq, A. M., Hossain, M. M., Sakib, S. A., Islam, M. N., Ali, M. H., Uddin, A. B. M. N., Hoque, M., Nasrin, M. S., Emran, T. B., Capasso, R., Reza, A. S. M. A., & Simal-Gandara, J. (2020). Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals, 13(9), 232. https://doi.org/10.3390/ph13090232















