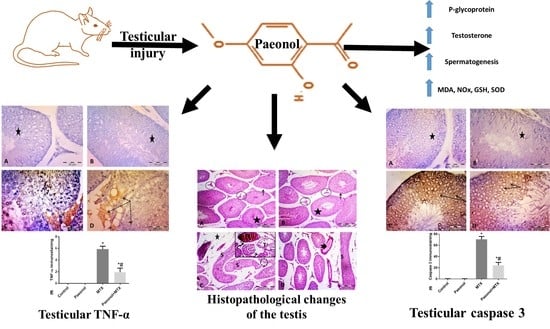
The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats
Abstract
1. Introduction
2. Results
2.1. Effect of Paeonol on Testicular Weight and Serum Testosterone Level
2.2. Effect of Paeonol on Testicular P-gp Level
2.3. Effect of Paeonol on Histopathological Changes and Spermatogenesis Scoring
2.4. Effect of Paeonol on Oxidative Stress Parameters
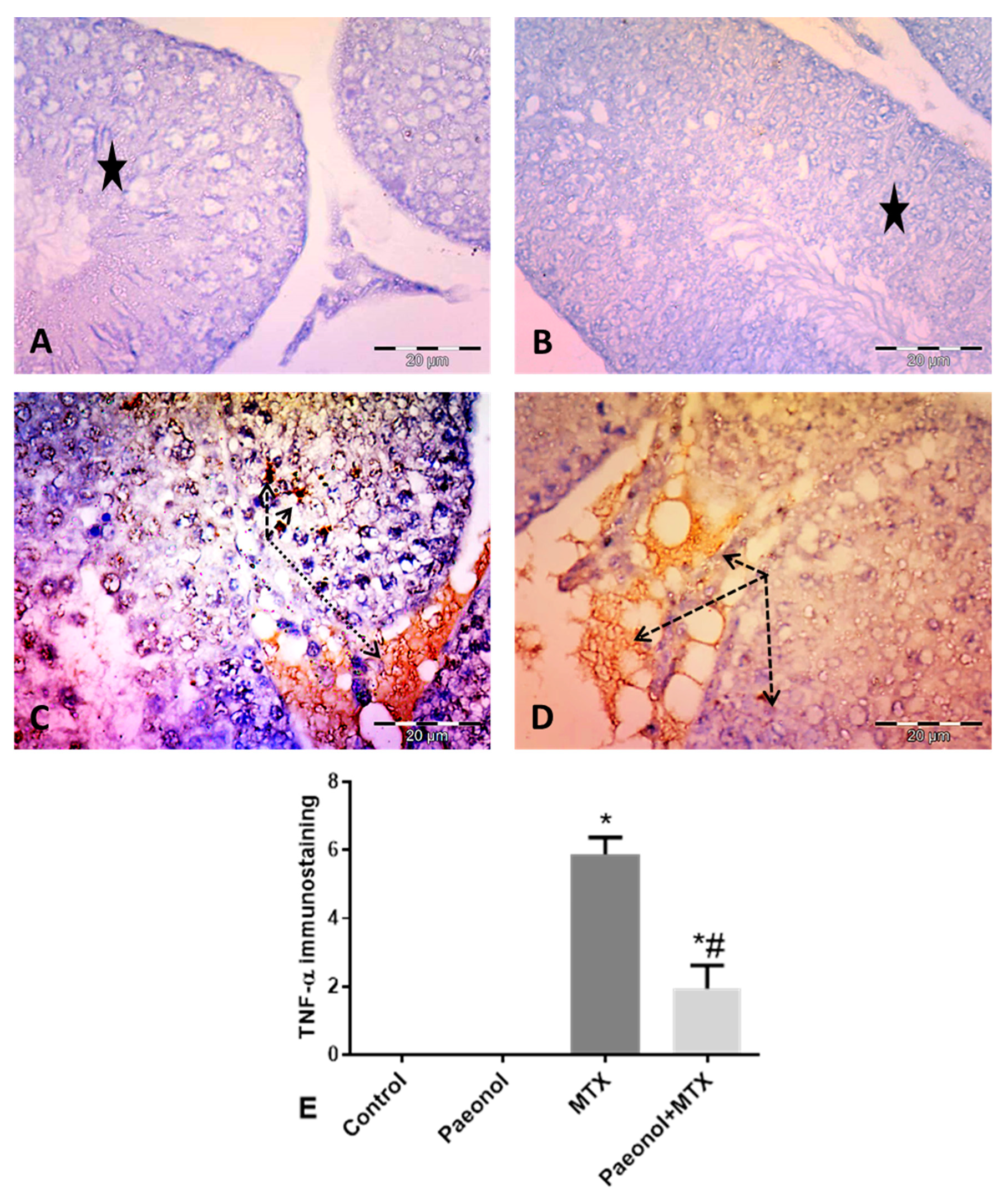
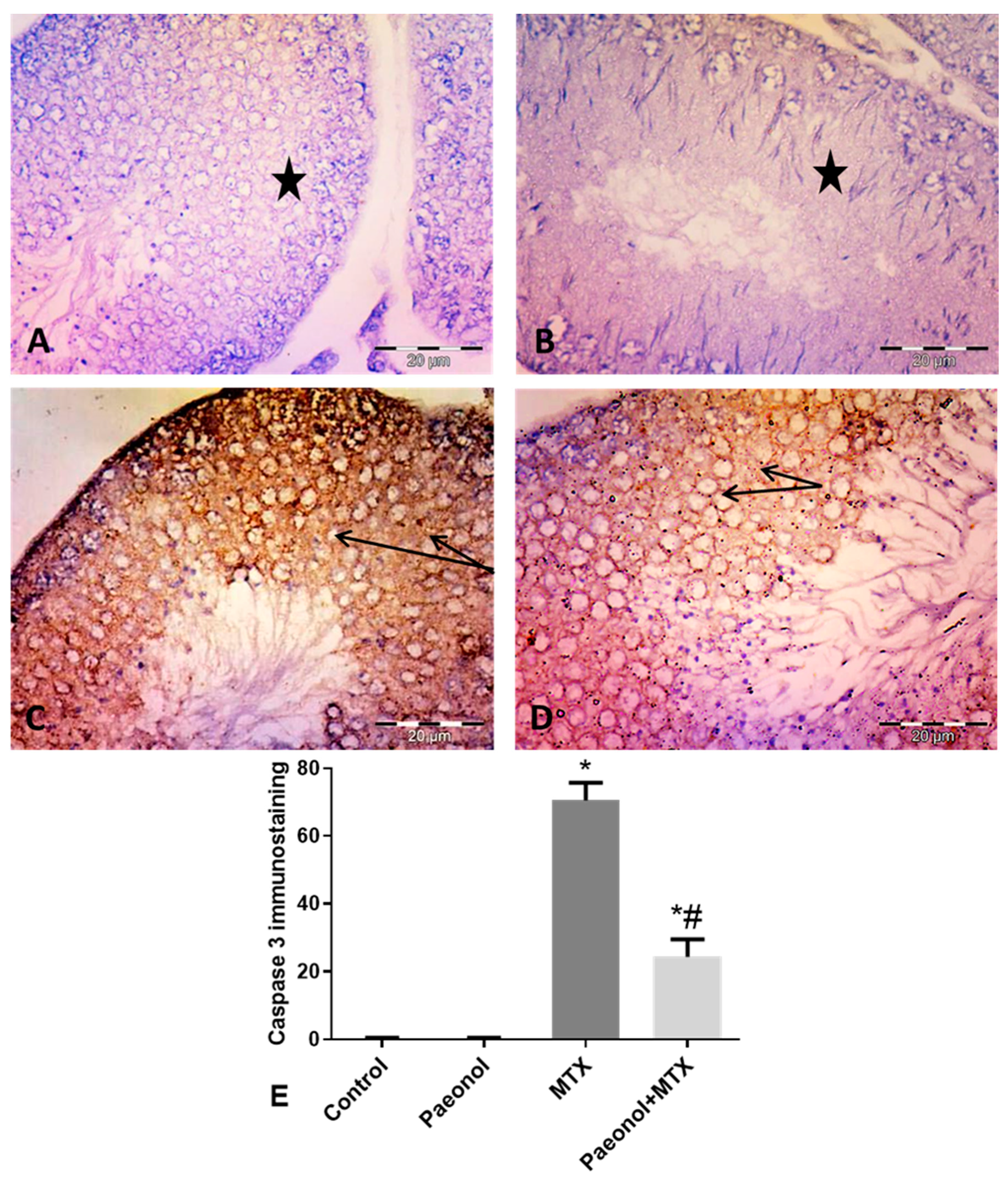
2.5. Effect of Paeonol on Tumor Necrosis Factor-Alpha (TNF-α) and Caspase 3 Immunostaining
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs, Chemicals, and Antibodies
4.3. Experimental Design
4.4. Biochemical Analysis
4.4.1. Determination of Serum Testosterone Level
4.4.2. Determination of Testicular P-gp Level
4.4.3. Determination of Testicular Oxidative Stress Biomarkers
4.5. Histopathological and Immunohistochemical Examinations
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Sramek, M.; Neradil, J.; Veselska, R. Much more than you expected: The non-DHFR-mediated effects of methotrexate. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 499–503. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of Thymoquinone Hepatorenal Protection in Methotrexate-Induced Toxicity in Rats. Mediat. Inflamm. 2015, 2015, 859383. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Hamouda, A.H. Protective Mechanisms of Thymoquinone on Methotrexate-induced Intestinal Toxicity in Rats. Pharmacogn. Mag. 2016, 12, S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, A.Y.; Morsy, M.A.; Rifaai, R.A.; Zenhom, N.M.; Abdel-Gaber, S.A. Paeonol Attenuates Methotrexate-Induced Cardiac Toxicity in Rats by Inhibiting Oxidative Stress and Suppressing TLR4-Induced NF-κB Inflammatory Pathway. Mediat. Inflamm. 2020, 2020, 8641026. [Google Scholar] [CrossRef]
- Pınar, N.; Çakırca, G.; Özgür, T.; Kaplan, M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed. Pharmacother. 2018, 97, 1486–1492. [Google Scholar] [CrossRef]
- Aslankoc, R.; Ozmen, O.; Ellidag, H.Y. Ameliorating effects of agomelatine on testicular and epididymal damage induced by methotrexate in rats. J. Biochem. Mol. Toxicol. 2020, 34, e22445. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Dhungel, S.; Bhattacharya, S.; Jha, C.B.; Srivastava, A.K. Effect of chronic low dose of methotrexate on cellular proliferation during spermatogenesis in rats. Arch. Androl. 2004, 50, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wu, Y.J.; Xiao, F.; Wang, B.; Asenso, J.; Wang, Y.; Sun, W.; Wang, C.; Wei, W. Regulation of CP-25 on P-glycoprotein in synoviocytes of rats with adjuvant arthritis. Biomed. Pharmacother. 2019, 119, 109432. [Google Scholar] [CrossRef]
- Mruk, D.D.; Su, L.; Cheng, C.Y. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol. Sci. 2011, 32, 99–106. [Google Scholar] [CrossRef]
- Su, L.; Mruk, D.D.; Cheng, C.Y. Drug transporters, the blood-testis barrier, and spermatogenesis. J. Endocrinol. 2011, 208, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Droździk, M.; Stefankiewicz, J.; Kurzawa, R.; Górnik, W.; Baczkowski, T.; Kurzawski, M. Association of the MDR1 (ABCB1) gene 3435C>T polymorphism with male infertility. Pharmacol. Rep. 2009, 61, 690–696. [Google Scholar] [CrossRef]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; de Lourdes Bastos, M.; Remião, F. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Morsy, M.A.; Mohamed, M.Z.; Zenhom, N.M. Mechanisms underlying gastroprotective effect of paeonol against indomethacin-induced ulcer in rats. Hum. Exp. Toxicol. 2019, 38, 510–518. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Z.; Zhang, L.; Ma, J.; Zhu, Q. Paeonol protects against hypertension in spontaneously hypertensive rats by restoring vascular endothelium. Biosci. Biotechnol. Biochem. 2019, 83, 1992–1999. [Google Scholar] [CrossRef]
- Chen, G.; Jia, P.; Yin, Z.Y.; Kong, S.Z.; Xiang, Z.B.; Zheng, X.X. Paeonol ameliorates monosodium urate-induced arthritis in rats through inhibiting nuclear factor-κB-mediated proinflammatory cytokine production. Phytother. Res. 2019, 33, 2971–2978. [Google Scholar] [CrossRef]
- Liu, D.H.; Agbo, E.; Zhang, S.H.; Zhu, J.L. Anticonvulsant and Neuroprotective Effects of Paeonol in Epileptic Rats. Neurochem. Res. 2019, 44, 2556–2565. [Google Scholar] [CrossRef]
- Mohamed, M.Z.; Morsy, M.A.; Mohamed, H.H.; Hafez, H.M. Paeonol protects against testicular ischaemia-reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia 2020, 52, e13599. [Google Scholar] [CrossRef]
- Feltrin, C.; Oliveira Simões, C.M. Reviewing the mechanisms of natural product-drug interactions involving efflux transporters and metabolic enzymes. Chem. Biol. Interact. 2019, 314, 108825. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wu, L.; Wu, H.; Dai, M. Paeonol nanoemulsion for enhanced oral bioavailability: Optimization and mechanism. Nanomedicine 2018, 13, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, T.T.; Zhao, H.Y.; Wang, H. Melatonin protects methotrexate-induced testicular injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7517–7525. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Khalaf, H.M.; El-Hussieny, M.; Bayoumi, A.; Shehata, S.; Refaie, M. Role of nitric oxide donor in methotrexate-induced testicular injury via modulation of pro-inflammatory mediators, eNOS and P-glycoprotein. Hum. Exp. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Morsy, M.A.; Al-Taher, A.Y. Multi-drug resistance protein (Mrp) 3 may be involved in resveratrol protection against methotrexate-induced testicular damage. Life Sci. 2014, 119, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Belhan, S.; Çomaklı, S.; Küçükler, S.; Gülyüz, F.; Yıldırım, S.; Yener, Z. Effect of chrysin on methotrexate-induced testicular damage in rats. Andrologia 2019, 51, e13145. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; De Graaf, D.; Haber, M.; Kavallaris, M.; Madafiglio, J.; Gilbert, J.; Kwan, E.; Stewart, B.W.; Mechetner, E.B.; Gudkov, A.V.; et al. Involvement of MDR1 P-glycoprotein in multifactorial resistance to methotrexate. Int. J. Cancer 1996, 65, 613–619. [Google Scholar] [CrossRef]
- Cai, J.; Chen, S.; Zhang, W.; Hu, S.; Lu, J.; Xing, J.; Dong, Y. Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2. Phytomedicine 2014, 21, 984–991. [Google Scholar] [CrossRef]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef]
- Babiak, R.M.; Campello, A.P.; Carnieri, E.G.; Oliveira, M.B. Methotrexate: Pentose cycle and oxidative stress. Cell Biochem. Funct. 1998, 16, 283–293. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel milk attenuates methotrexate-induced kidney injury via activation of PI3K/Akt/eNOS signaling and intervention with oxidative aberrations. Food Funct. 2018, 9, 2661–2672. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Al-Taher, A.Y. Protective mechanisms of resveratrol against methotrexate-induced renal damage may involve BCRP/ABCG2. Fundam. Clin. Pharmacol. 2016, 30, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Tagliamonte, M.C.; Catalani, S.; Primiterra, M.; Canestrari, F.; De Stefani, S.; Palini, S.; Bulletti, C. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod. Biomed. Online 2012, 25, 300–306. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Brodsky, S.V.; Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002, 61, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Walker, L.M.; Mayeux, P.R. Role of nitric oxide in lipopolysaccharide-induced oxidant stress in the rat kidney. Biochem. Pharmacol. 2000, 59, 203–209. [Google Scholar] [CrossRef]
- Ferreiro, M.E.; Amarilla, M.S.; Glienke, L.; Méndez, C.S.; González, C.; Jacobo, P.V.; Sobarzo, C.M.; De Laurentiis, A.; Ferraris, M.J.; Theas, M.S. The inflammatory mediators TNFα and nitric oxide arrest spermatogonia GC-1 cell cycle. Reprod. Biol. 2019, 19, 329–339. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Wan, R.Z.; Zhang, P.; Zhang, B. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure in mice. Int. Immunopharmacol. 2017, 46, 124–132. [Google Scholar] [CrossRef]
- Mariappan, N.; Soorappan, R.N.; Haque, M.; Sriramula, S.; Francis, J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2726–H2737. [Google Scholar] [CrossRef]
- Dempsey, P.W.; Doyle, S.E.; He, J.Q.; Cheng, G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003, 14, 193–209. [Google Scholar] [CrossRef]
- Fan, R.; Han, Y.; Han, H.; Chen, Z.; Yu, B.; Kou, J.; Zhang, Y. DT-13 ameliorates TNF-α-induced nitric oxide production in the endothelium in vivo and in vitro. Biochem. Biophys. Res. Commun. 2018, 495, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Park, J.H.; Ahn, R.S.; Im, S.Y.; Choi, H.S.; Soh, J.; Mellon, S.H.; Lee, K. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol. Cell. Biol. 2004, 24, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, M.; Jiang, N.; Zhang, M.; Feng, L.; Jia, X. Paeonol ameliorates lipopolysaccharides-induced acute lung injury by regulating TLR4/MyD88/ NF-κB signaling pathway. Pharmazie 2019, 74, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of Apoptosis. Biochemistry 2015, 80, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, F.; Khazaei, M.; Rabzia, A.; Mansouri, K.; Ghanbari, A. Protective Effects of Thymoquinone against Methotrexate-Induced Germ Cell Apoptosis in Male Mice. Int. J. Fertil. Steril. 2016, 9, 541–547. [Google Scholar] [CrossRef]
- Yao, W.; Tai, L.W.; Liu, Y.; Hei, Z.; Li, H. Oxidative Stress and Inflammation Interaction in Ischemia Reperfusion Injury: Role of Programmed Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 6780816. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cai, L.J.; Pang, K.; Dong, Y.; Zhang, Z.G.; Li, B.B.; Li, R.; Han, C.H. Paeonol inhibits proliferation and induces cell apoptosis of human T24 and 5637 bladder cancer cells in vitro and in vivo. Clin. Transl. Oncol. 2020. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.J.; Nishida, M.; Rabinowitz, R.; Cockett, A.T. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J. Urol. 1985, 133, 906–911. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]

| Group | Testicular Weight (g) | Serum Testosterone (nmol/mL) | P-Glycoprotein (ng/mL) |
|---|---|---|---|
| Control | 0.933 ± 0.07 | 2.93 ± 0.07 | 3.86 ± 0.32 |
| Paeonol | 0.969 ± 0.04 | 2.83 ± 0.09 | 3.70 ± 0.35 |
| MTX | 0.410 ± 0.08 *° | 1.18 ± 0.09 *° | 4.09 ± 0.39 |
| Paeonol + MTX | 0.738 ± 0.08 # | 2.16 ± 0.12 *°# | 5.52 ± 0.43 *°# |
| Group | Histopathological Score | Height of Germinal Lining Epithelium (nm) | Spermatogenesis Score |
|---|---|---|---|
| Control | 1.00 ± 0.00 | 145.0 ± 4.45 | 9.75 ± 0.16 |
| Paeonol | 1.00 ± 0.00 | 158.8 ± 2.70 | 9.87 ± 0.12 |
| MTX | 3.87 ± 0.12 *° | 78.25 ± 2.59 *° | 4.875 ± 0.35 *° |
| Paeonol + MTX | 1.62 ± 0.26 *°# | 137.3 ± 4.87 *°# | 7.12 ± 0.39 *°# |
| Group | MDA (nmol/g Tissue) | NOx (nmol/g Tissue) | GSH (nmol/g Tissue) | SOD (U/g Tissue) |
|---|---|---|---|---|
| Control | 14.30 ± 0.30 | 814.3 ± 66.57 | 309.9 ± 14.06 | 14019 ± 630.5 |
| Paeonol | 14.16 ± 0.37 | 807.5 ± 61.05 | 313.0 ± 12.42 | 14528 ± 546.4 |
| MTX | 45.63 ± 2.54 *° | 2825 ± 225.1 *° | 179.4 ± 10.67 *° | 5814 ± 320.6 *° |
| Paeonol + MTX | 16.23 ± 0.45 # | 968.4 ± 60.97 # | 262.1 ± 3.94 *°# | 10579 ± 404.6 *°# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, M.A.; Abdel-Aziz, A.M.; Abdel-Hafez, S.M.N.; Venugopala, K.N.; Nair, A.B.; Abdel-Gaber, S.A. The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals 2020, 13, 223. https://doi.org/10.3390/ph13090223
Morsy MA, Abdel-Aziz AM, Abdel-Hafez SMN, Venugopala KN, Nair AB, Abdel-Gaber SA. The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals. 2020; 13(9):223. https://doi.org/10.3390/ph13090223
Chicago/Turabian StyleMorsy, Mohamed A., Asmaa M. Abdel-Aziz, Sara M. N. Abdel-Hafez, Katharigatta N. Venugopala, Anroop B. Nair, and Seham A. Abdel-Gaber. 2020. "The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats" Pharmaceuticals 13, no. 9: 223. https://doi.org/10.3390/ph13090223
APA StyleMorsy, M. A., Abdel-Aziz, A. M., Abdel-Hafez, S. M. N., Venugopala, K. N., Nair, A. B., & Abdel-Gaber, S. A. (2020). The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals, 13(9), 223. https://doi.org/10.3390/ph13090223