Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury
Abstract
1. Introduction
2. Results
2.1. Extraction and Characterization of G. lucidum Pericarp
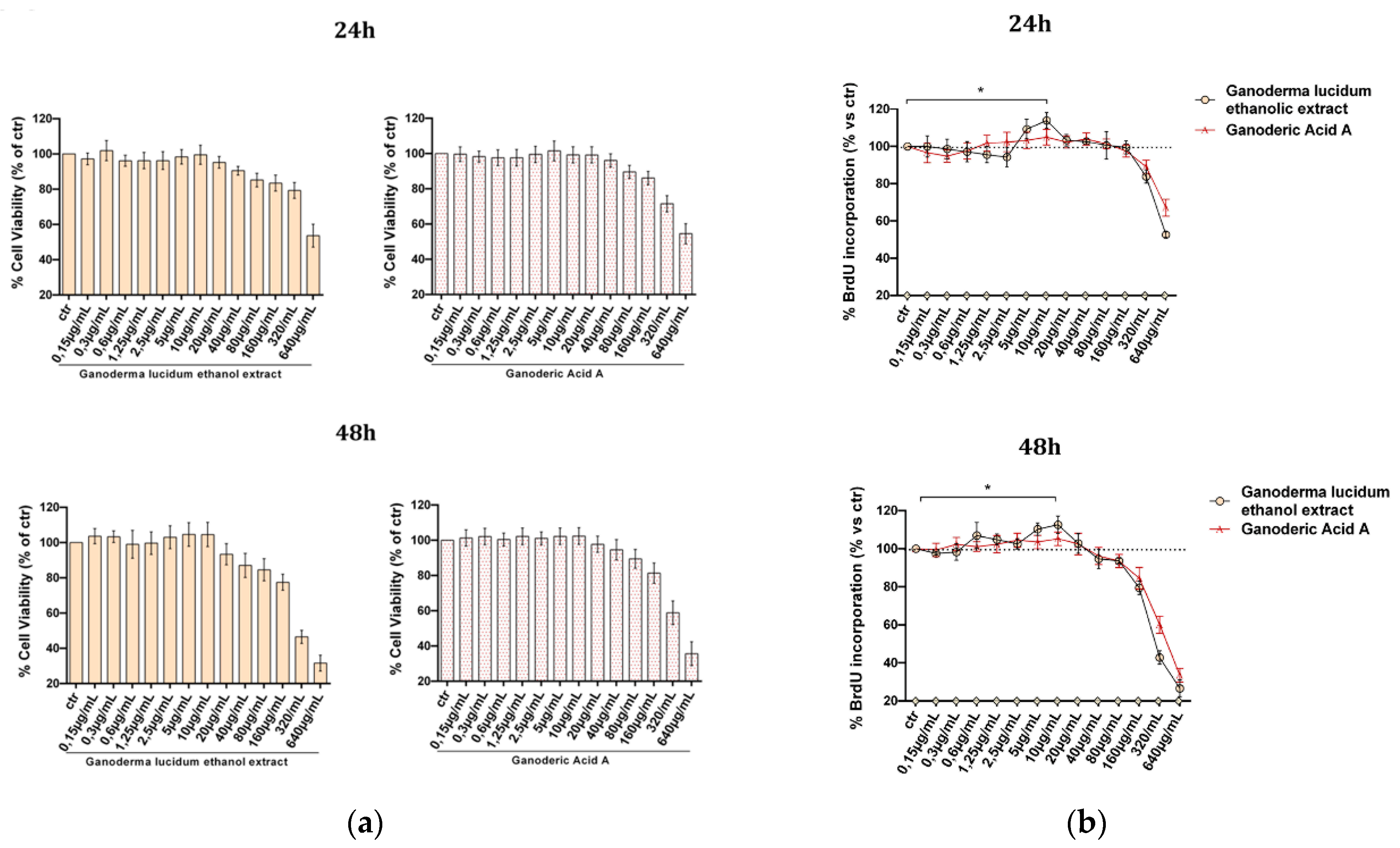
2.2. Evaluation of G. lucidum Extract Effect in HaCaT Cells
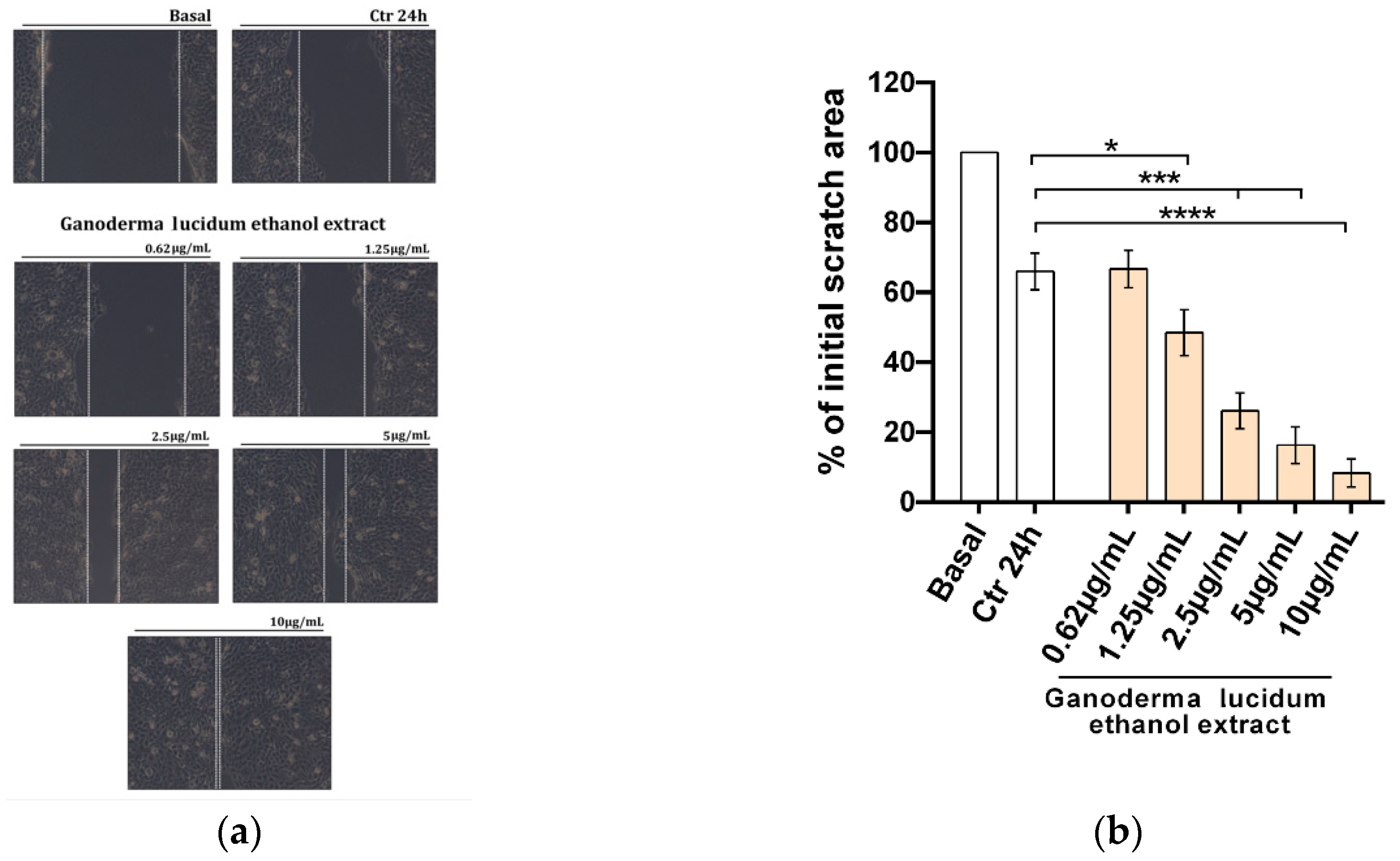
2.3. Improvement of the Migratory Capacity of Human Keratinocytes Exposed to G. lucidum Extract
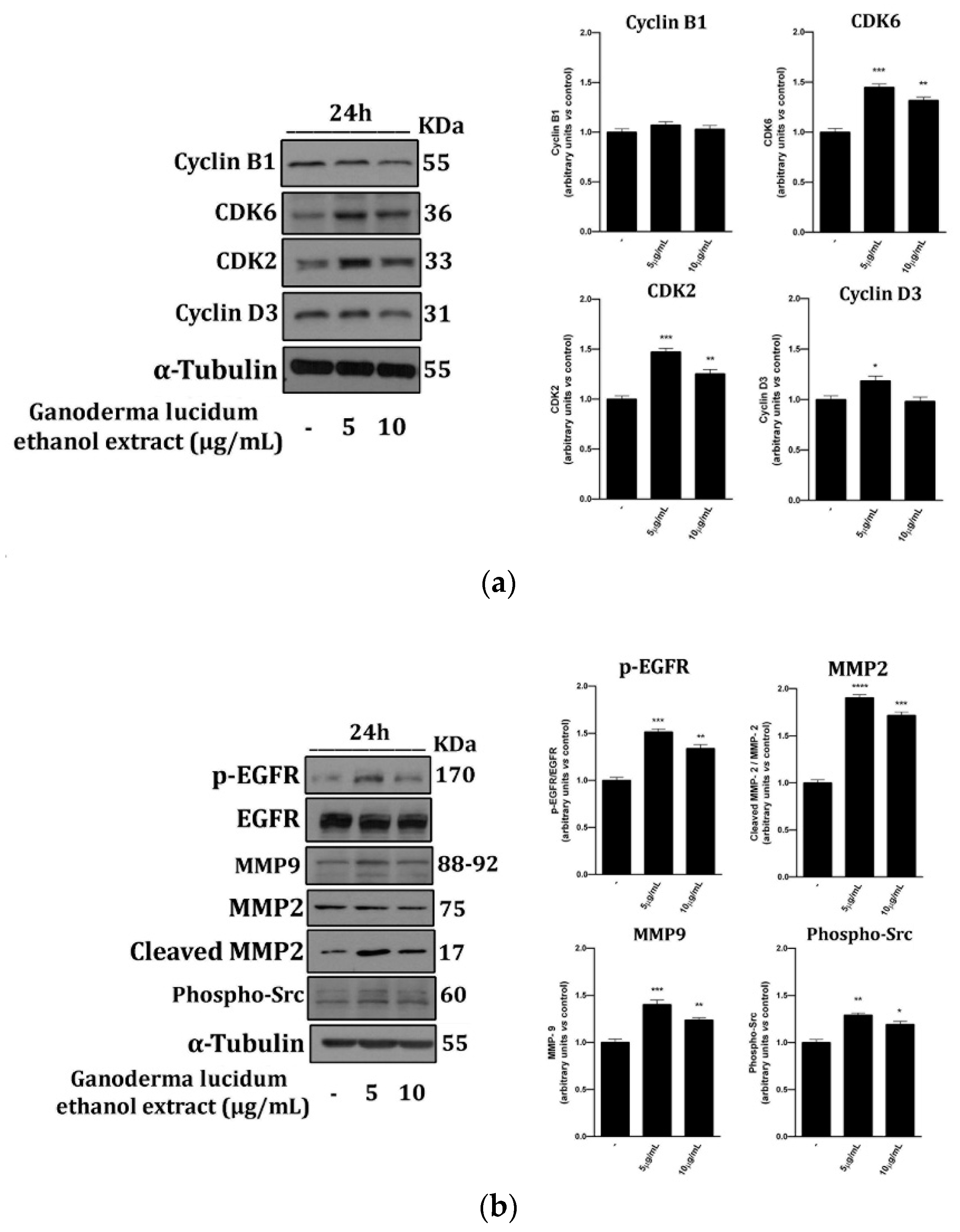
2.4. G. lucidum Ethanol Extract Induces Expression of Proteins Linked to the Control of Cell Cycle and Migration
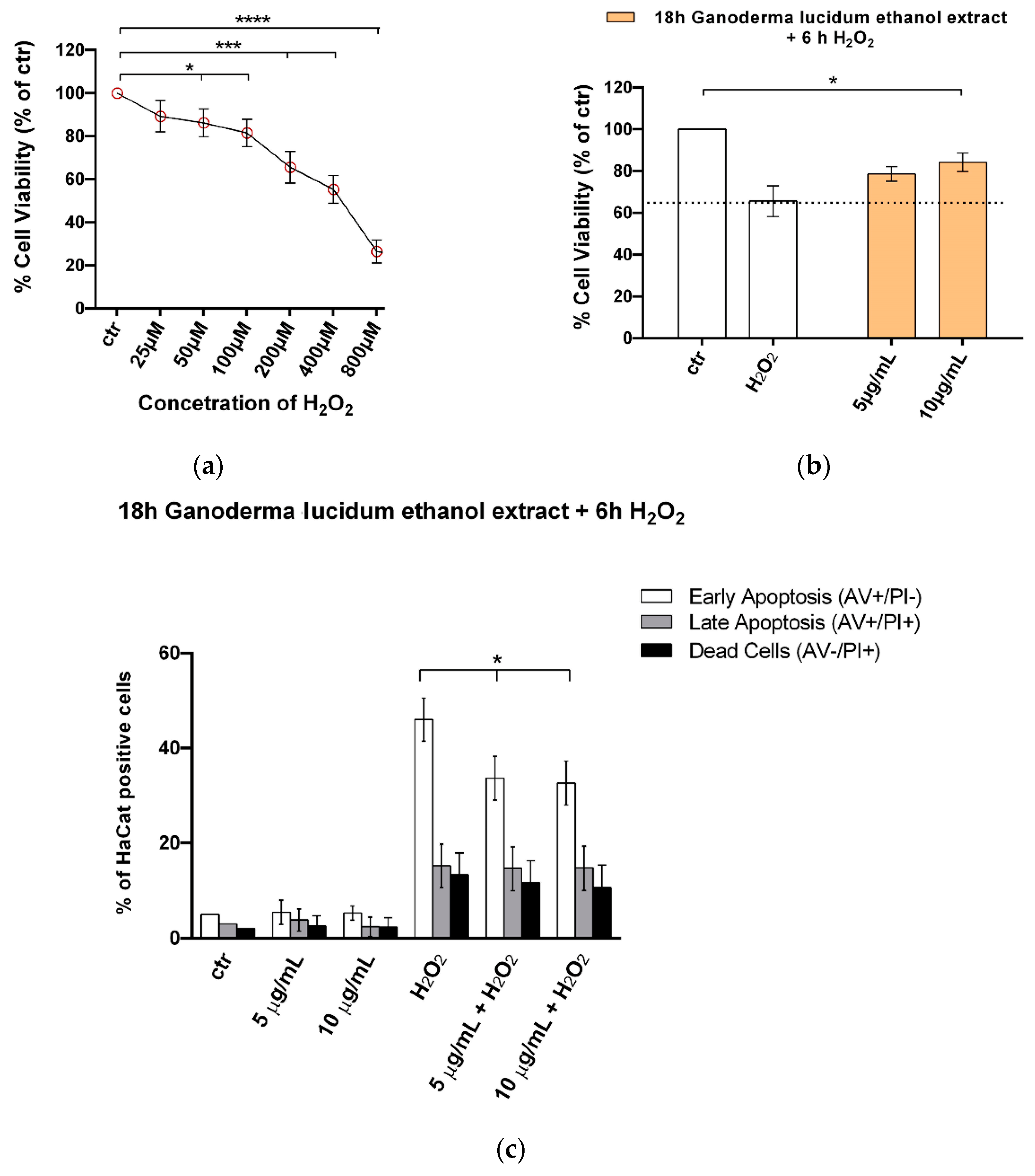
2.5. G. lucidum Ethanol Extract Ameliorates Cytotoxicity and Apoptosis Induced by H2O2 in HaCaT Cells
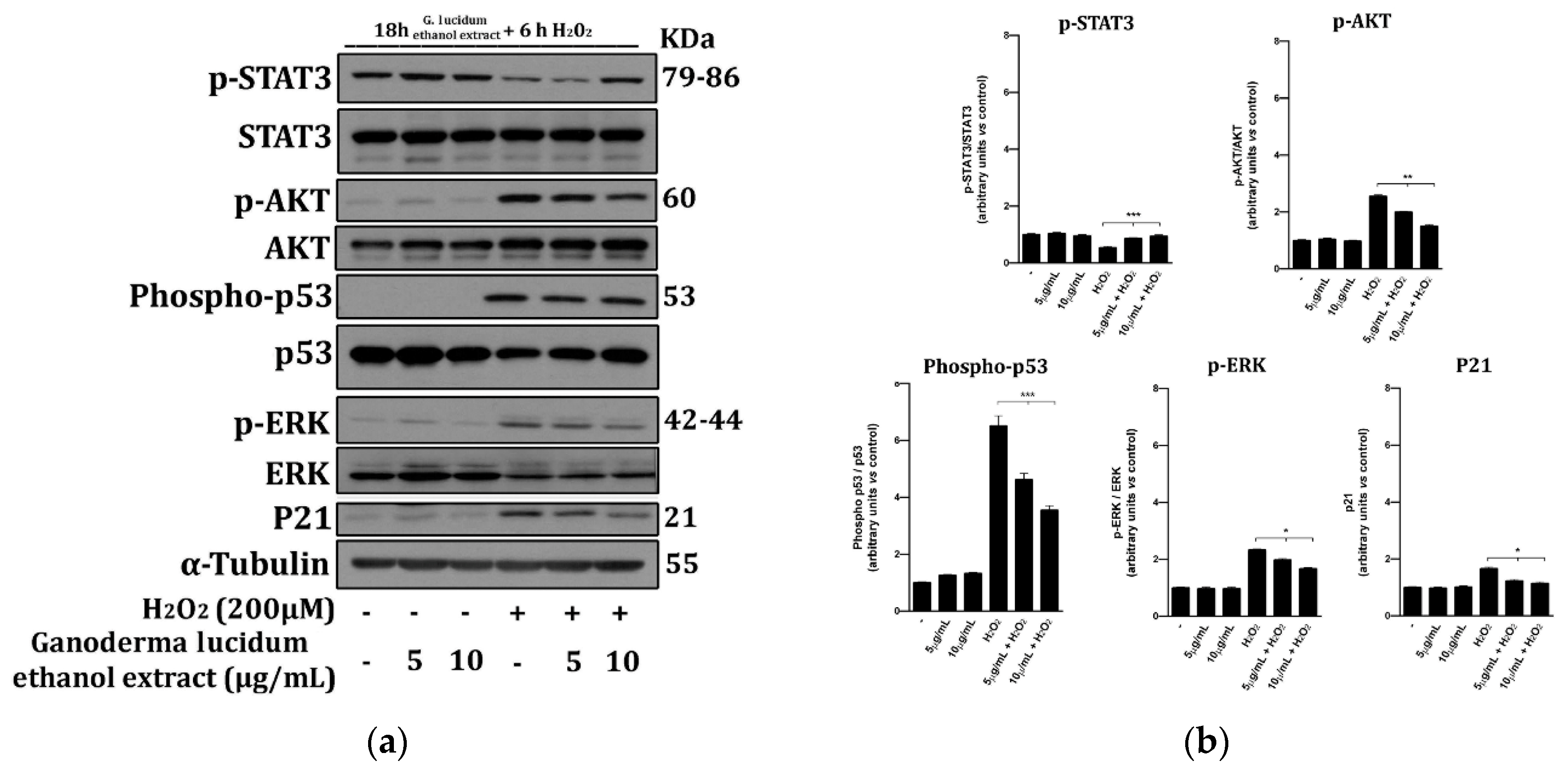
2.6. G. lucidum Extract Prevents the Activation of Cell Death Molecular Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Sample Preparation
4.3. LCMS-IT-TOF Analysis of G. lucidum Extract
4.4. Quantitative Analysis
4.5. Cells
4.6. Determination of Cells Viability, MTT Assay
4.7. Determination of Cells Proliferation, BrdU Assay
4.8. Scratch Wound Healing Assay
4.9. Apoptosis Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma Lucidum (Lingzhi or Reishi) Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Taylor and Francis Group: Milton Park, UK, 2011. [Google Scholar]
- Chu, T.T.; Benzie, I.F.; Lam, C.W.; Fok, B.S.; Lee, K.K.; Tomlinson, B. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): Results of a controlled human intervention trial. Br. J. Nutr. 2012, 107, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.W.; Gohel, M.D. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer 2005, 53, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and Their Potential Anti-inflammatory Effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Peng, X.R.; Dong, J.R.; Yan, H.; Kong, Q.H.; Shi, Q.Q.; Li, D.S.; Zhou, L.; Li, Z.R.; Qiu, M.H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti-inflammatory activities. Fitoterapia 2019, 134, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cuong, V.T.; Chen, W.; Shi, J.; Zhang, M.; Yang, H.; Wang, N.; Yang, S.; Li, J.; Yang, P.; Fei, J. The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol. 2019, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Zhao, H.; Feng, J. Emerging Roles of Ganoderma Lucidum in Anti-Aging. Aging Dis. 2017, 8, 691–707. [Google Scholar] [CrossRef]
- Bishop, K.S.; Kao, C.H.J.; Xu, Y.; Glucina, M.P.; Paterson, R.R.M.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Zhao, X.R.; Zhang, B.J.; Deng, S.; Zhang, H.L.; Huang, S.S.; Huo, X.K.; Wang, C.; Liu, F.; Ma, X.C. Isolation and identification of oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. Phytochem. Lett. 2016, 16, 87–91. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, J.; Zheng, S.; Bo, R.; Luo, L.; Huang, Y.; Niu, Y.; Li, Z.; Wang, D.; Hu, Y.; et al. Ganoderma lucidum polysaccharides encapsulated in liposome as an adjuvant to promote Th1-bias immune response. Carbohydr. Polym. 2016, 142, 141–148. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.H.; Nie, X.T.; Jiang, W.R.; Zhang, Y.S. Sodium butyrate ameliorates lipopolysaccharide-induced cow mammary epithelial cells from oxidative stress damage and apoptosis. J. Cell. Biochem. 2018, 120, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.V.; Gavarić, N.S.; Božin, B.N. Ganoderma: Insights into anticancer effects. Eur. J. Cancer Prev. 2016, 25, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lou, B.; Zhang, Y.; Zhang, C. Ganoderic Acid A exerts the cytoprotection against hypoxia-triggered impairment in PC12 cells via elevating microRNA-153. Phytother. Res. 2020, 34, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Chao, H.H.; Chan, P.; Chang, L.P.; Liu, C.F. Antioxidant activity of Ganoderma lucidum in acute ethanol-induced heart toxicity. Phytother. Res. 2004, 18, 1024–1026. [Google Scholar] [CrossRef]
- Ruan, W.; Wei, Y.; Popovich, D.G. Distinct Responses of Cytotoxic Ganoderma lucidum Triterpenoids in Human Carcinoma Cells. Phytother. Res. 2015, 29, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Tung, N.T.; Cuong, T.D.; Hung, T.M.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Lee, J.H.; Min, B.S. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 2015, 12, 69–74. [Google Scholar] [CrossRef]
- Stojkovic, D.S.; Barros, L.; Calhelha, R.C.; Glamoclija, J.; Ciric, A.; van Griensven, L.J.; Sokovic, M.; Ferreira, I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.H.; Li, J.; Yang, H.; Shin, H.J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Guo, W.L.; Pan, Y.Y.; Li, L.; Li, T.T.; Liu, B.; Lv, X.C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef]
- Taofiq, O.; Gonzalez-Paramas, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Turner, N.A.; Xia, F.; Azhar, G.; Zhang, X.; Liu, L.; Wei, J.Y. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J. Mol. Cell. Cardiol. 1998, 30, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, C.; Liu, L.; Zhao, L.; Cui, W.; Tian, Y.; Liu, B.; Li, J. Taurine Protects Primary Neonatal Cardiomyocytes Against Apoptosis Induced by Hydrogen Peroxide. Int. Heart J. 2018, 59, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.F.; Hurst, J.S.; Godley, B.F. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr. Eye Res. 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Yang, Y.; Yi, W.; Yan, J.; Liang, Z.; Wang, N.; Li, Y.; Chen, W.; Yu, S.; Jin, Z.; et al. New Role of JAK2/STAT3 Signaling in Endothelial Cell Oxidative Stress Injury and Protective Effect of Melatonin. PLoS ONE 2013, 8, e57941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Xu, J.; Wang, Y.; Ma, F.; Li, Z.; Liu, N. Stat3 activation is critical for pluripotency maintenance. J. Cell. Physiol. 2018, 234, 1044–1051. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Barreiro, M.F.; Ferreira, I.C.; Oliveira, M. Mushroom ethanolic extracts as cosmeceuticals ingredients: Safety and ex vivo skin permeation studies. Food Chem. Toxicol. 2019, 127, 228–236. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Gonzalez-Paramas, A.M.; Barreiro, M.F.; Ferreira, I.C. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem. Toxicol. 2017, 108 Pt A, 139–147. [Google Scholar] [CrossRef]
- Ruan, W.; Lim, A.H.; Huang, L.G.; Popovich, D.G. Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth. Nat. Prod. Res. 2014, 28, 2264–2272. [Google Scholar] [CrossRef]
- Chang, Y.; Kong, R. Ganoderic acid A alleviates hypoxia-induced apoptosis, autophagy, and inflammation in rat neural stem cells through the PI3K/AKT/mTOR pathways. Phytother. Res. 2019, 33, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.R.; Feng, L.; Ye, L.H.; Wang, L.S.; Xiao, B.X.; Tao, X.; Chang, Q. Ganoderic Acid A Metabolites and Their Metabolic Kinetics. Front. Pharmacol. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Cai, L.; Li, W.; Zhang, L.; Guo, S.; Zhang, R.; Zheng, Y.; Liu, X.; Wang, M.; Zhou, X.; et al. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes 2018, 67, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, Z.; Zhou, Q.; Bai, X.; Fan, L.; Yang, C.; Su, L.; Hu, D. miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2. J. Mol. Histol. 2017, 48, 147–155. [Google Scholar] [CrossRef]
- Vuong, T.T.; Rønning, S.B.; Ahmed, T.A.E.; Brathagen, K.; Høst, V.; Hincke, M.T.; Suso, H.P.; Pedersen, M.E. Processed eggshell membrane powder regulates cellular functions and increase MMP-activity important in early wound healing processes. PLoS ONE 2018, 13, e0201975. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Hu, Z.; Du, R.; Xiu, L.; Bian, Z.; Ma, C.; Sato, N.; Hattori, M.; Zhang, H.; Liang, Y.; Yu, S.; et al. Protective effect of triterpenes of Ganoderma lucidum on lipopolysaccharide-induced inflammatory responses and acute liver injury. Cytokine 2020, 127, 154917. [Google Scholar] [CrossRef]
- Ciaglia, E.; Malfitano, A.M.; Laezza, C.; Fontana, A.; Nuzzo, G.; Cutignano, A.; Abate, M.; Pelin, M.; Sosa, S.; Bifulco, M.; et al. Immuno-Modulatory and Anti-Inflammatory Effects of Dihydrogracilin A, a Terpene Derived from the Marine Sponge Dendrilla membranosa. Int. J. Mol. Sci. 2017, 18, 1643. [Google Scholar] [CrossRef]
- Pisanti, S.; Picardi, P.; Ciaglia, E.; Margarucci, L.; Ronca, R.; Giacomini, A.; Malfitano, A.M.; Casapullo, A.; Laezza, C.; Gazzerro, P.; et al. Antiangiogenic effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, mediated by AMPK activation. FASEB J. 2014, 28, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Abate, M.; Laezza, C.; Pisanti, S.; Vitale, M.; Seneca, V.; Torelli, G.; Franceschelli, S.; Catapano, G.; Gazzerro, P.; et al. Antiglioma effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int. J. Cancer 2017, 140, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Laezza, C.; Pisanti, S.; Torelli, G.; Seneca, V.; Catapano, G.; Montella, F.; Ranieri, R.; Notarnicola, M.; Gazzerro, P.; et al. Deregulated expression and activity of Farnesyl Diphosphate Synthase (FDPS) in Glioblastoma. Sci. Rep. 2017, 7, 14123. [Google Scholar] [CrossRef] [PubMed]
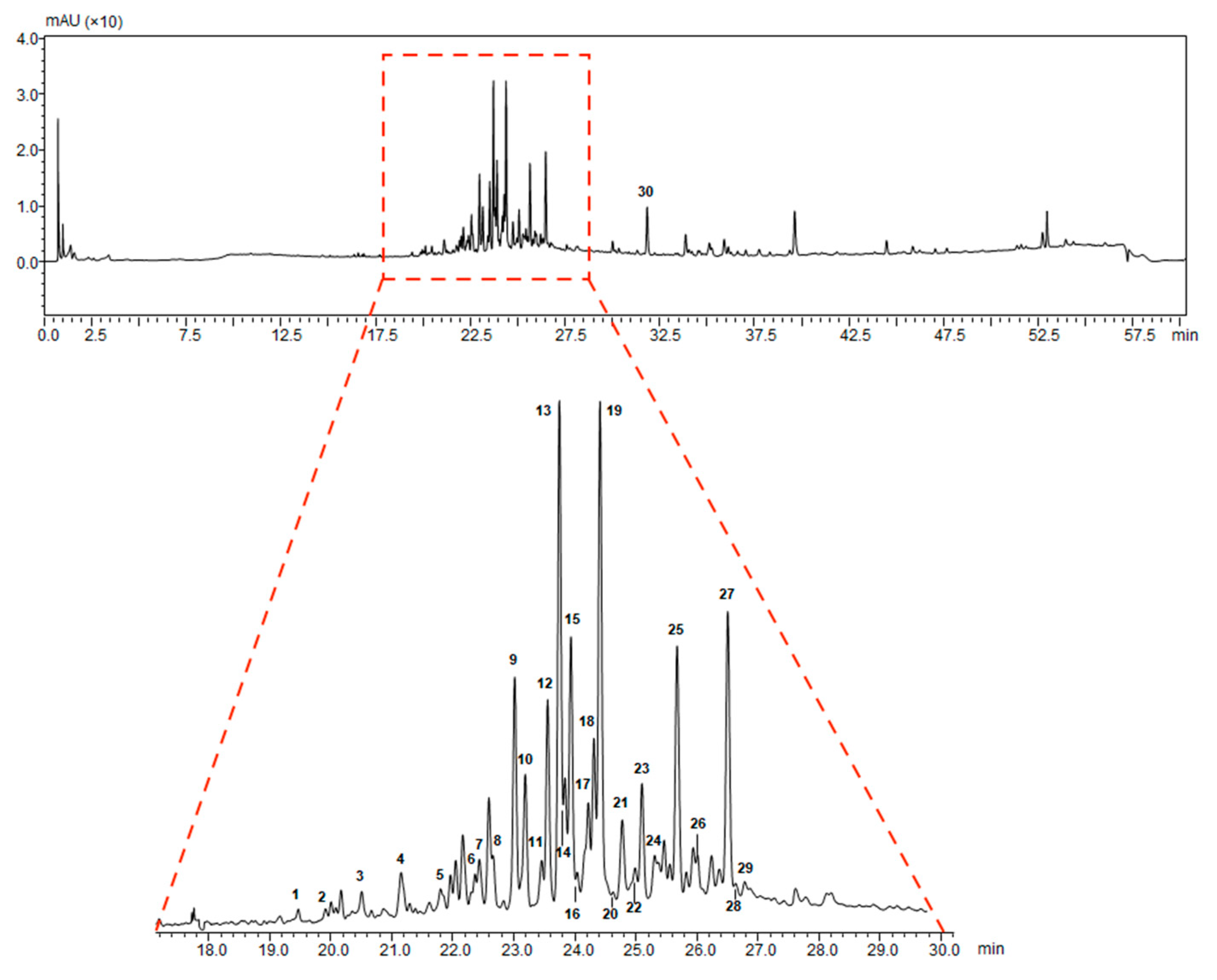
| Peak | Retention Time (min) | Molecular Formula | [M − H]− Observed | [M − H]− Calculated | Error (ppm) | MS2 m/z | Tentative Identification |
|---|---|---|---|---|---|---|---|
| 1 | 19.49 | C30H46O8 | 533.3106 | 533.3120 | −2.63 | 515.3096 | 12-hydroxyganoderic acid C2 |
| 2 | 19.94 | C30H42O8 | 529.2807 | 529.2807 | 0.00 | 511.2468 | 20-hydroxyganoderic acid AM1 |
| 3 | 20.55 | C30H42O8 | 529.2843 | 529.2807 | 4.89 | 511.2698 | 12-deacetylganoderic acid H |
| 4 | 21.19 | C30H44O8 | 531.2962 | 531.2963 | −0.19 | 513.2930 | Ganoderic acid η |
| 5 | 21.75 | C30H42O8 | 529.2774 | 529.2807 | −6.23 | 511.2707; 467.2884 | 12-hydroxyganoderic acid D |
| 6 | 22.42 | C30H46O7 | 517.3189 | 517.3171 | 3.48 | 499.3131 | Ganoderic acid C2 |
| 7 | 22.49 | C30H46O8 | 529.2801 | 529.2807 | −1.13 | 511.2640; 467.2661 | Ganoderic acid C6 |
| 8 | 22.78 | C27H40O6 | 459.2761 | 459.2752 | 1.96 | 441.2709 | Lucidenic acid N |
| 9 | 23.07 | C30H44O8 | 531.2991 | 531.2963 | 5.27 | 513.2849; 469.2961 | Ganoderic acid G |
| 10 | 23.27 | C30H42O7 | 513.2864 | 513.2858 | 1.17 | 495.2737 | Ganoderenic acid B |
| 11 | 23.52 | C30H44O7 | 515.2979 | 515.3014 | −6.79 | 497.2841 | Ganoderic acid B |
| 12 | 23.61 | C29H40O8 | 515.2651 | 515.2650 | 0.19 | 473.2539 | Lucidenic acid E |
| 13 | 23.79 | C32H44O9 | 571.2936 | 571.2913 | 4.03 | 553.2818 | Ganoderenic acid K |
| 14 | 23.85 | C30H42O7 | 513.2847 | 513.2858 | −2.14 | 495.2708 | Ganoderic acid AM1 |
| 15 | 23.95 | C32H46O9 | 573.3067 | 573.3069 | −0.35 | 555.2977 | Ganoderic acid K |
| 16 | 24.09 | C30H42O8 | 529.2825 | 529.2807 | 3.40 | 511.2753 | Ganoderic acid derivative |
| 17 | 24.25 | C32H42O9 | 569.2750 | 569.2756 | −1.05 | 551.2746 | Ganoderic acid F |
| 18 | 24.32 | C30H44O7 | 515.3061 | 515.3014 | 4.12 | 497.2940 | Ganoderic acid A |
| 19 | 24.49 | C32H44O9 | 571.2909 | 571.2913 | −0.70 | 553.2850 | Ganoderic acid H |
| 20 | 24.68 | C30H40O8 | 527.2648 | 527.2650 | −0.38 | 509.2487 | Elfvingic acid A |
| 21 | 24.81 | C27H38O6 | 457.2620 | 457.2596 | 5.25 | 439.2390 | Lucidenic acid A |
| 22 | 24.89 | C30H44O6 | 499.3001 | 499.3065 | −6.40 | 481.3798 | Ganolucidic acid A |
| 23 | 25.17 | C30H40O7 | 511.2717 | 511.2701 | 3.13 | 493.2400 | Ganoderenic acid D |
| 24 | 25.31 | C27H36O6 | 455.2449 | 455.2439 | 2.20 | 380.2072; 301.1813 | Lucidenic acid F |
| 25 | 25.72 | C29H38O8 | 513.2508 | 513.2494 | 2.73 | 471.2400 | Lucidenic acid D |
| 26 | 26.04 | C34H46O10 | 613.2997 | 613.3018 | −3.42 | 595.2916; 553.2831 | 3-acetylganoderic acid H |
| 27 | 26.58 | C32H42O9 | 569.2736 | 569.2697 | 3.85 | 551.2649 | 12-acetoxyganoderic acid F |
| 28 | 26.81 | C30H42O7 | 513.2859 | 513.2858 | 0.19 | 451.2844 | Ganoderic acid J |
| 29 | 28.20 | C30H44O6 | 499.3089 | 499.3065 | 4.81 | 437.2981 | Ganolucidic acid D |
| 30 | 31.98 | C30H44O5 | 483.3122 | 483.3116 | 1.24 | 439.3309; 409.2717 | Ganolucidic acid E |
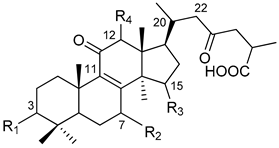 | 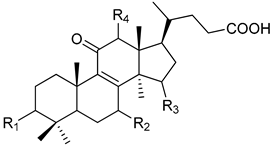 |  | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Peak | Compound Name | Type | R1 | R2 | R3 | R4 | R5 | Double Bond |
| 1 | 12-hydroxyganoderic acid C2 | A | β-OH | β-OH | α-OH | OH | - | |
| 4 | Ganoderic acid η | C | β-OH | β-OH | =O | β-OH | β-OH | |
| 5 | 12-hydroxyganoderic acid D | A | =O | β-OH | =O | OH | - | |
| 6 | Ganoderic acid C2 | A | β-OH | β-OH | α-OH | H | - | |
| 7 | Ganoderic acid C6 | A | β-OH | =O | =O | β-OH | - | |
| 8 | Lucidenic acid N | B | β-OH | β-OH | =O | H | - | |
| 9 | Ganoderic acid G | A | β-OH | β-OH | =O | β-OH | - | |
| 10 | Ganoderenic acid B | A | β-OH | β-OH | =O | H | - | Δ20,22 |
| 11 | Ganoderic acid B | A | β-OH | β-OH | =O | H | - | |
| 12 | Lucidenic acid E | B | β-OH | =O | =O | β-OAc | - | |
| 13 | Ganoderenic acid K | A | β-OH | β-OH | =O | β-OAc | - | Δ20,22 |
| 14 | Ganoderic acid AM1 | A | β-OH | =O | =O | H | - | |
| 15 | Ganoderic acid K | A | β-OH | β-OH | =O | β-OAc | - | |
| 17 | Ganoderic acid F | A | =O | =O | =O | H | - | |
| 18 | Ganoderic acid A | A | =O | β-OH | α-OH | H | - | |
| 19 | Ganoderic acid H | A | β-OH | =O | =O | β-OAc | - | |
| 20 | Elfvingic acid A | A | =O | =O | β-OH | α-OH | - | Δ20,22 |
| 21 | Lucidenic acid A | B | =O | β-OH | =O | H | - | |
| 22 | Ganolucidic acid A | A | =O | H | α-OH | H | - | |
| 23 | Ganoderenic acid D | A | =O | β-OH | =O | H | - | Δ20,22 |
| 24 | Lucidenic acid F | B | =O | =O | =O | H | - | |
| 25 | Lucidenic acid D | B | =O | =O | =O | β-OAc | - | |
| 26 | 3-acetylganoderic acid H | A | β-OAc | =O | =O | β-OAc | - | |
| 27 | 12-acetoxyganoderic acid F | A | =O | =O | =O | β-OAc | - | |
| 28 | Ganoderic acid J | A | =O | =O | α-OH | H | - | |
| 29 | Ganolucidic acid D | C | =O | H | α-OH | H | β-OH | |
| 30 | Ganolucidic acid E | C | =O | H | α-OH | H | H | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, M.; Pepe, G.; Randino, R.; Pisanti, S.; Basilicata, M.G.; Covelli, V.; Bifulco, M.; Cabri, W.; D’Ursi, A.M.; Campiglia, P.; et al. Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury. Pharmaceuticals 2020, 13, 224. https://doi.org/10.3390/ph13090224
Abate M, Pepe G, Randino R, Pisanti S, Basilicata MG, Covelli V, Bifulco M, Cabri W, D’Ursi AM, Campiglia P, et al. Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury. Pharmaceuticals. 2020; 13(9):224. https://doi.org/10.3390/ph13090224
Chicago/Turabian StyleAbate, Mario, Giacomo Pepe, Rosario Randino, Simona Pisanti, Manuela Giovanna Basilicata, Verdiana Covelli, Maurizio Bifulco, Walter Cabri, Anna Maria D’Ursi, Pietro Campiglia, and et al. 2020. "Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury" Pharmaceuticals 13, no. 9: 224. https://doi.org/10.3390/ph13090224
APA StyleAbate, M., Pepe, G., Randino, R., Pisanti, S., Basilicata, M. G., Covelli, V., Bifulco, M., Cabri, W., D’Ursi, A. M., Campiglia, P., & Rodriquez, M. (2020). Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury. Pharmaceuticals, 13(9), 224. https://doi.org/10.3390/ph13090224