Automated GMP-Compliant Production of [68Ga]Ga-DO3A-Tuna-2 for PET Microdosing Studies of the Glucagon Receptor in Humans
Abstract
1. Introduction
2. Material and Methods
2.1. Facilities, Equipment, and Materials
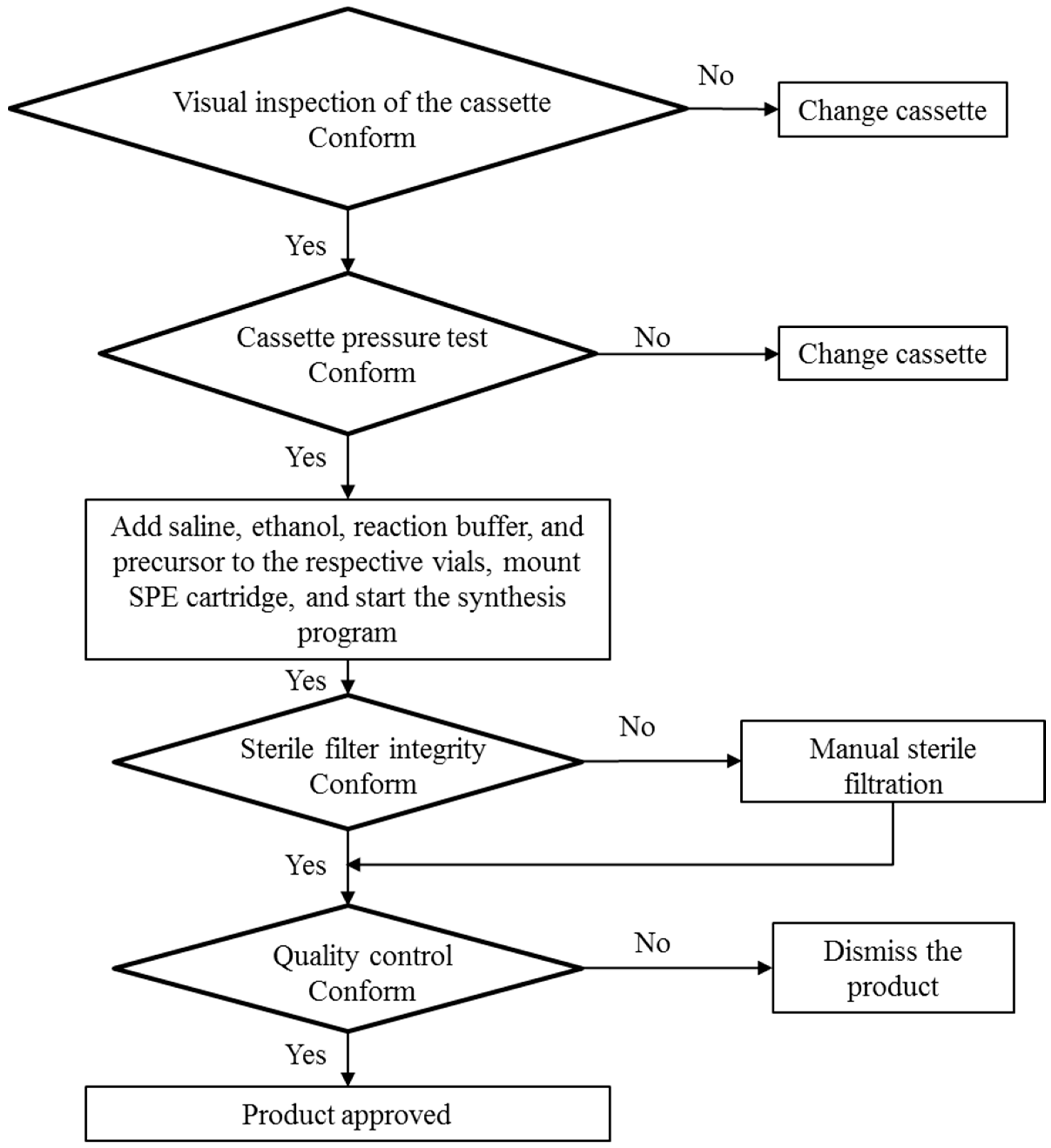
2.2. Production and Quality Control of [68Ga]Ga-DO3A-VS-Cys40-Tuna-2
2.3. Clinical Examination
3. Results
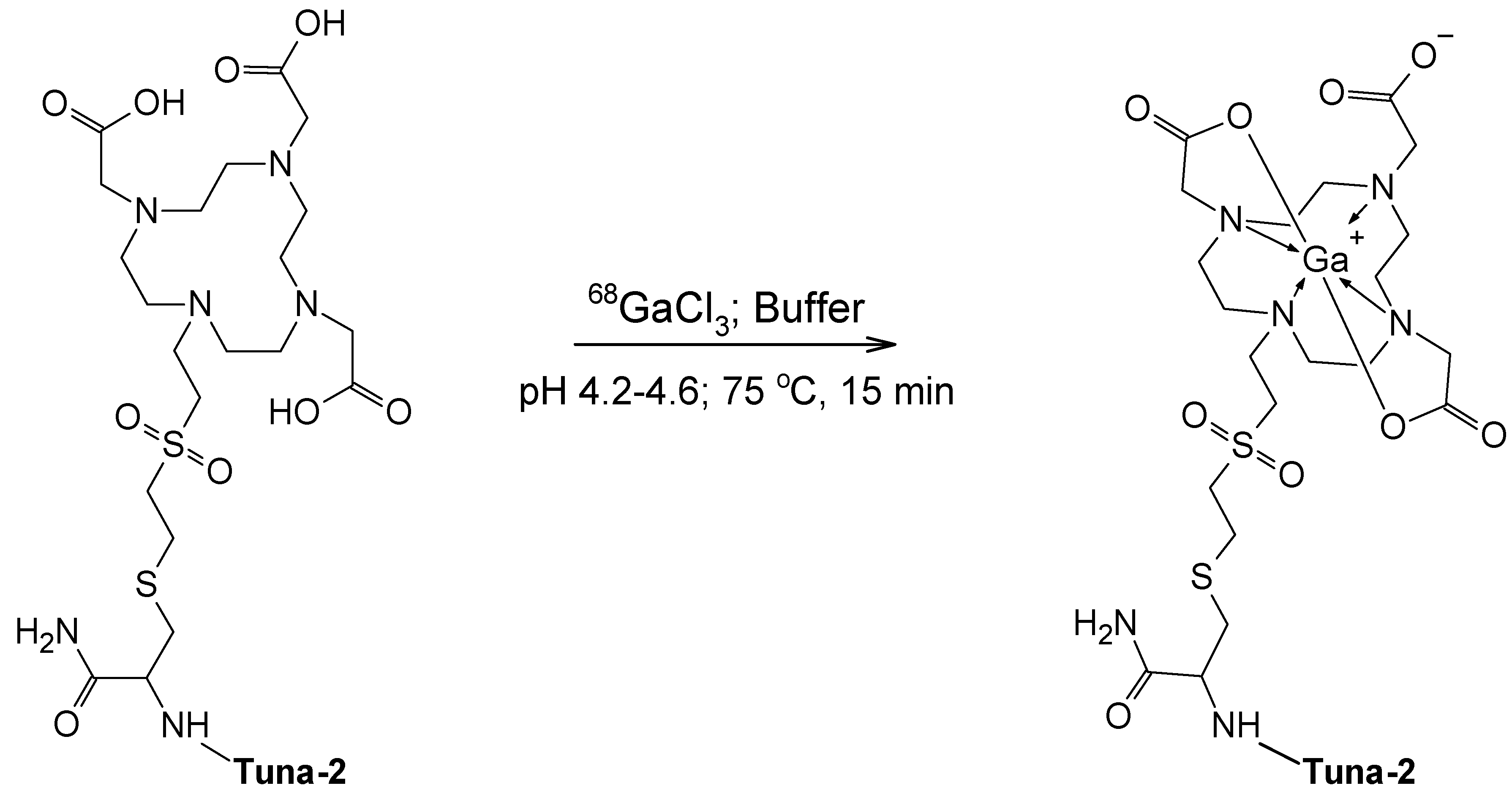
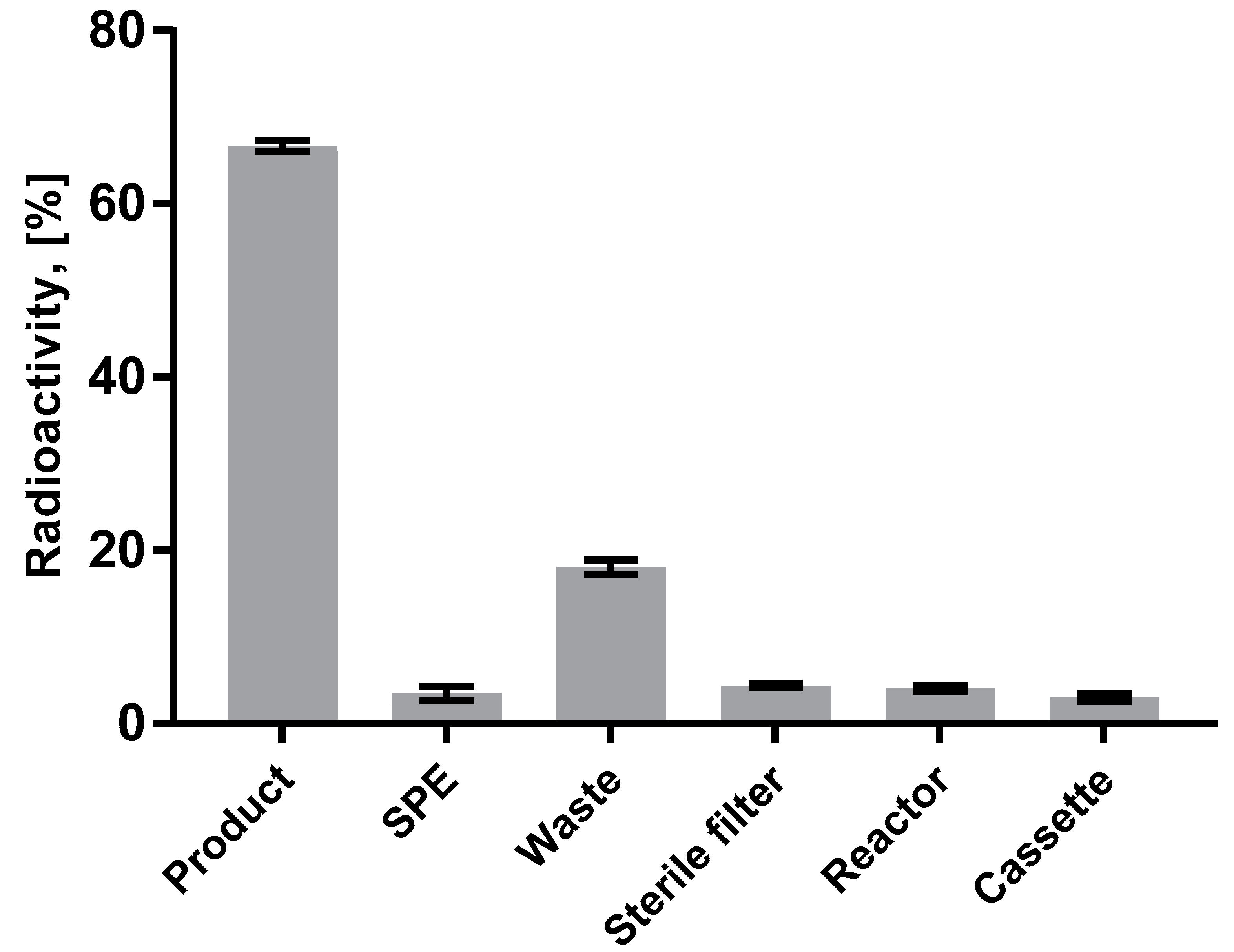
3.1. Production of [68Ga]Ga-DO3A-VS-Cys40-Tuna-2
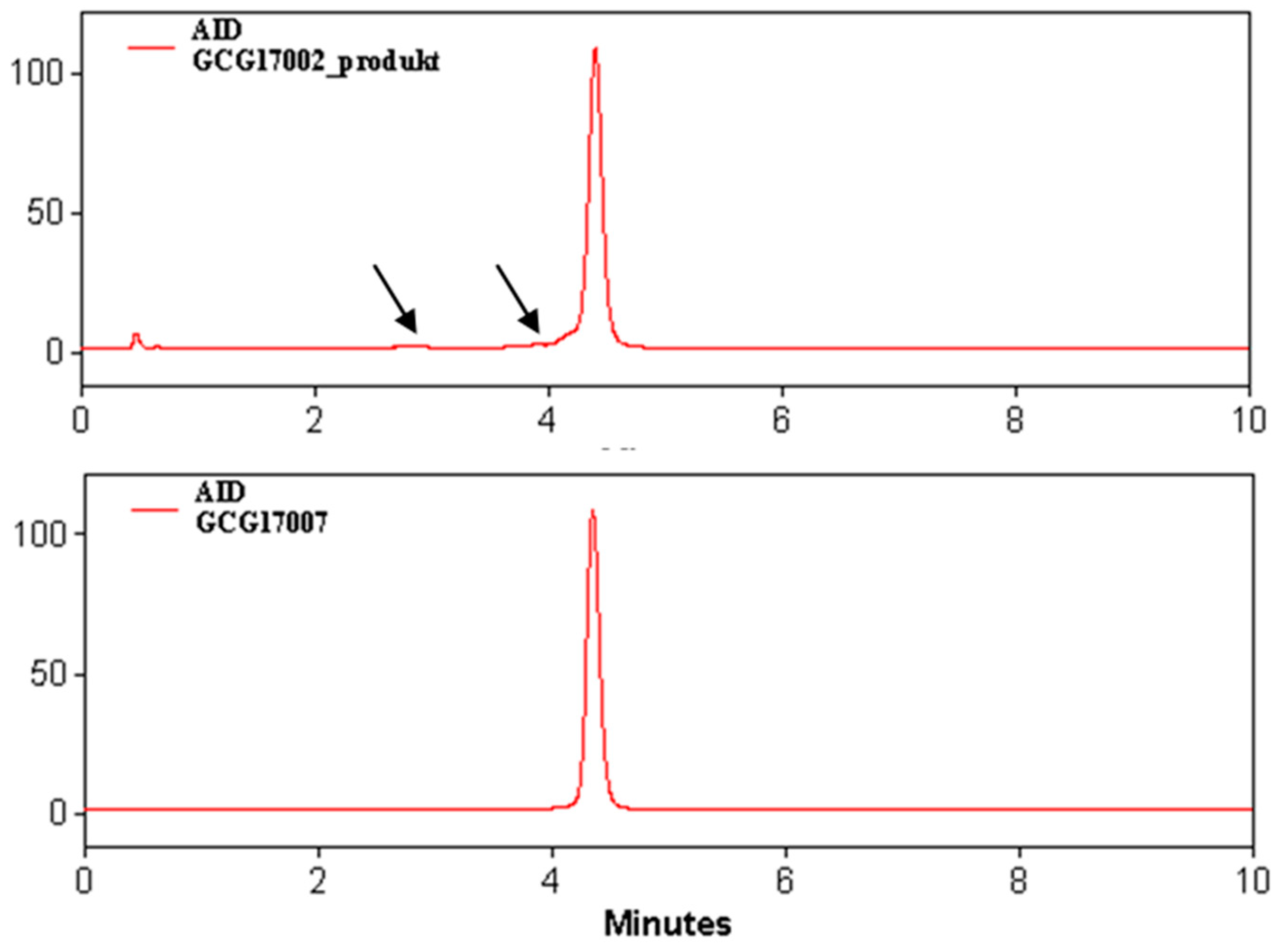
3.2. Quality Control
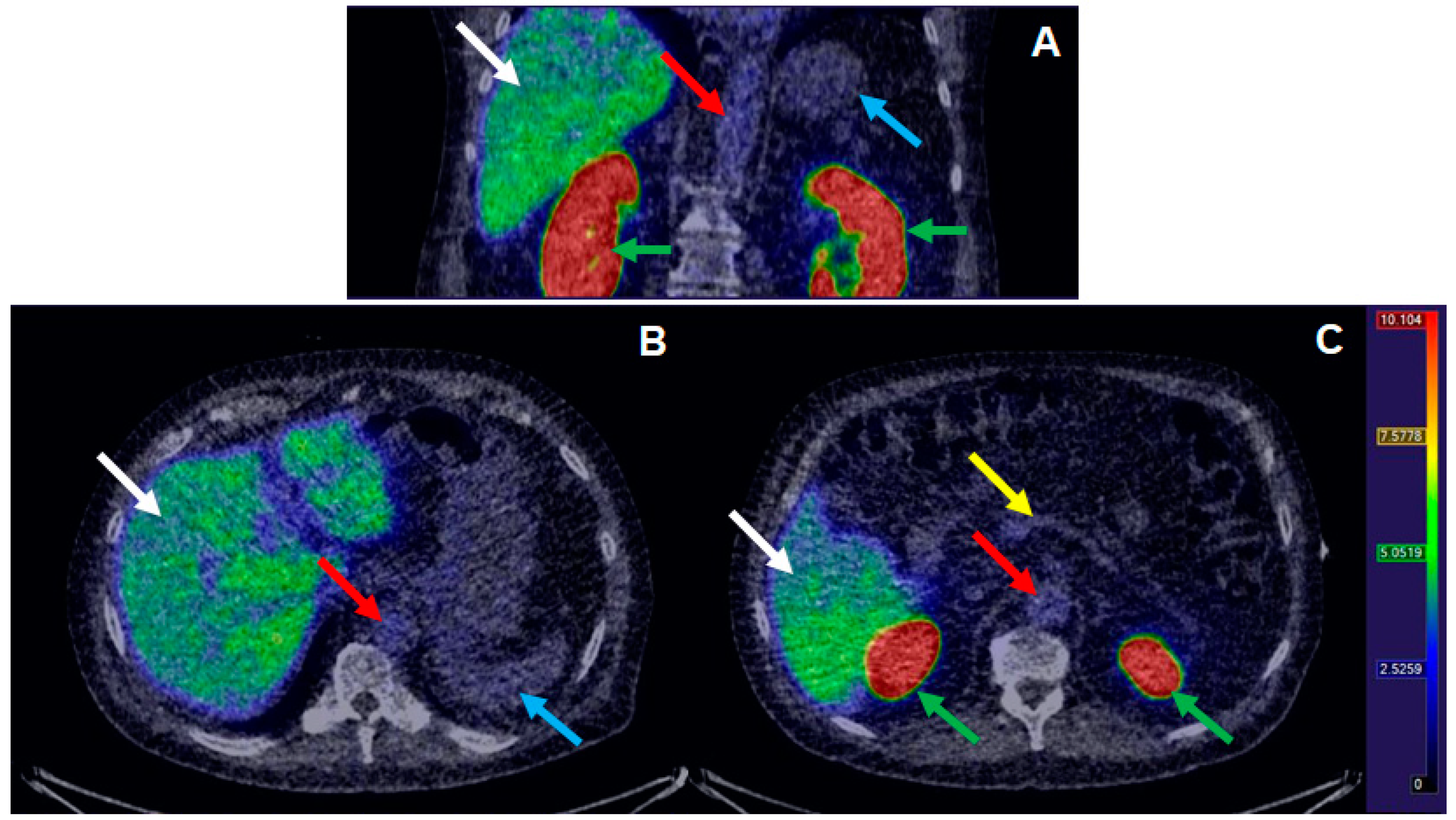
3.3. Clinical Examination
4. Discussion
4.1. Automated Production of DO3A-VS-Cys40-Tuna-2
4.2. Quality Control and Clinical Use Aspects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimasi, J.A. Risks in new drug development: Approval success rates for investigational drugs. Clin. Pharmacol. Ther. 2001, 69, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Lappin, G.; Kuhnz, W.; Jochemsen, R.; Kneer, J.; Chaudhary, A.; Oosterhuis, B.; Drijfhout, W.J.; Rowland, M.; Garner, R.C. Use of microdosing to predict pharmacokinetics at the therapeutic dose: Experience with 5 drugs. Clin. Pharmacol. Ther. 2006, 80, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.C.; Lappin, G. The phase 0 microdosing concept. Br. J. Clin. Pharmacol. 2006, 61, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M.; Grahnen, A.; Langstrom, B. Positron emission tomography microdosing: A new concept with application in tracer and early clinical drug development. Eur. J. Clin. Pharmacol. 2003, 59, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, S.; Schellens, J.H.M. The impact of FDA and EMEA guidelines on drug development in relation to Phase 0 trials. Br. J. Cancer 2007, 97, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Mills, G. The exploratory IND. J. Nucl. Med. 2008, 49, 45N–47N. [Google Scholar] [PubMed]
- Hesse, B.; Vinberg, N.; Berthelsen, A.K.; Ballinger, J.R. Adverse events in nuclear medicine—cause for concern? Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 782–785. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Diabetes; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Brandt, S.J.; Clemmensen, C.; Muller, T.D.; DiMarchi, R.D.; Tschop, M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 2017, 60, 1851–1861. [Google Scholar] [CrossRef]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Tillner, J.; Posch, M.G.; Wagner, F.; Teichert, L.; Hijazi, Y.; Einig, C.; Keil, S.; Haack, T.; Wagner, M.; Bossart, M.; et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes. Metab. 2019, 21, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Haack, T.; Bossart, M.; Evers, A.; Laitinen, I.; Larsen, P.; Plettenburg, O.; Johansson, L.; Pierrou, S.; Wagner, M.; et al. First-in-class positron emission tomography tracer for the glucagon receptor. EJNMMI Res. 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Haack, T.; Lorenz, M.; Bossart, M.; Elvert, R.; Henkel, B.; Stengelin, S.; Kurz, M.; Glien, M.; Dudda, A.; et al. Design of Novel Exendin-Based Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists. J. Med. Chem. 2017, 60, 4293–4303. [Google Scholar] [PubMed]
- Gallium (68Ga) edotreotide injection. Eur. Pharm. 2014, 1062–1064.
- Velikyan, I.; Rosenstrom, U.; Eriksson, O. Fully automated GMP production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 for clinical use. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 111–125. [Google Scholar] [PubMed]
- Eriksson, O.; Velikyan, I.; Haack, T.; Bossart, M.; Evers, A.; Laitinen, I.; Larsen, P.J.; Plettenburg, O.; Takano, A.; Halldin, C.; et al. Assessment of glucagon receptor occupancy by Positron Emission Tomography in non-human primates. Sci. Rep. 2019, 9, 14960. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical development. Theranostics 2014, 4, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Beyer, G.J.; Langstrom, B. Microwave-supported preparation of 68Ga-bioconjugates with high specific radioactivity. Bioconjug. Chem. 2004, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]

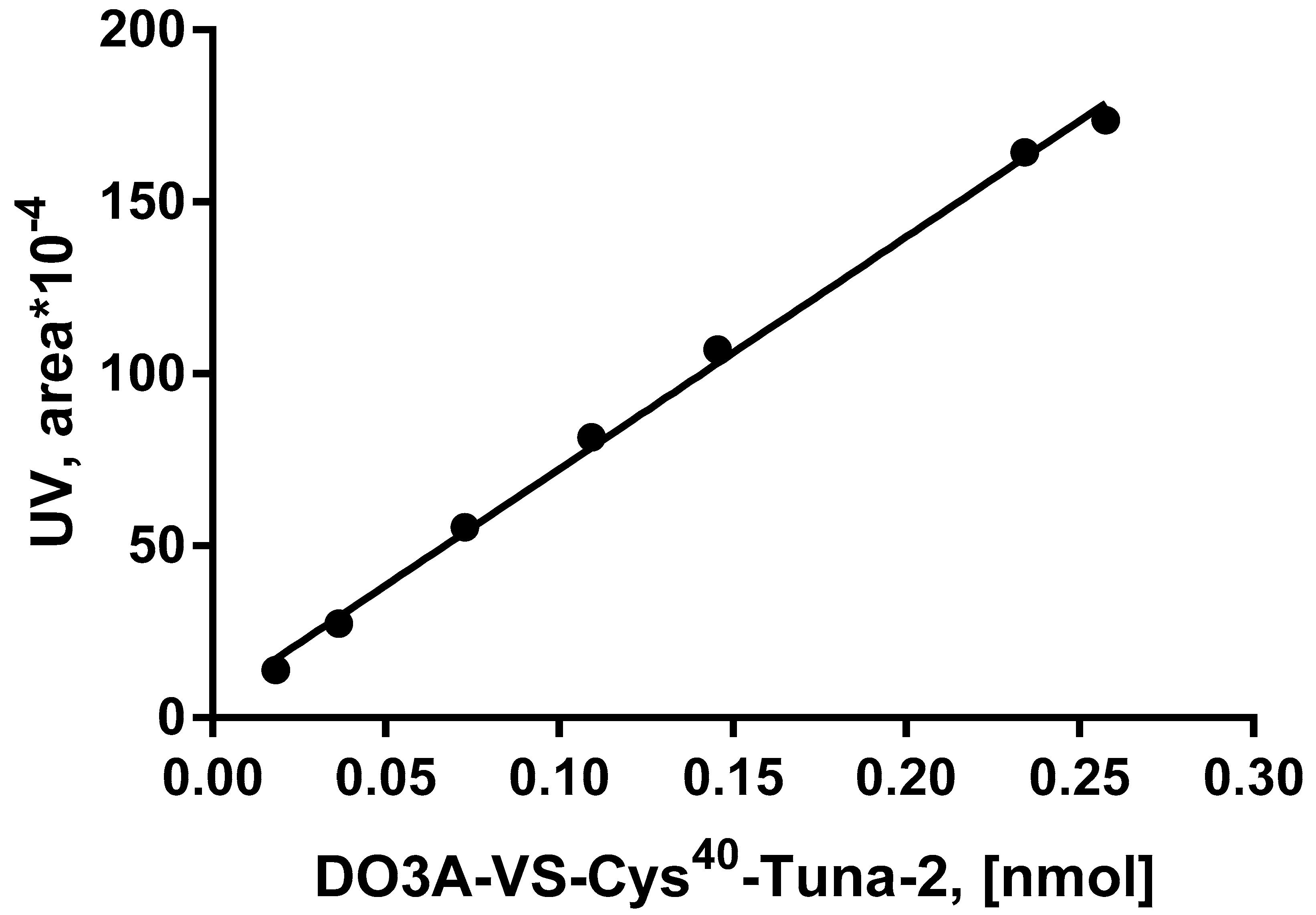
| Test Parameter | Test Method | Acceptance Criteria | GCG17008 | GCG17009 | GCG17010 |
|---|---|---|---|---|---|
| Radiochemical purity | HPLC (retention time) | ≥90% no impurity > 5% | 98.83 | 99.88 | 99.10 |
| pH | pH paper | 4.0–8.0 | 6 | 6 | 6 |
| System suitability test (SST) | HPLC (retention time) | SST corresponds to reference chromatogram | 3.69 | 4.38 | 4.38 |
| Identity | HPLC (retention time) | Agreement between radioactivity and SST UV signals | 3.74 | 4.41 | 4.42 |
| Chemical Purity | HPLC | Absence of unknown UV signals | Absent | Absent | Absent |
| Ethanol | GC chromatography | ≤10% | 5.1 | 4.9 | 4.8 |
| Filter-Integrity | Pressure test | * ≥ 1 bar; ** > 3.45 bar | 1.75 | >3.45 | 2.0 |
| Sterility | Absence of anaerobe or aerobe bacterial growth | Absent | Absent | Absent | |
| Endotoxins | LaL | ≤0.25 EU/mL | ≤0.25 EU/mL | ≤0.25 EU/mL | ≤0.25 EU/mL |
| 68Ge-Break through | Radioactivity measurement | <0.001% | 0.8 × 10−5 | 0.4 × 10−5 | 0.1 × 10−5 |
| Radionuclide Purity | >99.9% | >99.9999 | >99.9999 | >99.999 | |
| Stability | HPLC | ≥90%, no impurity > 5% within 120 min | 97.51 | 98.89 | 97.26 |
| Radioactivity Concentration | 5–500 MBq/mL | 60 | 65 | 62 | |
| Radio Activity | 50–1000 MBq | 350 | 384 | 350 | |
| Volume | Syringe or balance | 5–7 mL | 5.8 | 5.9 | 5.64 |
| Color | Visual | Colorless | Colorless | Colorless | Colorless |
| Test | Acceptance Criteria | [68Ga]Ga DO3A-VS-Cys40-Tuna-2 |
|---|---|---|
| Radiochemical purity | >90%; no unknown impurity corresponds to > 5% | 99.3 ± 0.5 |
| pH | 4–8.5 | 6.0 |
| Radioactivity concentration | 5–100 MBq/mL | 62.5 ± 2.4 |
| Radioactivity | 50–500 MBq | 361 ± 20 |
| Volume | 2–10 mL | 5.78 ± 0.13 |
| Color | colorless | colorless |
| Radionuclidic purity | >99.9% | 99.99999 ± 0.000006 |
| 68Ge breakthrough | <0.001% | 0.000043 ± 0.000004 |
| Stability | RCP ** >91% within 120 min | 97.9 ± 0.9 |
| Ethanol content | <10% | 4.9 ± 0.2 |
| Description | Acceptance Criteria | Result |
|---|---|---|
| Linearity—UV detector Pearson correlation coefficient (R2) | >0.99 | >0.999 |
| Precision as repeatability—UV (Relative standard deviation) | ≤5% | 4 (n = 6) |
| Specificity—Radio detector (Relative standard deviation) | ≤5% | 1.0 (n = 6) |
| Column recovery ([68Ga]Ga- DO3A-VS-Cys40-Tuna-2 spiked with [68Ga]GaCl3) | ≥95% | >97 |
| Column recovery ([68Ga]Ga- DO3A-VS-Cys40-Tuna-2) | ≥95% | >99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, M.; Doverfjord, J.G.; Tillner, J.; Antoni, G.; Haack, T.; Bossart, M.; Laitinen, I.; Johansson, L.; Pierrou, S.; Eriksson, O.; et al. Automated GMP-Compliant Production of [68Ga]Ga-DO3A-Tuna-2 for PET Microdosing Studies of the Glucagon Receptor in Humans. Pharmaceuticals 2020, 13, 176. https://doi.org/10.3390/ph13080176
Wagner M, Doverfjord JG, Tillner J, Antoni G, Haack T, Bossart M, Laitinen I, Johansson L, Pierrou S, Eriksson O, et al. Automated GMP-Compliant Production of [68Ga]Ga-DO3A-Tuna-2 for PET Microdosing Studies of the Glucagon Receptor in Humans. Pharmaceuticals. 2020; 13(8):176. https://doi.org/10.3390/ph13080176
Chicago/Turabian StyleWagner, Michael, Johan G. Doverfjord, Joachim Tillner, Gunnar Antoni, Torsten Haack, Martin Bossart, Iina Laitinen, Lars Johansson, Stefan Pierrou, Olof Eriksson, and et al. 2020. "Automated GMP-Compliant Production of [68Ga]Ga-DO3A-Tuna-2 for PET Microdosing Studies of the Glucagon Receptor in Humans" Pharmaceuticals 13, no. 8: 176. https://doi.org/10.3390/ph13080176
APA StyleWagner, M., Doverfjord, J. G., Tillner, J., Antoni, G., Haack, T., Bossart, M., Laitinen, I., Johansson, L., Pierrou, S., Eriksson, O., & Velikyan, I. (2020). Automated GMP-Compliant Production of [68Ga]Ga-DO3A-Tuna-2 for PET Microdosing Studies of the Glucagon Receptor in Humans. Pharmaceuticals, 13(8), 176. https://doi.org/10.3390/ph13080176






