Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches
Abstract
1. Introduction
2. Results
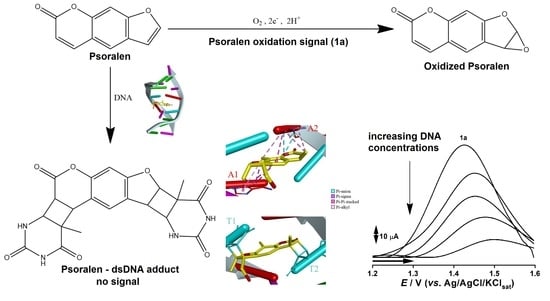
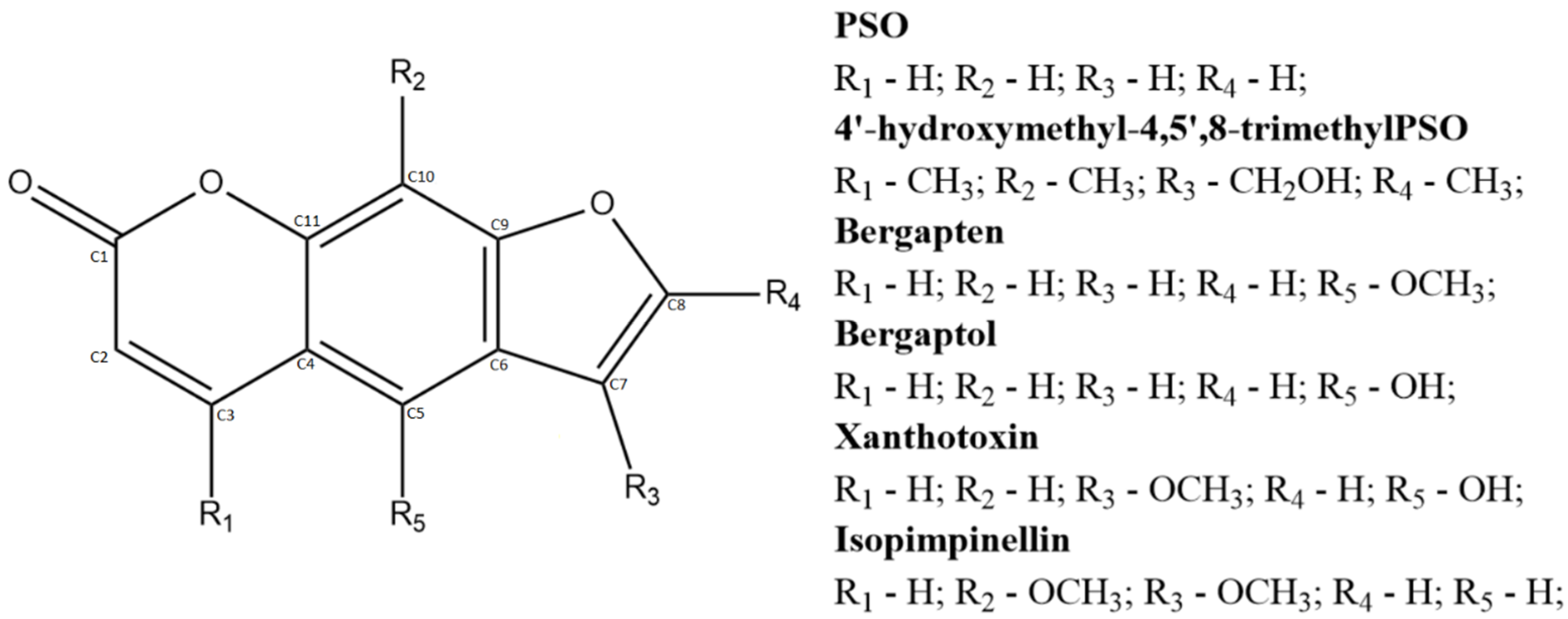
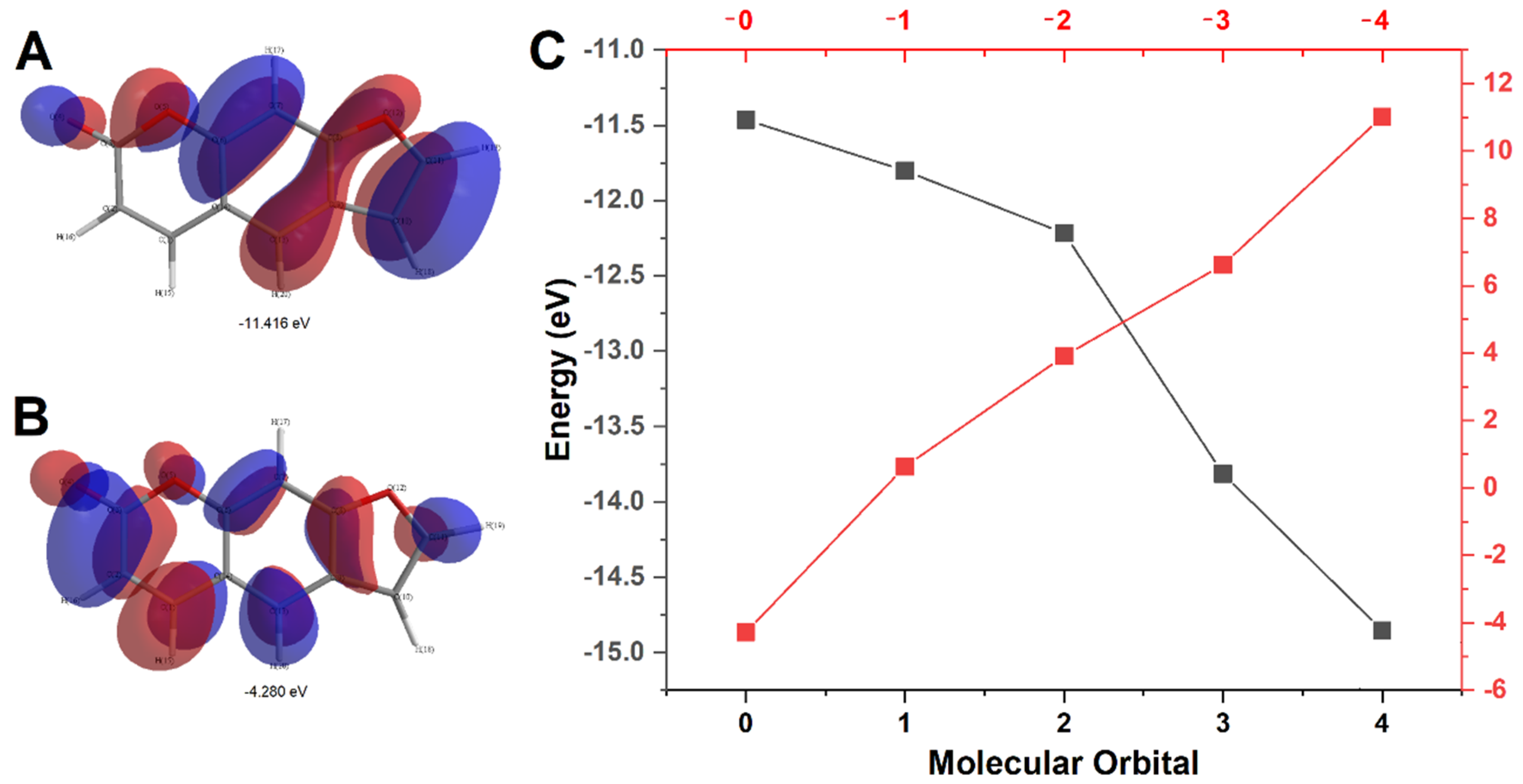
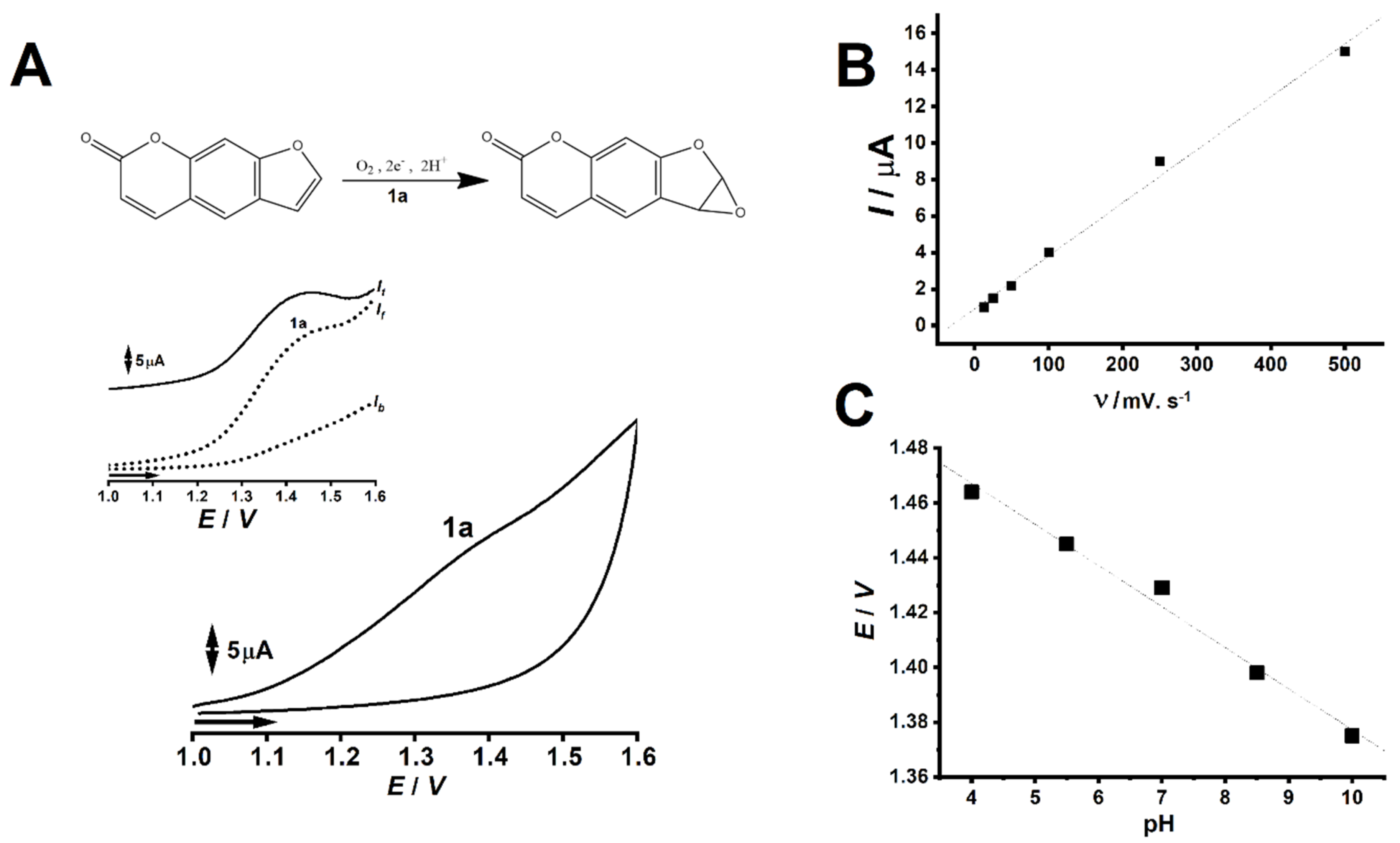
2.1. PSO Redox Behavior without DNA
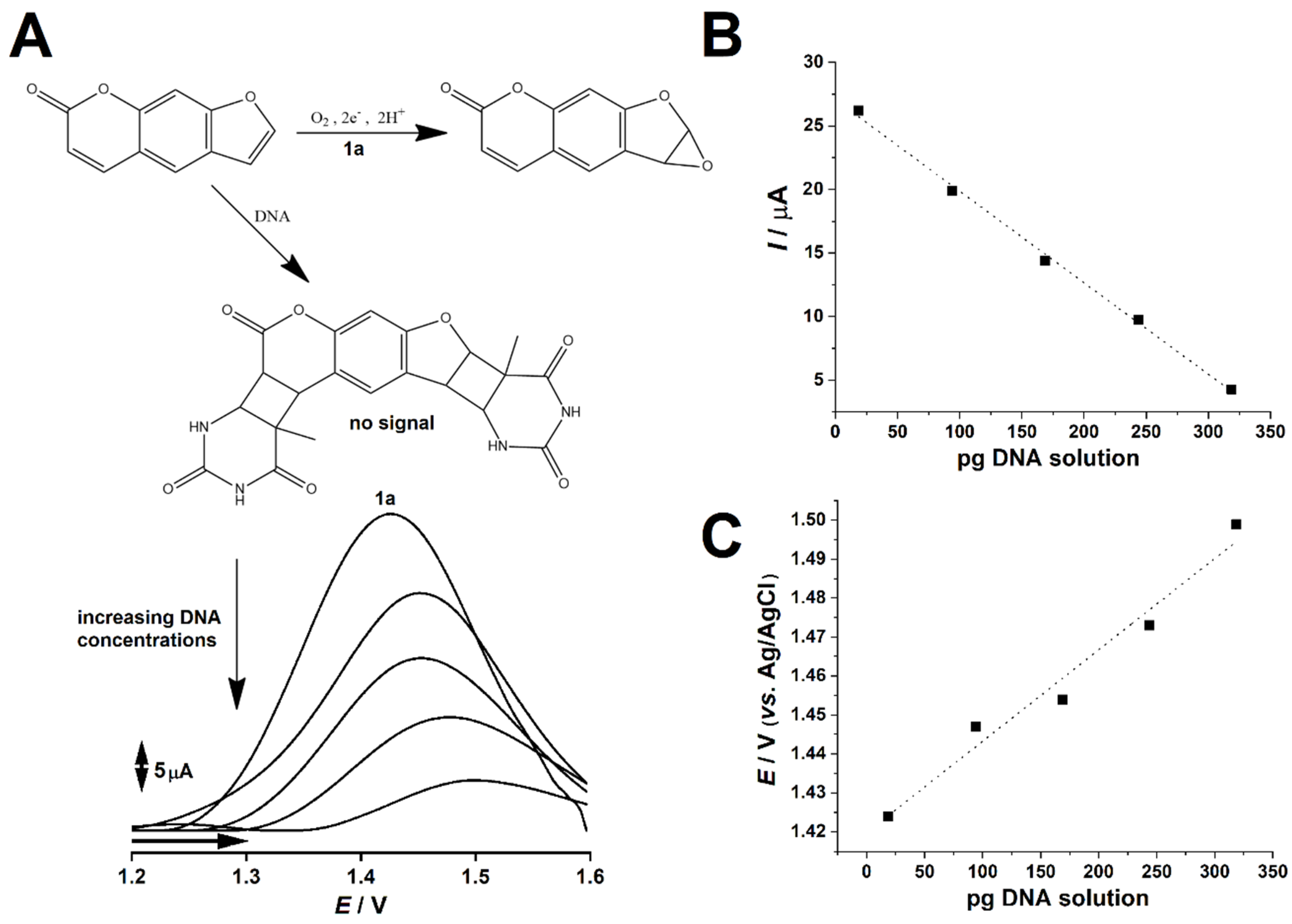
2.2. PSO-dsDNA Redox Study
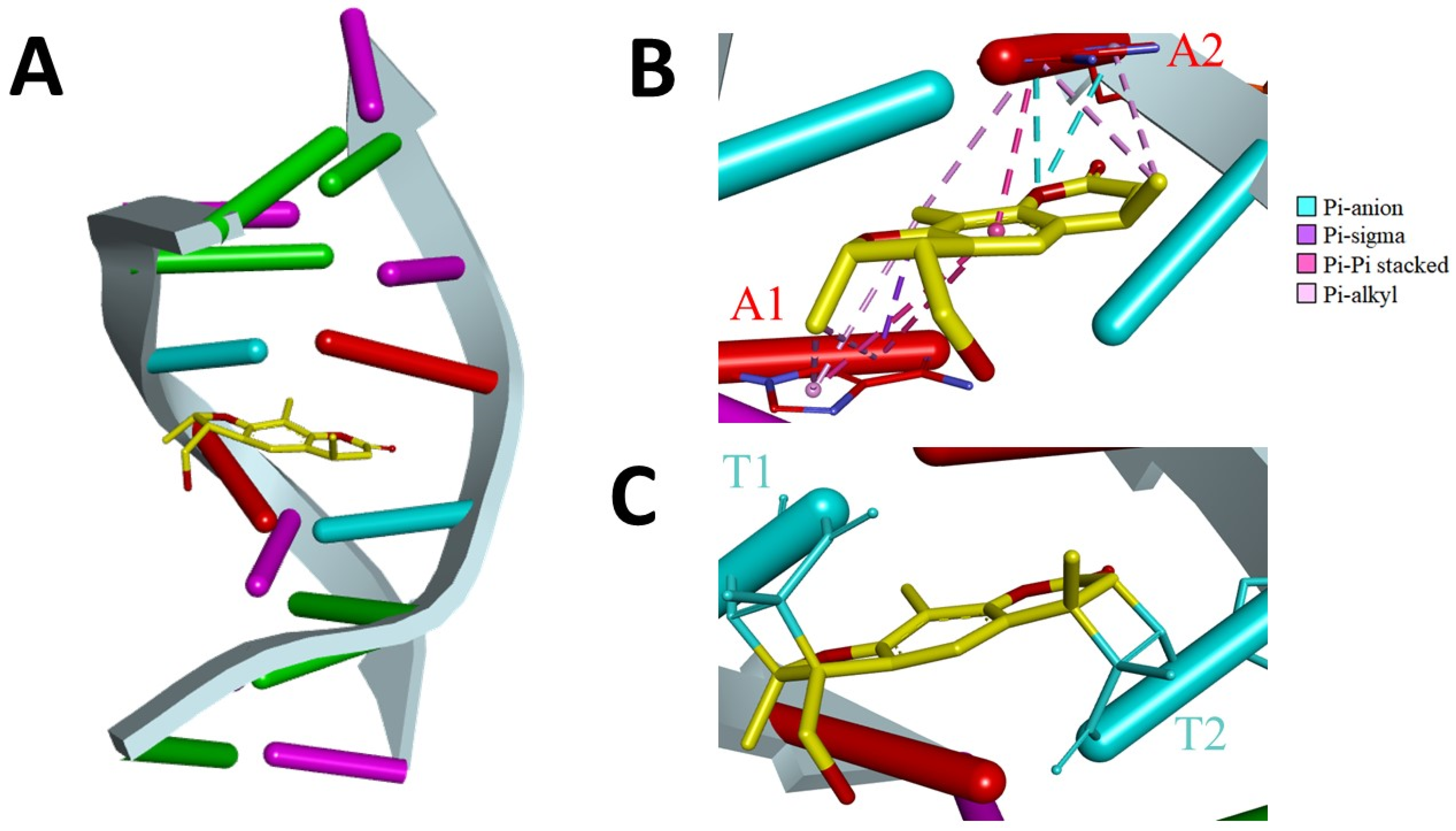
2.3. Description of PSO-dsDNA Interactions
3. Discussion
4. Materials and Methods
4.1. Solutions and Reagents
4.2. Electrochemical Assays
4.3. PSO Redox Behavior without DNA
4.4. PSO-dsDNA Redox Study
4.5. Description of PSO Oxidation and PSO-dsDNA Interactions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer-lipid hybrid nanoparticles: A novel drug delivery system for enhancing the activity of Psoralen against breast cancer. Int. J. Pharm. 2019, 561, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, Z.; Ye, Y.; Han, X.; Song, X.; Liu, S. Psoralen inhibits bone metastasis of breast cancer in mice. Fitoterapia 2013, 91, 205–210. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Salvador, J.M.; Pérez-Ferriols, A.; Alegre de Miquel, V.; Saneleuterio Temporal, M.; Vilata Corell, J.J. Incidence of non-melanoma skin cancer in patients treated with psoralen and ultraviolet A therapy. Med. Clínica 2019, 152, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Mahira, S.; Kommineni, N.; Doppalapudi, S.; Khan, W. Edge activated ultradeformable liposomes of psoralen and its derivatives: Development and comparative evaluation for vitiligo therapy. J. Drug Deliv. Sci. Technol. 2019, 52, 83–95. [Google Scholar] [CrossRef]
- Stanjek, V.; Piel, J.; Boland, W. Biosynthesis of furanocoumarins: Mevalonate-independent prenylation of umbelliferone in Apium graveolens (Apiaceae). Phytochemistry 1999, 50, 1141–1146. [Google Scholar] [CrossRef]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef]
- De Morais, M.C.; de Almeida, P.H.G.; Ferreira, N.L.O.; Arruda, R.L.; Borges, L.L.; de Freitas, O.; da Conceição, E.C. Validation of a photostability indicating method for quantification of furanocoumarins from Brosimum gaudichaudii soft extract. Rev. Bras. Farmacogn. 2018, 28, 118–123. [Google Scholar] [CrossRef]
- Ji, L.; Lu, D.; Cao, J.; Zheng, L.; Peng, Y.; Zheng, J. Psoralen, a mechanism-based inactivator of CYP2B6. Chem. Biol. Interact. 2015, 240, 346–352. [Google Scholar] [CrossRef]
- Laskin, J.D. Cellular and molecular mechanisms in photochemical sensitization: Studies on the mechanism of action of psoralens. Food Chem. Toxicol. 1994, 32, 119–127. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Wang, L. Probing the binding mode of psoralen to calf thymus DNA. Int. J. Biol. Macromol. 2014, 67, 228–237. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, T.M.; Koehler, G. Interaction of psoralens with DNA-bases (I). An ab initio quantum chemical, density functional theory and second-order Møller–Plesset perturbational study. J. Mol. Struct. THEOCHEM 2007, 808, 97–109. [Google Scholar] [CrossRef]
- Deepa, P.; Kolandaivel, P.; Senthilkumar, K. Theoretical investigation of interaction between psoralen and altretamine with stacked DNA base pairs. Mater. Sci. Eng. C 2012, 32, 423–431. [Google Scholar] [CrossRef]
- Evison, B.J.; Actis, M.L.; Fujii, N. A clickable psoralen to directly quantify DNA interstrand crosslinking and repair. Bioorg. Med. Chem. 2016, 24, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.B.; Kawano, D.F.; Carvalho, I.; da Conceição, E.C.; de Freitas, O.; da Silva, C.H.T.P. Psoralen and bergapten: In silico metabolism and toxicophoric analysis of drugs used to treat vitiligo. J. Pharm. Pharm. Sci. 2009, 12, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lozano Untiveros, K.; da Silva, E.G.; de Abreu, F.C.; da Silva-Júnior, E.F.; de Araújo-Junior, J.X.; Mendoça de Aquino, T.; Armas, S.M.; de Moura, R.O.; Mendonça-Junior, F.J.B.; Serafim, V.L.; et al. An electrochemical biosensor based on Hairpin-DNA modified gold electrode for detection of DNA damage by a hybrid cancer drug intercalation. Biosens. Bioelectron. 2019, 133, 160–168. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Shipovskov, S.; Ferapontova, E.E. Electrocatalysis of ferricyanide reduction mediated by electron transfer through the DNA duplex: Kinetic analysis by thin layer voltammetry. Electrochim. Acta 2019, 318, 703–710. [Google Scholar] [CrossRef]
- Nigar, A.; Shabbir, M.; Akhter, Z.; Sabahat, S.; Fatmi, M.Q.; Bolte, M.; Ahmad, I.; Janjua, N.K.; Mehmood, S. Synthesis, characterization, docking and electrochemical studies of nitroaromatic amides. J. Mol. Struct. 2019, 1176, 791–797. [Google Scholar] [CrossRef]
- Rodrigues, E.S.B.; de Macêdo, I.Y.L.; da Silva Lima, L.L.; Thomaz, D.V.; da Cunha, C.E.P.; Teles de Oliveira, M.; Ballaminut, N.; Alecrim, M.F.; Ferreira de Carvalho, M.; Isecke, B.G.; et al. Electrochemical Characterization of Central Action Tricyclic Drugs by Voltammetric Techniques and Density Functional Theory Calculations. Pharmaceuticals 2019, 12, 116. [Google Scholar] [CrossRef]
- Yates, K. Hückel Molecular Orbital Theory; Academic Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Zinola, C.F. Density functional theory. In Electrocatalysis: Computational, Experimental, and Industrial Aspects; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420045451. [Google Scholar]
- Spielmann, H.P.; Dwyer, T.J.; Hearst, J.E.; Wemmer, D.E. Solution Structures of Psoralen Monoadducted and Cross-Linked DNA Oligomers by NMR Spectroscopy and Restrained Molecular Dynamics. Biochemistry 2002, 34, 12937–12953. [Google Scholar] [CrossRef]
- Lazzeretti, P. Assessment of aromaticity via molecular response properties. Phys. Chem. Chem. Phys. 2004, 35. [Google Scholar] [CrossRef]
- Matito, E.; Feixas, F.; Solà, M. Electron delocalization and aromaticity measures within the Hückel molecular orbital method. J. Mol. Struct. THEOCHEM 2007, 811, 3–11. [Google Scholar] [CrossRef]
- Thomaz, D.V.; Peixoto, L.F.; de Oliveira, T.S.; Fajemiroye, J.O.; da Silva Neri, H.F.; Xavier, C.H.; Costa, E.A.; Alcantara dos Santos, F.C.; de Souza Gil, E.; Ghedini, P.C. Antioxidant and neuroprotective properties of Eugenia dysenterica leaves. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Thomaz, D.V.; de Filho, A.M.A.; Macedo, I.Y.L.; Rodrigues, E.S.B.; de Gil, E.S. Predictive Modelling to Study the Electrochemical Behaviour of PdO, TiO2 and Perovskite-Type LaFeO3 Modified Carbon Paste Electrodes. Path Sci. 2019, 5, 4001–4007. [Google Scholar] [CrossRef]
- Gonçalves, A.-M.; Mathieu, C.; Herlem, M.; Etcheberry, A. The pH response of the InP/liquid ammonia interface at 223 K: A pure nernstian behavior. Electrochim. Acta 2010, 55, 7413–7418. [Google Scholar] [CrossRef]
- Wang, L.H.; Jiang, S.Y.; Lan, Y.Z. Voltammetric behavior of coumarins and psoralens at a carbon fiber ultramicroelectrode and their determination in citrus essential oils. Bull. Electrochem. 2004, 20, 445–451. [Google Scholar]
- Rodenko, I.N.; Osipov, A.N.; Lysenko, E.P.; Potapenko, A.Y. Degradation of psoralen photo-oxidation products induced by ferrous ions. J. Photochem. Photobiol. B Biol. 1993, 19, 39–48. [Google Scholar] [CrossRef]
- Yang, A.; Chen, J.; Ma, Y.; Wang, L.; Fan, Y.; He, X. Studies on the metabolites difference of psoralen/isopsoralen in human and six mammalian liver microsomes in vitro by UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 141, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, C.E.P.; Rodrigues, E.S.B.; Fernandes Alecrim, M.; Thomaz, D.V.; Macêdo, I.Y.L.; Garcia, L.F.; de Oliveira Neto, J.R.; Moreno, E.K.G.; Ballaminut, N.; de Souza Gil, E. Voltammetric Evaluation of Diclofenac Tablets Samples through Carbon Black-Based Electrodes. Pharmaceuticals 2019, 12, 83. [Google Scholar] [CrossRef]
- Leite, K.C.D.S.; Garcia, L.F.; Lobón, G.S.; Thomaz, D.V.; Moreno, E.K.G.; Carvalho, M.F.D.; Rocha, M.L.; Santos, W.T.P.D.; Gil, E.D.S. Antioxidant activity evaluation of dried herbal extracts: An electroanalytical approach. Brazilian J. Pharmacogn. 2018, 28, 325–332. [Google Scholar] [CrossRef]
- Thomaz, D.V. Electrochemical Study of Commercial Black Tea Samples. Int. J. Electrochem. Sci. 2018, 13, 5433–5439. [Google Scholar] [CrossRef]
- Thomaz, D.V. The Potential of Nanostructured Electrode Materials in Analytical Sciences: A Short Commentary. Mater. Sci. 2019, 1, 1–3. [Google Scholar]
- Alévêque, O.; Blanchard, P.-Y.; Gautier, C.; Dias, M.; Breton, T.; Levillain, E. Electroactive self-assembled monolayers: Laviron’s interaction model extended to non-random distribution of redox centers. Electrochem. Commun. 2010, 12, 1462–1466. [Google Scholar] [CrossRef]
- Inamdar, P.R.; Sheela, A. Spectroscopic investigations on partial intercalative binding behaviour of terpyridine based copper(II) complexes with DNA. J. Photochem. Photobiol. B Biol. 2016, 159, 133–141. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.S. Label-free quantitative detection of nucleic acids based on surface-immobilized DNA intercalators. Sens. Actuators B Chem. 2017, 241, 1310–1315. [Google Scholar] [CrossRef]
- Gnapareddy, B.; Deore, P.M.; Dugasani, S.R.; Kim, S.; Park, S.H. Synthesis and characterization of doxorubicin hydrochloride drug molecule-intercalated DNA nanostructures. Curr. Appl. Phys. 2018, 18, 1294–1299. [Google Scholar] [CrossRef]
- Jafari, F.; Baghayi, H.; Lavaee, P.; Hadizadeh, F.; Soltani, F.; Moallemzadeh, H.; Mirzaei, S.; Aboutorabzadeh, S.M.; Ghodsi, R. Design, synthesis and biological evaluation of novel benzo- and tetrahydrobenzo-[h]quinoline derivatives as potential DNA-intercalating antitumor agents. Eur. J. Med. Chem. 2019, 164, 292–303. [Google Scholar] [CrossRef]
- Eichman, B.F.; Mooers, B.H.; Alberti, M.; Hearst, J.E.; Ho, P.S. The crystal structures of psoralen cross-linked DNAs: Drug-dependent formation of Holliday junctions. J. Mol. Biol. 2001, 308, 15–26. [Google Scholar] [CrossRef]
- Sastry, S.S.; Spielmann, H.P.; Dwyer, T.J.; Wemmer, D.E.; Hearst, J.E. Recent advances in the synthesis and structure determination of site specifically psoralen-modified DNA oligonucleotides. J. Photochem. Photobiol. B Biol. 1992, 14, 65–79. [Google Scholar] [CrossRef]
- Thomaz, D.V. Flavonoid chemistry and neuroprotection. Front. Drug Chem. Clin. Res. 2020, 3, 1–3. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomaz, D.V.; de Oliveira, M.G.; Rodrigues, E.S.B.; da Silva, V.B.; dos Santos, P.A. Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches. Pharmaceuticals 2020, 13, 108. https://doi.org/10.3390/ph13060108
Thomaz DV, de Oliveira MG, Rodrigues ESB, da Silva VB, dos Santos PA. Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches. Pharmaceuticals. 2020; 13(6):108. https://doi.org/10.3390/ph13060108
Chicago/Turabian StyleThomaz, Douglas Vieira, Matheus Gabriel de Oliveira, Edson Silvio Batista Rodrigues, Vinicius Barreto da Silva, and Pierre Alexandre dos Santos. 2020. "Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches" Pharmaceuticals 13, no. 6: 108. https://doi.org/10.3390/ph13060108
APA StyleThomaz, D. V., de Oliveira, M. G., Rodrigues, E. S. B., da Silva, V. B., & dos Santos, P. A. (2020). Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches. Pharmaceuticals, 13(6), 108. https://doi.org/10.3390/ph13060108