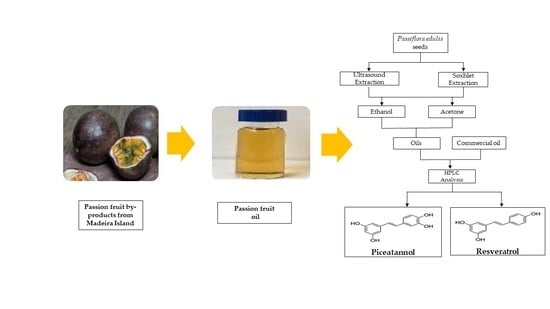
Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products
Abstract
1. Introduction
2. Results and Discussion
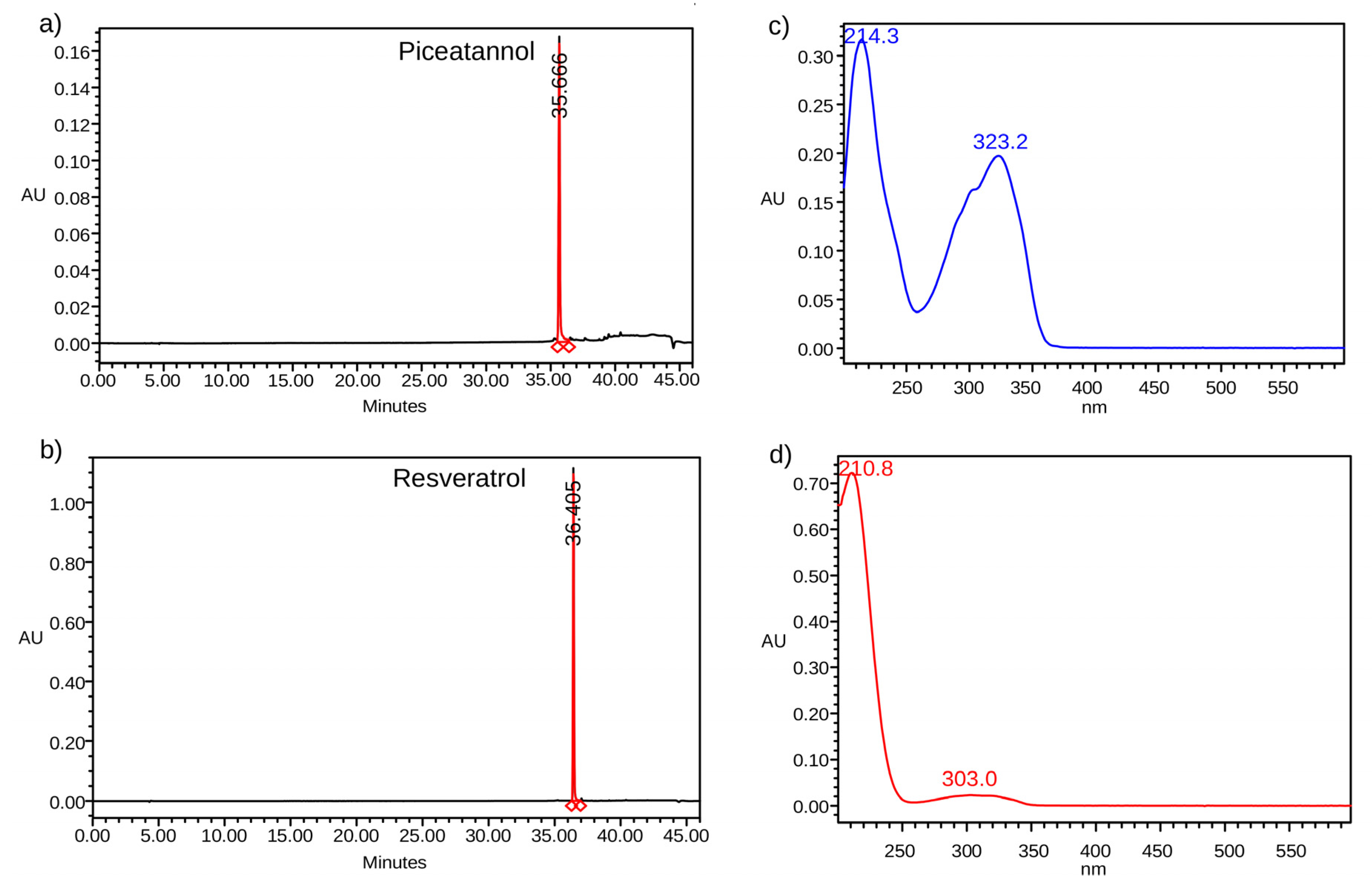
2.1. Standard Samples
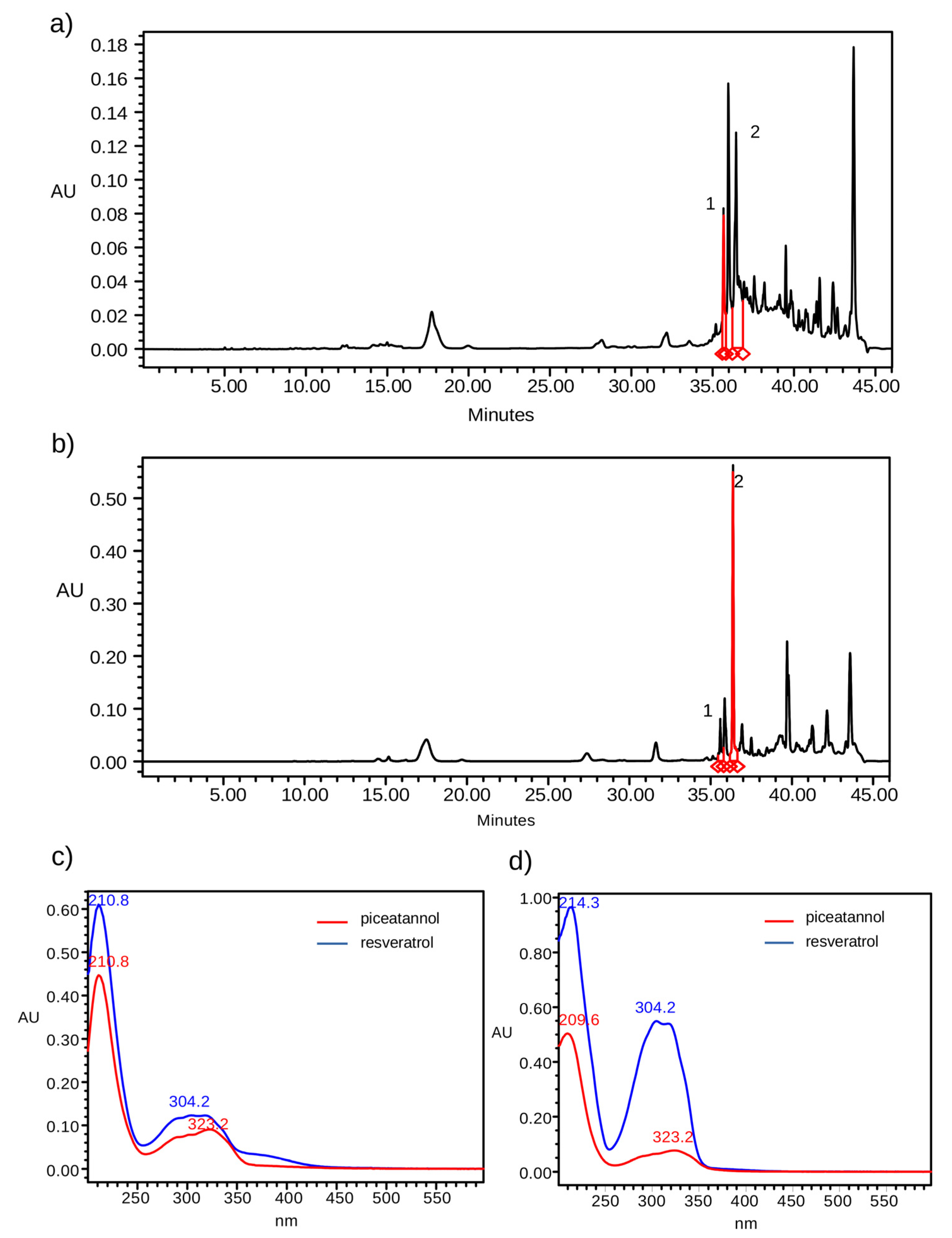
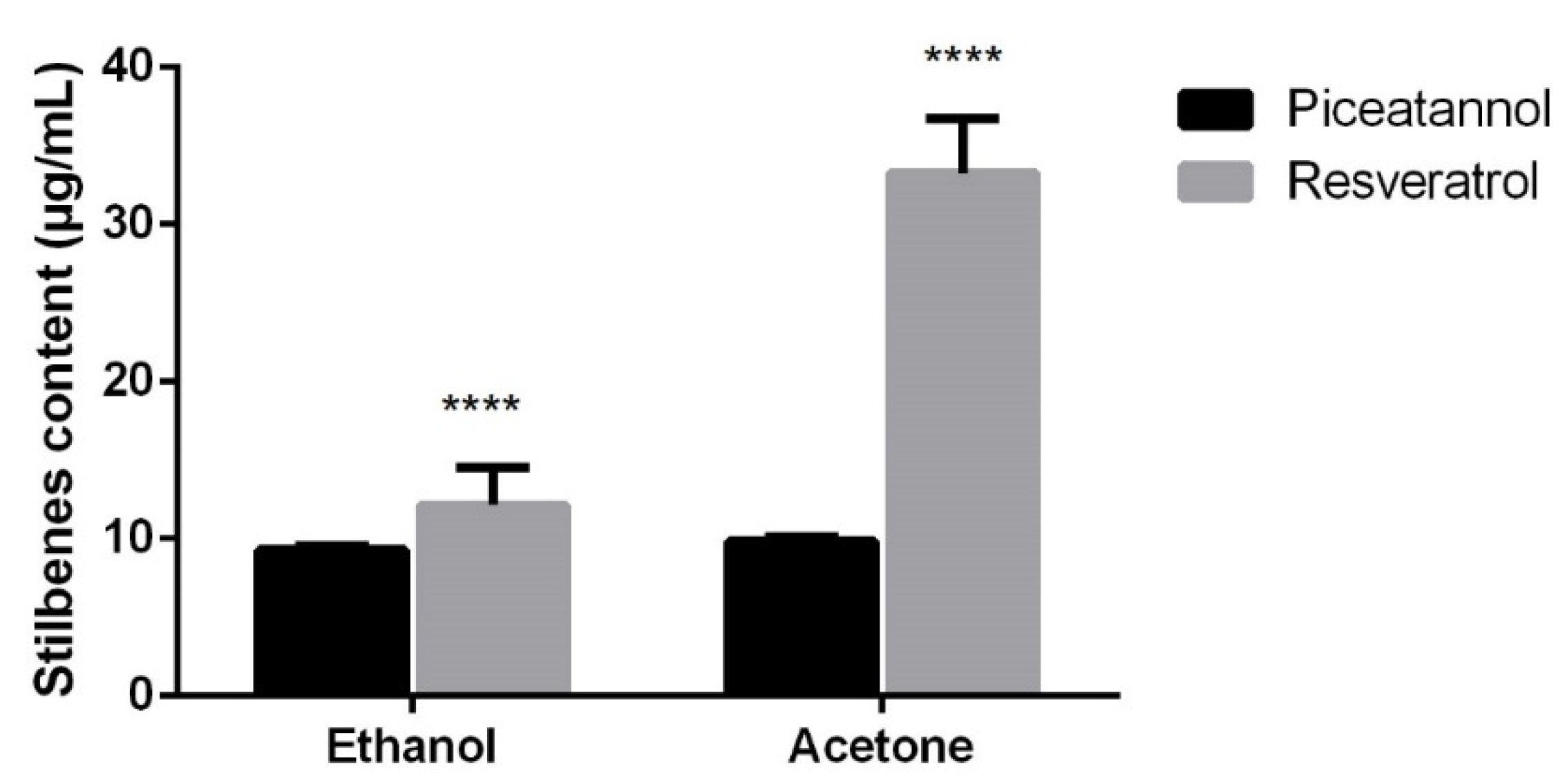
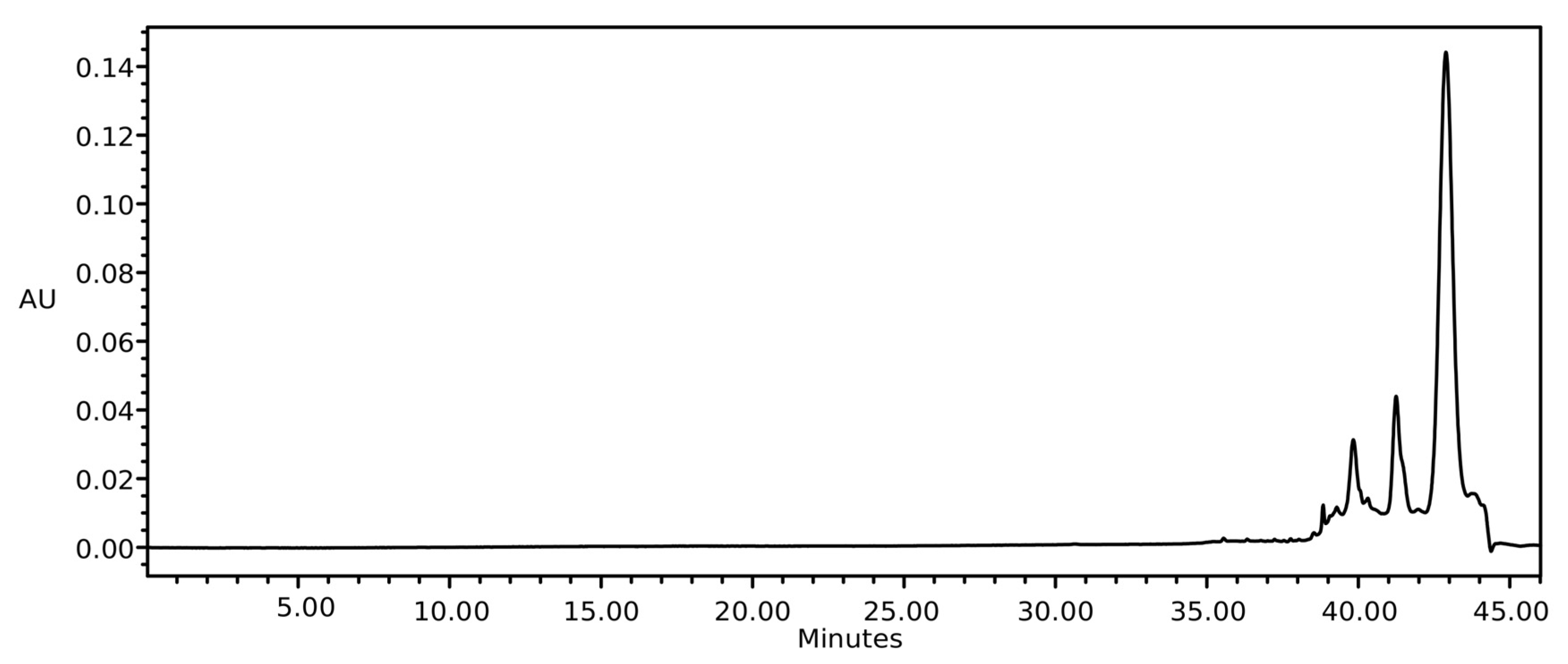
2.2. Soxhlet Extraction
2.3. Ultrasound Extraction
2.4. Commercial Oil
3. Materials and Methods
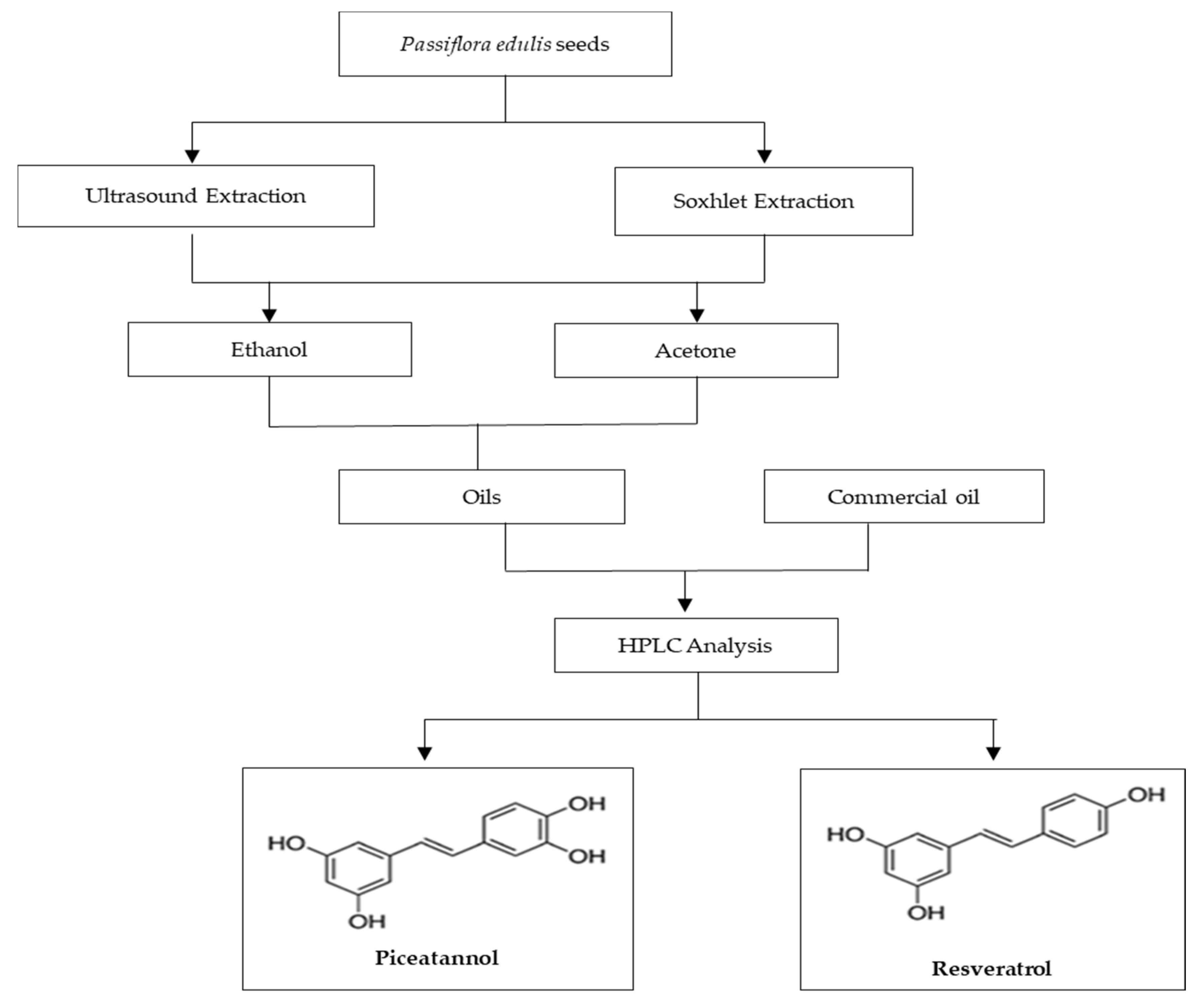
3.1. Samples Preparation
3.2. Chemicals and Standards
3.3. Methods
Reverse Phase High-Performance Liquid Chromatography (RP-HPLC)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Viganó, J.; Martinez, J. Trends for the Application of Passion Fruit Industrial By-products: A Review on the Chemical Composition and Extraction Techniques of Phytochemicals. Food Public Heal. 2015, 5, 164–173. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Raffo, A.; Giovannini, A.; Kiefer, J. Passion Fruit (Passiflora spp.) Seed Oil. In Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019; pp. 577–603. [Google Scholar]
- De Toledo, N.M.V.; De Camargo, A.C.; Ramos, P.B.M.; Button, D.C.; Granato, D.; Canniatti-Brazaca, S.G. Potentials and pitfalls on the use of passion fruit by-products in drinkable yogurt: Physicochemical, technological, microbiological, and sensory aspects. Beverages 2018, 4, 47. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.H.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carriers and moisture scavengers. Sci. Total Environ. 2020, 706, 135747. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-food Waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Davantel De Barros, S.T.; Gimenes, M.L. The extraction of passion fruit oil with green solvents. J. Food Eng. 2013, 117, 458–463. [Google Scholar] [CrossRef]
- Krambeck, K.; Santos, D.; Oliveira, A.; Pintado, M.E.; Silva, J.B.; Sousa Lobo, J.M.; Amaral, M.H. Optimization of extraction parameters on the antioxidant activity of passion fruit waste. Acad. J. Med. Plants 2018, 6, 209–213. [Google Scholar]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Fan, G.; Tang, J.J.; Bhadauria, M.; Nirala, S.K.; Dai, F.; Zhou, B.; Li, Y.; Liu, Z.L. Resveratrol ameliorates carbon tetrachloride-induced acute liver injury in mice. Environ. Toxicol. Pharmacol. 2009, 28, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, C.W.; Tan, Y.H.; Foo, J.P.Y.; Wong, S.K.; Chan, H.T. Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J. Appl. Pharm. Sci. 2019, 9, 124–129. [Google Scholar]
- Aires, V.; Colin, D.J.; Doreau, A.; Di Pietro, A.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients 2019, 11, 2098. [Google Scholar] [CrossRef]
- Frazzi, R.; Guardi, M. Cellular and molecular targets of resveratrol on lymphoma and leukemia cells. Molecules 2017, 22, 1–15. [Google Scholar]
- Levenson, A.S. Metastasis-associated protein 1-mediated antitumor and anticancer activity of dietary stilbenes for prostate cancer chemoprevention and therapy. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.-M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Vasdekis, E.P.; Karkabounas, A.; Giannakopoulos, I.; Savvas, D.; Lekka, M.E. Screening of mushrooms bioactivity: piceatannol was identified as a bioactive ingredient in the order Cantharellales. Eur. Food Res. Technol. 2018, 244, 861–871. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Maruki-Uchida, H.; Morita, M.; Yonei, Y.; Sai, M. Effect of passion fruit seed extract rich in piceatannol on the skin of women: A randomized, placebo-controlled, double-blind trial. J. Nutr. Sci. Vitaminol. (Tokyo). 2018, 64, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, Y.J. Piceatannol Inhibits Melanogenesis by Its Antioxidative Actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Assessment of alcoholic distillates for the extraction of bioactive polyphenols from grapevine canes. Ind. Crops Prod. 2018, 111, 99–106. [Google Scholar] [CrossRef]
- Viganó, J.; Aguiar, A.C.; Moraes, D.R.; Jara, J.L.P.; Eberlin, M.N.; Cazarin, C.B.B.; Maróstica, M.R.; Martínez, J. Sequential high pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Res. Int. 2016, 85, 51–58. [Google Scholar] [CrossRef]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.-T.; Zhou, Z.; Rosen, R.T. Isolation and Identification of Stilbenes in Two Varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Ku, K.-L.; Chang, P.-S.; Cheng, Y.-C.; Lien, C.-Y. Production of Stilbenoids from the Callus of Arachis hypogaea: A Novel Source of the Anticancer Compound Piceatannol. J. Agric. Food Chem. 2005, 53, 3877–3881. [Google Scholar] [CrossRef]
- Beňová, B.; Adam, M.; Onderková, K.; Královský, J.; Krajíček, M. Analysis of selected stilbenes in Polygonum cuspidatum by HPLC coupled with CoulArray detection. J. Sep. Sci. 2008, 31, 2404–2409. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Fernández, M.J.; Oliva, J.; Tomás-Barberán, F.A. Postharvest UV-C-Irradiated Grapes as a Potential Source for Producing Stilbene-Enriched Red Wines. J. Agric. Food Chem. 2003, 51, 1208–1214. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.G.; Cao, Y.P.; Tian, Y.; Li, X.H. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Santos, D.T.; Cavalcanti, R.N.; Rostagno, M.A.; Queiroga, C.L.; Eberlin, M.N.; Meireles, M.A.A. Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels. Food Public Heal. 2013, 3, 12–20. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; dos Santos Lima, B.; de Souza Araújo, A.A.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans-Júnior, L.J.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.N.H.; Herent, M.-F.; Quetin-Leclercq, J.; Nguyen, T.B.T.; Rogez, H.; Larondelle, Y.; André, C.M. Piceatannol, a potent bioactive stilbene, as major phenolic component in Rhodomyrtus tomentosa. Food Chem. 2013, 138, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Baptista Silva, J.; Sousa Lobo, J.M.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals 2020, 13, 73. https://doi.org/10.3390/ph13040073
Krambeck K, Oliveira A, Santos D, Pintado MM, Baptista Silva J, Sousa Lobo JM, Amaral MH. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals. 2020; 13(4):73. https://doi.org/10.3390/ph13040073
Chicago/Turabian StyleKrambeck, Karolline, Ana Oliveira, Delfim Santos, Maria Manuela Pintado, João Baptista Silva, José Manuel Sousa Lobo, and Maria Helena Amaral. 2020. "Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products" Pharmaceuticals 13, no. 4: 73. https://doi.org/10.3390/ph13040073
APA StyleKrambeck, K., Oliveira, A., Santos, D., Pintado, M. M., Baptista Silva, J., Sousa Lobo, J. M., & Amaral, M. H. (2020). Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals, 13(4), 73. https://doi.org/10.3390/ph13040073