Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells
Abstract
1. Introduction
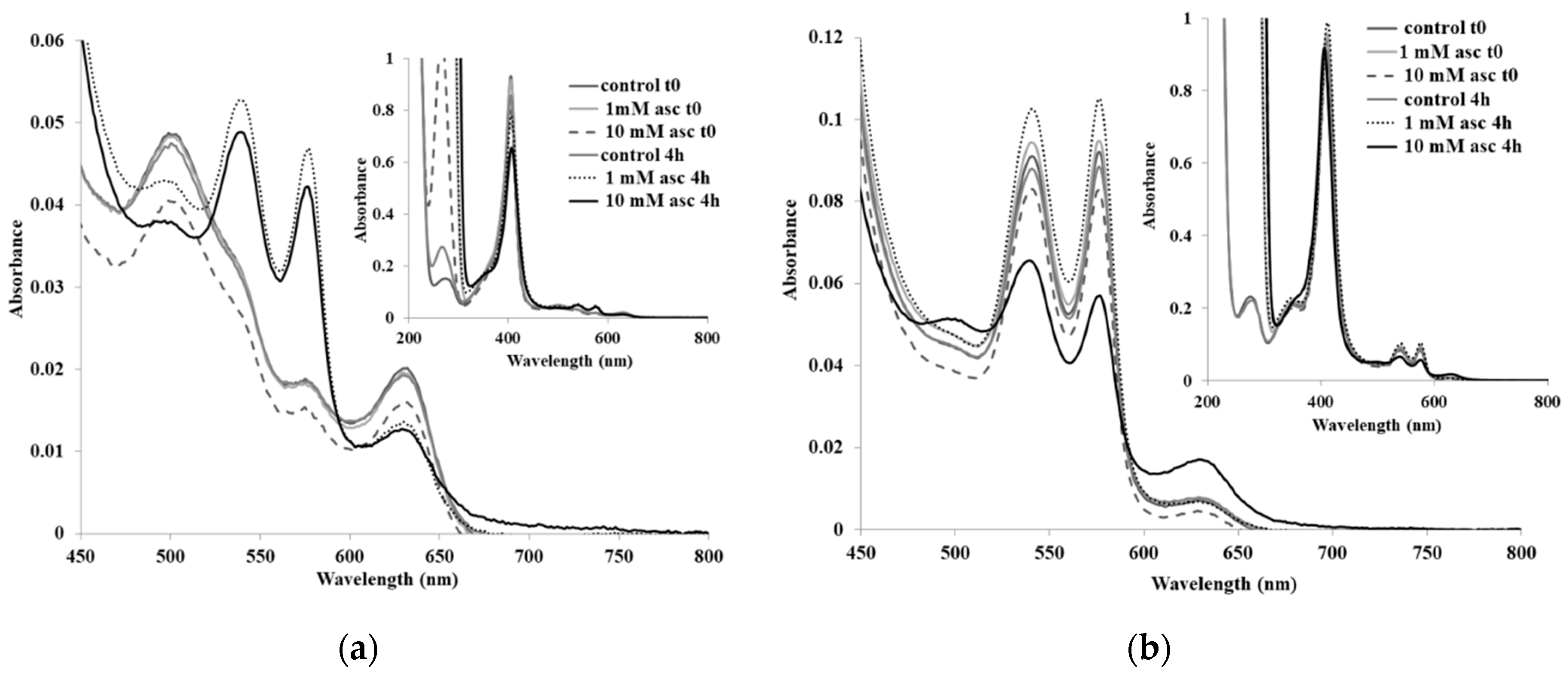
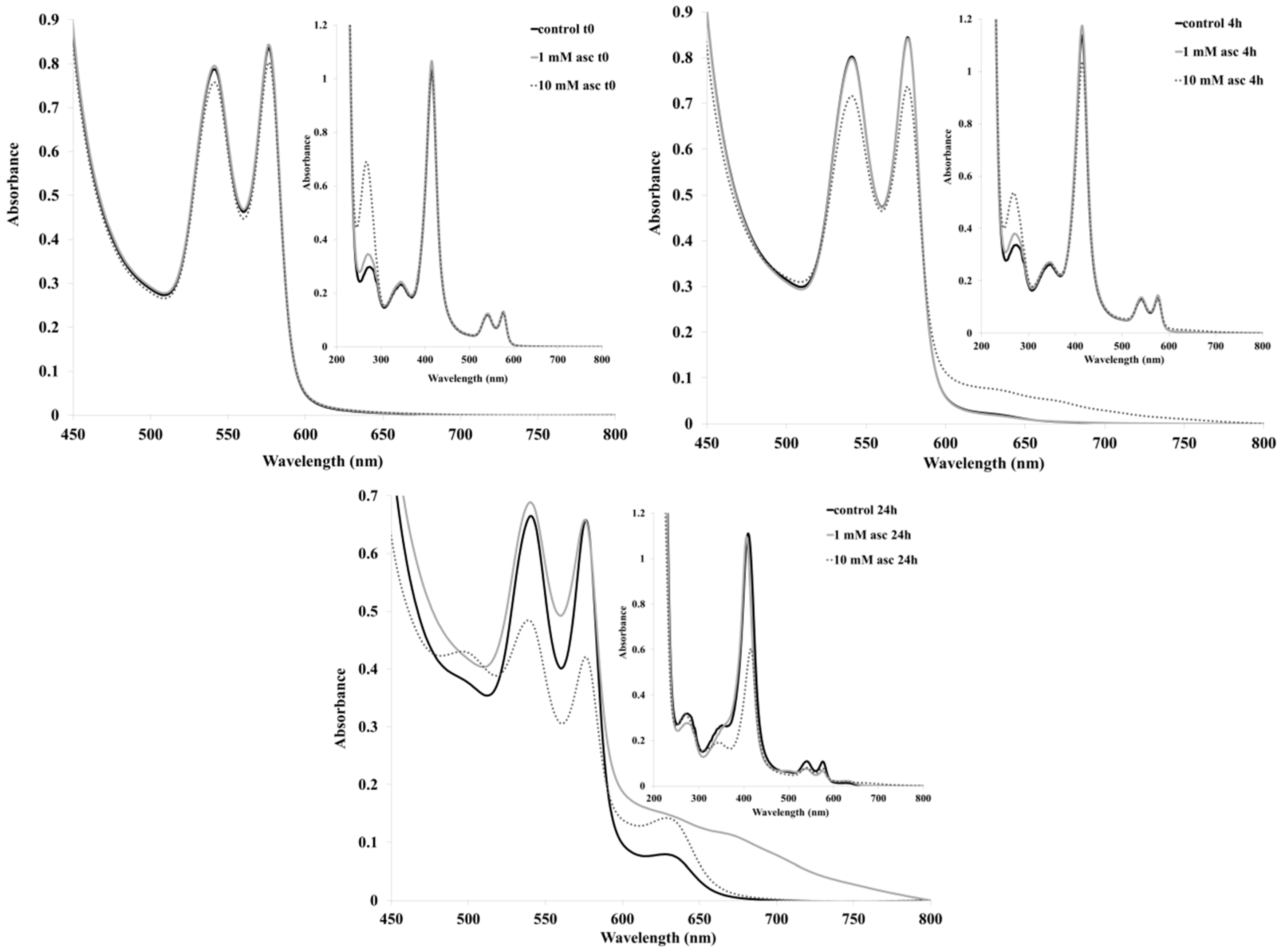
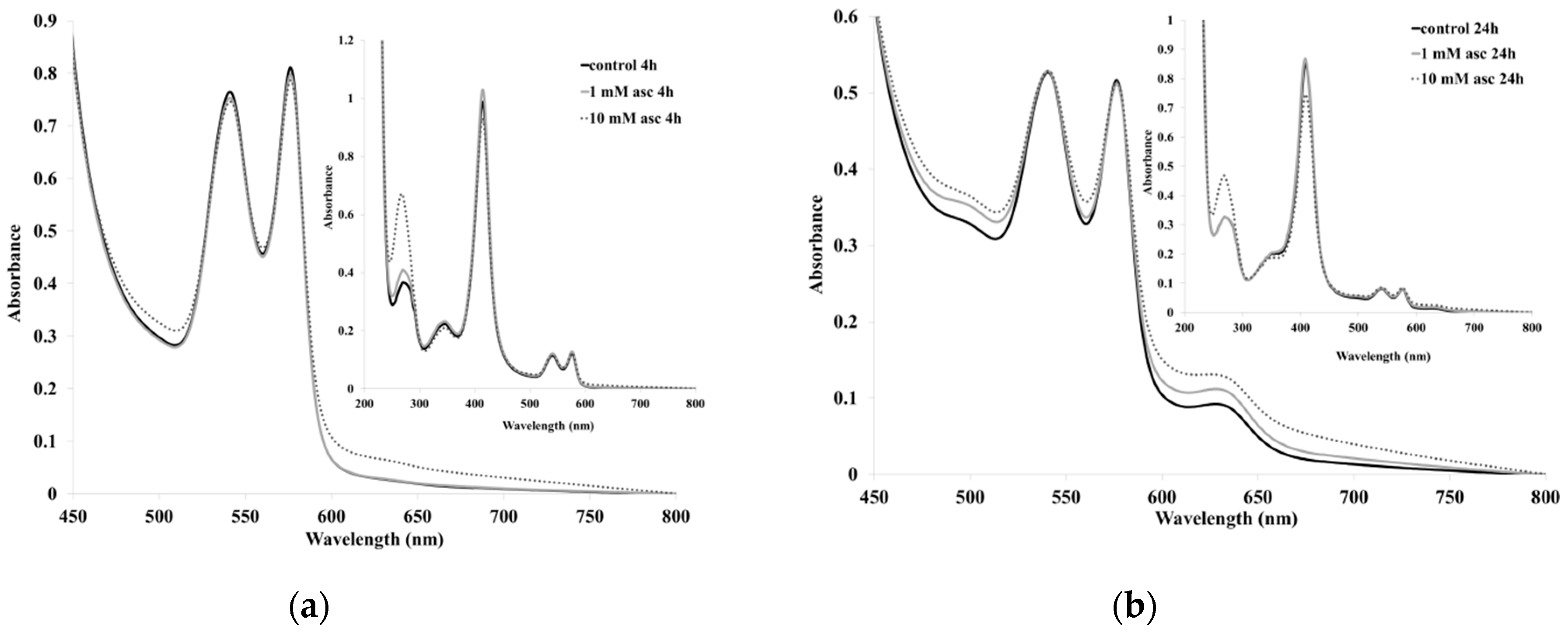
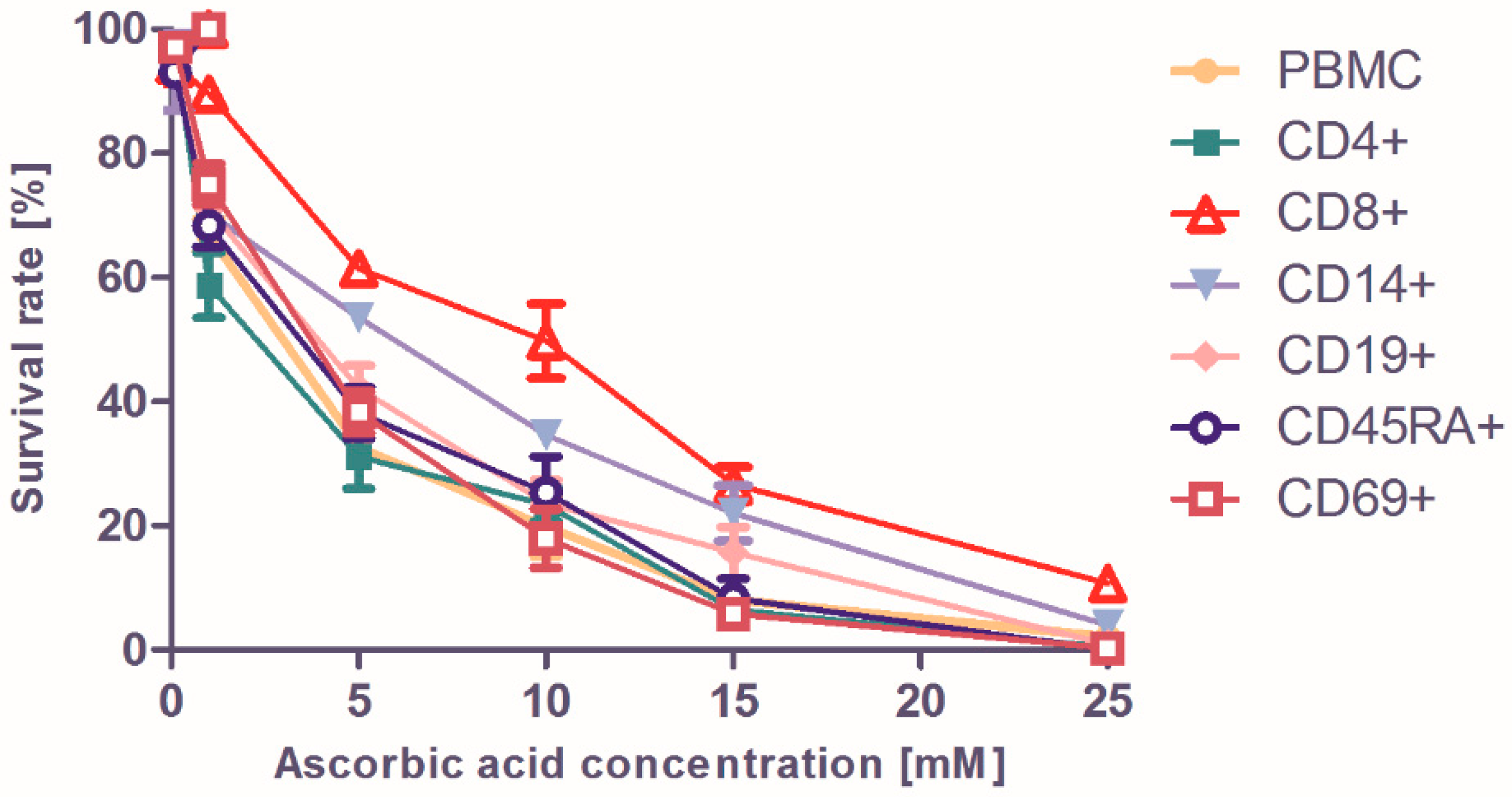
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cameron, E.; Pauling, L.; Leibovitz, B. Ascorbic Acid and Cancer: A Review. Cancer Res. 1979, 39, 663–681. [Google Scholar]
- Arabi, Y.M.; Fowler, R.; Hayden, F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020, 46, 315–328. [Google Scholar] [CrossRef]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020, 100028. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 1–15. [Google Scholar] [CrossRef]
- Dunne, J.; Caron, A.; Menu, P.; Alayash, A.I.; Buehler, P.W.; Wilson, M.T.; Silaghi-Dumitrescu, R.; Faivre, B.; Cooper, C.E. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem. J. 2006, 399, 513–524. [Google Scholar] [CrossRef]
- Mehlhorn, R.J. Ascorbate and Dehydroascorbate Acid Mediated Reduction of Free Radicals in the Human Erythrocyte. J. Biol. Chem. 1991, 266, 2724–2731. [Google Scholar]
- Cooper, C.E.E.; Silaghi-Dumitrescu, R.; Rukengwa, M.; Alayash, A.I.I.; Buehler, P.W.W. Peroxidase activity of hemoglobin towards ascorbate and urate: A synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC). Biochim. Biophys. Acta 2008, 1784, 1415–1420. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Cobb, C.E. Recycling of the Ascorbate Free Radical by Human Erythrocyte Membranes. Free Rad. Biol. Med. 2001, 31, 117–124. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Kroger-Ohlsen, M.V.; Bellio, R.; Skibsted, L.H. Protein binding in deactivation of ferrylmyoglobin by chlorogenate and ascorbate. J Agric. Food Chem. 2000, 48, 204–212. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Allen, A.O.; Schwartz, H.A. Mechanism of Disproportionation of Ascorbate Radicals. J. Am. Chem. Soc. 1981, 103, 3516–3518. [Google Scholar] [CrossRef]
- VanDuijin, M.M.; Tijssen, K.; VanSteveninck, J.; Van den Broek, P.J.A.; Van der Zee, J. Erythrocytes Reduce Extracellular Ascorbate Free Radicals using Intracellular Ascorbate as an Electron Donor. J. Biol. Chem. 2000, 275, 27720–27725. [Google Scholar]
- May, J.M.; Qu, Z.C.; Cobb, C.E. Extracellular Reduction of the Ascorbate Free Radical by Human Erythrocytes. Biochem. Biophys. Res. Commun. 2000, 267, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Stern, A. Effects of Ascorbate on Methemoglobin Reduction in Intact Red- Cells. Arch. Biochem. Biophys. 1982, 213, 590–594. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Puscas, C.; Radu, L.; Carrascoza, F.; Mot, A.C.; Amariei, D.; Lungu, O.; Scurtu, F.; Podea, P.; Septelean, R.; Matei, A.; et al. The high affinity of small-molecule antioxidants for hemoglobin. Free Radic. Biol. Med. 2018, 124, 260–274. [Google Scholar] [CrossRef]
- Mot, A.C.; Bischin, C.; Damian, G.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation involving hemoglobin-related free radical reactivity. Methods Mol. Biol. 2015, 1208, 247–255. [Google Scholar]
- Irwin, J.A.; Ostdal, H.; Davies, M.J.; Østdal, H.; Davies, M.J. Myoglobin-induced oxidative damage: Evidence for radical transfer from oxidized myoglobin to other proteins and antioxidants. Arch. Biochem. Biophys. 1999, 362, 94–104. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Giulivi, C.; Cadenas, E. The reaction of ascorbic acid with different heme iron redox states of myoglobin. Antioxidant and prooxidant aspects. FEBS Lett. 1993, 332, 287–290. [Google Scholar] [CrossRef]
- Vollaard, N.B.; Reeder, B.J.; Shearman, J.P.; Menu, P.; Wilson, M.T.; Cooper, C.E. A new sensitive assay reveals that hemoglobin is oxidatively modified in vivo. Free Radic. Biol. Med. 2005, 39, 1216–1228. [Google Scholar] [CrossRef]
- Bischin, C.; Contra, G.; Tusan, C.; Miclea, P.; Taciuc, V.; Parvu, M.; Silaghi-Dumitrescu, R. Free-radical reactions: The fine line between the anti- and pro-oxidant reactivities. Oxid. Commun. 2018, 41, 130–140. [Google Scholar]
- Gibson, Q.H. The Reduction of Methaemoglobin by Ascorbic Acid. Biochem. J. 1943, 37, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Farcas, A.D.; Mot, A.C.; Zagrean-Tuza, C.; Toma, V.; Cimpoiu, C.; Hosu, A.; Parvu, M.; Roman, I.; Silaghi-Dumitrescu, R. Chemo-mapping and biochemical-modulatory and antioxidant/prooxidant effect of galium verum extract during acute restraint and dark stress in female rats. PLoS ONE 2018, 13, e0200022. [Google Scholar] [CrossRef]
- Cooper, C.E.; Green, E.S.R.; Rice-Evans, C.A.; Davies, M.J.; Wriggleswort, J.M. A hydrogen-donating monohydroxamate scavenges ferryl myoglobin radicals. Free Radic. Res. 1994, 20, 219–227. [Google Scholar] [CrossRef]
- Moţ, A.C.; Coman, C.; Miron, C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. An assay for pro-oxidant reactivity based on phenoxyl radicals generated by laccase. Food Chem 2014, 143, 214–222. [Google Scholar] [CrossRef]
- Cox, B.M.; Leslie, F.M.; Dunlap, C.E. The use of ascorbate as a probe of opioid receptor structure: Evidence for two independent mechanisms of receptor destruction by ascorbate. J. Recept. Signal Transduct. 1980, 1, 329–354. [Google Scholar] [CrossRef]
- Miura, K.; Yazama, F.; Tai, A. Oxidative stress-mediated antitumor activity of erythorbic acid in high doses. Biochem. Biophys. Reports 2015, 3, 117–122. [Google Scholar] [CrossRef]
- Makino, Y.; Sakagami, H.; Takeda, M. Induction of cell death by ascorbic acid derivatives in human renal carcinoma and glioblastoma cell lines. Anticancer Res. 1999, 19, 3125–3132. [Google Scholar]
- Iheanacho, E.N.; Hunt, N.H.; Stocker, R. Vitamin C redox reactions in blood of normal and malaria-infected mice studied with isoascorbate as a nonisotopic marker. Free Radic. Biol. Med. 1995, 18, 543–552. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Song, J.H.; Simons, C.; Cao, L.; Shin, S.H.; Hong, M.; Chung, I.M. Rapid uptake of oxidized ascorbate induces loss of cellular glutathione and oxidative stress in liver slices. Exp. Mol. Med. 2003, 35, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Reports 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Kawasaki, M.; Motomatsu, H.; Katoil, F. Virus-Inactivating Effect of D-Isoascorbic Acid. J. Nutr. Sci. Vitaminol. (Tokyo). 1986, 32, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Ensing, B.; Buda, F.; Blochl, P.; Baerends, E.J. Chemical Involvement of Solvent Water Molecules in Elementary Steps of the Fenton Oxidation Reaction. Angew. Chemie - Int. Ed. 2001, 40, 2893–2895. [Google Scholar] [CrossRef]
- Liu, W.; Li, H. COVID-19:Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism 1 Introduction. Chemarxiv.org 2020. [Google Scholar] [CrossRef]
- Dolhnikoff, M.; Duarte-Neto, A.N.; de Almeida Monteiro, R.A.; Ferraz da Silva, L.F.; Pierre de Oliveira, E.; Nascimento Saldiva, P.H.; Mauad, T.; Marcia Negri, E. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in their Reaction with Ligands; Neuberger, A., Tatum, E.L., Eds.; North-Holland: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Hathazi, D.; Scurtu, F.; Bischin, C.; Mot, A.; Attia, A.; Kongsted, J.; Silaghi-Dumitrescu, R. The Reaction of Oxy Hemoglobin with Nitrite: Mechanism, Antioxidant-Modulated Effect, and Implications for Blood Substitute Evaluation. Molecules 2018, 23, E350. [Google Scholar] [CrossRef]
- Mot, A.C.; Puscas, C.; Dorneanu, S.A.; Silaghi-Dumitrescu, R. EPR detection of sulfanyl radical during sulfhemoglobin formation – Influence of catalase. Free Radic. Biol. Med. 2019, 137, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020, nwaa041. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, ciaa248. [Google Scholar] [CrossRef]
- Hathazi, D.; Mot, A.C.; Vaida, A.; Scurtu, F.; Lupan, I.; Fischer-Fodor, E.; Damian, G.; Kurtz, D.M., Jr.; Silaghi-Dumitescu, R. Oxidative protection of hemoglobin and hemerythrin by cross-linking with a nonheme iron peroxidase: Potentially improved oxygen carriers for use in blood substitutes. Biomacromolecules 2014, 15, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Andreicu, A.D.; Fischer-Fodor, E.; Pârvu, A.E.; Ţigu, A.B.; Cenariu, M.; Pârvu, M.; Cǎtoi, F.A.; Irimie, A. Antitumoral and Immunomodulatory Effect of Mahonia aquifolium Extracts. Oxid. Med. Cell. Longev. 2019, 6439021. [Google Scholar] [CrossRef] [PubMed]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzǎu, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells in Vitro. J. Nanomater. 2019, 6090259. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehene, M.; Fischer-Fodor, E.; Scurtu, F.; Hădade, N.D.; Gal, E.; Mot, A.C.; Matei, A.; Silaghi-Dumitrescu, R. Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells. Pharmaceuticals 2020, 13, 107. https://doi.org/10.3390/ph13060107
Lehene M, Fischer-Fodor E, Scurtu F, Hădade ND, Gal E, Mot AC, Matei A, Silaghi-Dumitrescu R. Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells. Pharmaceuticals. 2020; 13(6):107. https://doi.org/10.3390/ph13060107
Chicago/Turabian StyleLehene, Maria, Eva Fischer-Fodor, Florina Scurtu, Niculina D. Hădade, Emese Gal, Augustin C. Mot, Alina Matei, and Radu Silaghi-Dumitrescu. 2020. "Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells" Pharmaceuticals 13, no. 6: 107. https://doi.org/10.3390/ph13060107
APA StyleLehene, M., Fischer-Fodor, E., Scurtu, F., Hădade, N. D., Gal, E., Mot, A. C., Matei, A., & Silaghi-Dumitrescu, R. (2020). Excess Ascorbate is a Chemical Stress Agent against Proteins and Cells. Pharmaceuticals, 13(6), 107. https://doi.org/10.3390/ph13060107






