Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv.
Abstract
1. Introduction
2. Results
2.1. Extraction and 1H NMR Characterization of the Extracts
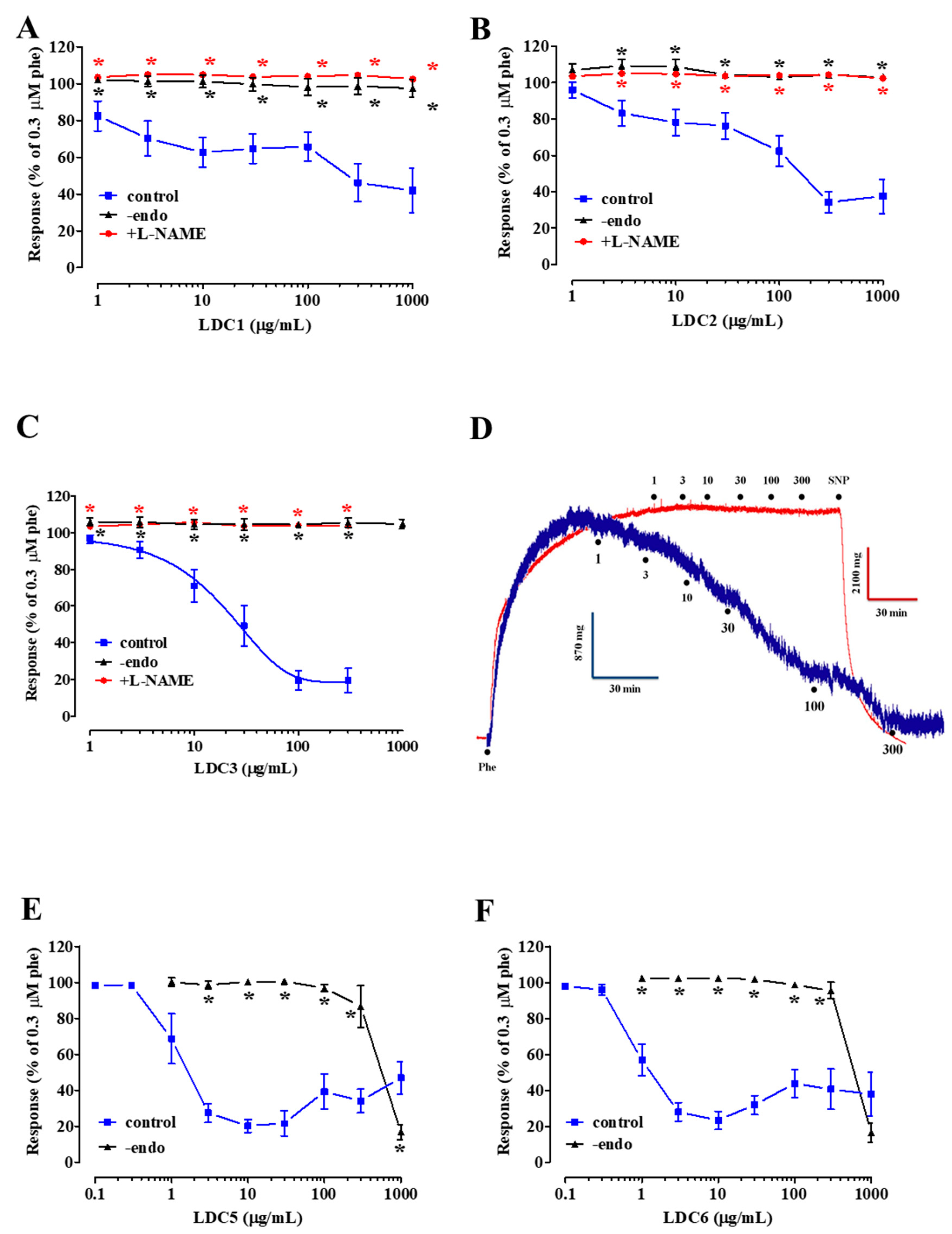
2.2. Effect of LDC Extracts on Phenylephrine-Induced Contraction
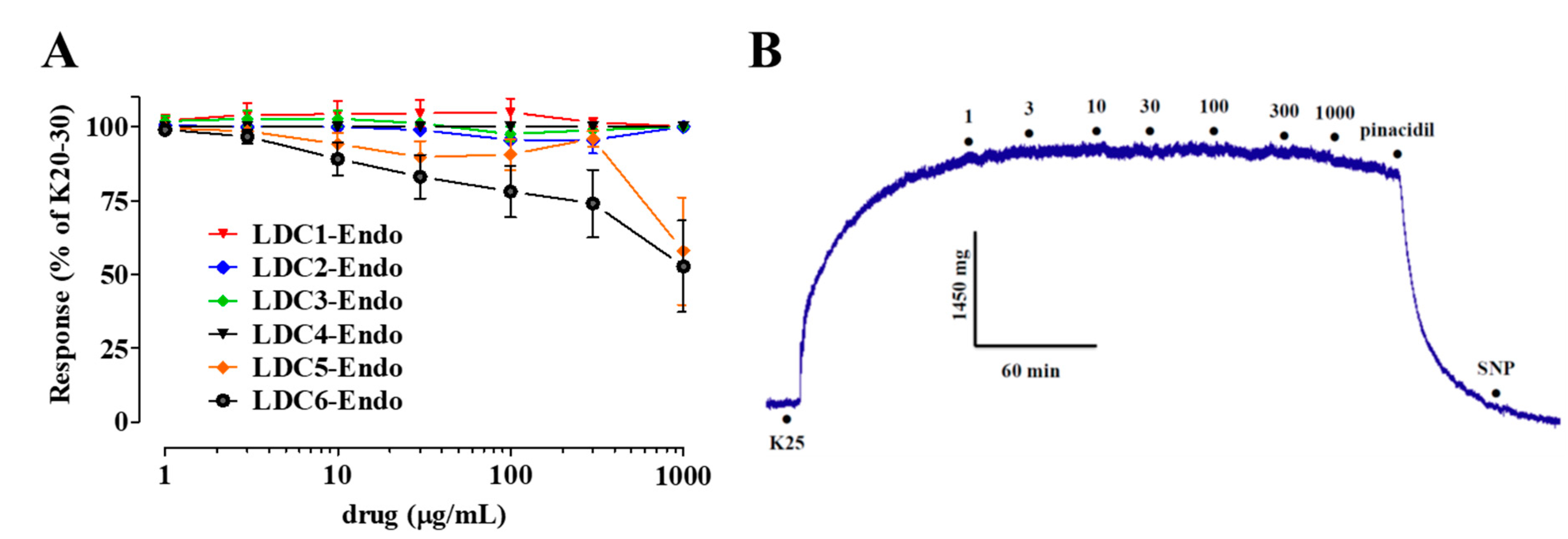
2.3. Effects of LDC Extracts on High KCl-Induced Contraction
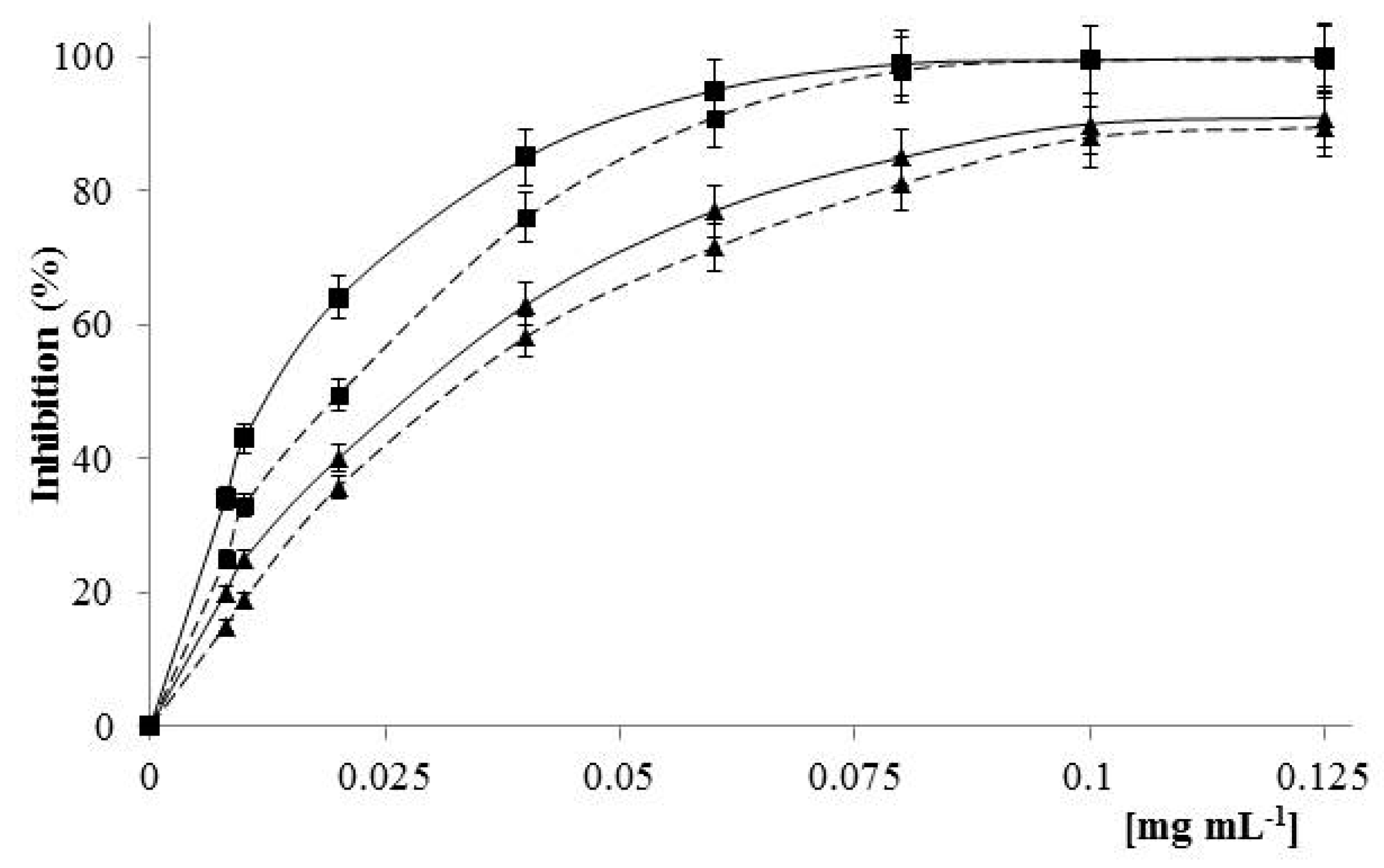
2.4. Antioxidant Activity
3. Discussion
- Magliocco dolce wine and pomaces (obtained from different steps during the winemaking process) comprise a range of valuable bioactive entities (e.g., polyphenols);
- LDC extracts, except LDC4, contain vasorelaxant agents capable of relaxing in vitro vascular preparations in an endothelium-dependent manner;
- relaxation induced by LDC1-LDC3 is dependent on the activation of eNOS;
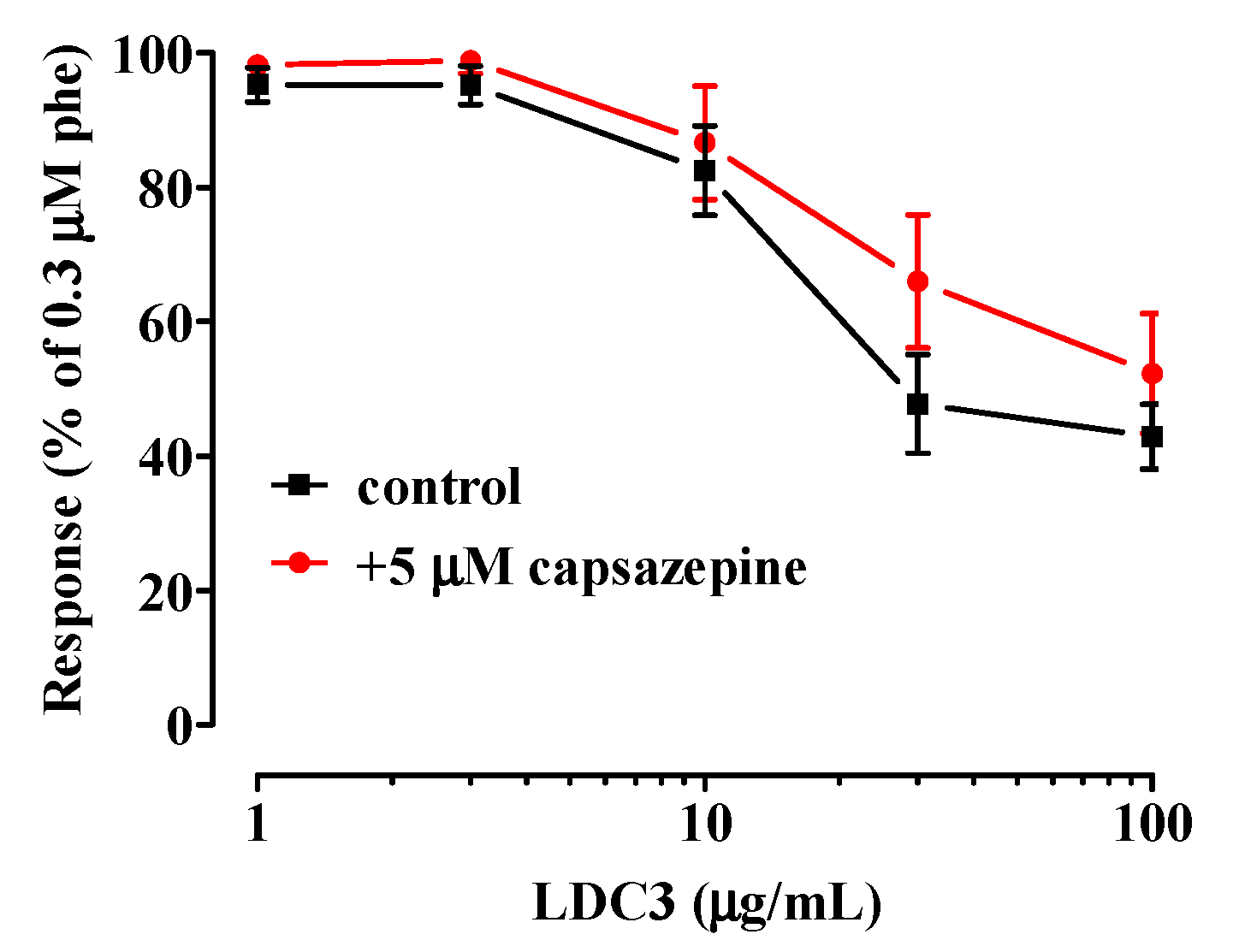
- TRPV1 channels are not involved in the relaxation caused by LDC3.
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of the Arvino Grape Pomace Extracts
4.3. NMR Analysis
4.4. Vasoactivity Assessments of LDC Extracts
4.4.1. Animals
4.4.2. Preparation of Rat Aortic Rings
4.4.3. Effect of LDC Extracts on Phenylephrine- and High K+-Induced Contraction
4.5. Antioxidant Activity of LDC3
4.5.1. Total Phenolic Equivalents (TPE) by Folin–Ciocalteu Procedure
4.5.2. Assessment of Total Antioxidant Capacity (TAC)
4.5.3. Assessment of Scavenging Activity against DPPH Radicals
4.5.4. Assessment of Scavenging Activity against the ABTS Radical Cations
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alabas, O.A.; Jernberg, T.; Pujades-Rodriguez, M.; Rutherford, M.J.; West, R.M.; Hall, M.; Timmis, A.; Lindahl, B.; Fox, K.A.A.; Hemingway, H.; et al. Statistics on mortality following acute myocardial infarction in 842 897 Europeans. Cardiovasc. Res. 2020, 116, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Noale, M.; Limongi, F.; Maggi, S. Epidemiology of Cardiovascular Diseases in the Elderly. In Frailty and Cardiovascular Deseases, Advances in Experimental Medicine and Biology; Veronese, N., Ed.; Springer: Cham, Switzerland, 2020; Volume 1216, pp. 29–38. [Google Scholar]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Fantin, F.; Macchi, F.; Giani, A.; Bissoli, L. The importance of nutrition in hypertension. Nutrients 2019, 11, 2542. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Treatment of hypertension with nutrition and nutraceutical supplements: Part 1. Altern. Complement. Ther. 2018, 24, 260–275. [Google Scholar] [CrossRef]
- Fumagalli, F.; Rossoni, M.; Iriti, M.; Di Gennaro, A.; Faoro, F.; Borroni, E.; Borgo, M.; Scienza, A.; Sala, A.; Folco, G. From field to health: A simple way to increase the nutraceutical content of grape as shown by NO-dependent vascular relaxation. J. Agric. Food Chem. 2006, 54, 5344–5349. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.; Jiao, R.; Womg, Y.M.; Yang, N.; Huang, H. Anti-hypertensive nutraceuticals and functional foods. J. Agric. Food Chem. 2009, 57, 4485–4499. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Carolyn Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.J.; et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- De Figueiredo, E.A.; Ferraz Bandeira Alves, N.; de Oliveira Monteiro, M.M.; de Oliveira Cavalcanti, C.; Sarmento da Silva, T.M.; Guedes da Silva, T.M.; de Andrade Braga, V.; de Jesus Oliveira, E. Antioxidant and antihypertensive effects of a chemically defined fraction of Syrah red wine on spontaneously hypertensive rats. Nutrients 2017, 9, 574. [Google Scholar] [CrossRef]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights 2017, 9, 51–57. [Google Scholar] [CrossRef]
- Carullo, G.; Durante, M.; Sciubba, F.; Restuccia, D.; Spizzirri, U.G.; Ahmed, A.; Di Cocco, M.E.; Saponara, S.; Aiello, F.; Fusi, F. Vasoactivity of Mantonico and Pecorello grape pomaces on rat aorta rings: An insight into nutraceutical development. J. Funct. Foods 2019, 57, 328–334. [Google Scholar] [CrossRef]
- Fusi, F.; Saponara, S.; Pessina, F.; Gorelli, B.; Sgaragli, G. Effects of quercetin and rutin on vascular preparations: A comparison between mechanical and electrophysiological phenomena. Eur. J. Nutr. 2003, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Jimenez, R.; Moreno, L.; Kroon, P.A.; Needs, P.W.; Hughes, D.A.; Santos-Buelga, C.; Gonzalez-Paramas, A.; Cogolludo, A.; Lopez-Sepulveda, R.; et al. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis 2009, 204, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Liu, X.H.; Rayment, S.; Hughes, D.A.; Kroon, P.A.; Needs, P.W.; Taylor, M.A.; Tribolo, S.; Wilson, V.G. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br. J. Pharmacol. 2010, 159, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Duarte, J.; Perez-Vizcaino, F. Epicatechin: Endothelial function and blood pressure. J. Agric. Food Chem. 2012, 60, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.; Salisu Usman, N.; Dewa, A.; Abubakar, K.; Aminu, N.; Zaini Asmawi, M.; Mahmud, R. Blood pressure lowering effect and vascular activity of Phyllanthus niruri extract: The role of NO/cGMP signaling pathway and β-adrenoceptor mediated relaxation of isolated aortic rings. J. Ethnopharmacol. 2020, 250, 112461. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Yeap, Z.Q.; Ng, C.H.; Yam, M.F. Overview of the microenvironment of vasculature in vascular tone regulation. Int. J. Mol. Sci. 2018, 19, 120. [Google Scholar]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Heiss, E.H.; Dirsch, V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharm. Des. 2014, 20, 3503–3513. [Google Scholar] [CrossRef]
- Ghayur, M.N.; Khan, H.; Hassan Gilani, A. Antispasmodic, bronchodilator and vasodilator activities of (+)-catechin, a naturally occurring flavonoid. Arch. Pharm. Res. 2007, 30, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Perri, M.; Manetti, F.; Aiello, F.; Caroleo, M.C.; Cione, E. Quercetin-3-oleoyl derivatives as new GPR40 agonists: Molecular docking studies and functional evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the in vitro wound-healing activity of Calabrian honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (Licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Tousoulis, D.; Katsargyris, A.; Charakida, M.; Androulakis, E.; Siasos, G.; Tentolouris, C.; Stefanadis, C. Antioxidant treatment and endothelial dysfunction: Is it time for flavonoids? Recent Pat. Cardiovasc. Drug Discov. 2013, 8, 81–92. [Google Scholar] [CrossRef]
- Tundis, R.; Frattaruolo, L.; Carullo, G.; Armentano, B.; Badolato, M.; Loizzo, M.R.; Aiello, F.; Cappello, A.R. An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Nat. Prod. Res. 2019, 33, 162–168. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Impellizzeri, D.; Mannina, L.; De Salvador, F.R.; Venditti, A.; Delfini, M. Metabolic profile of different Italian cultivars of hazelnut (Corylus avellana) by nuclear magnetic resonance spectroscopy. Nat. Prod. Res. 2014, 28, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Sticozzi, C.; Frosini, M.; Perrone, M.G.; Colabufo, N.A.; Saponara, S. Vascular toxicity risk assessment of MC18 and MC70, novel potential diagnostic tools for in vivo PET studies. Basic Clin. Pharmacol. Toxicol. 2018, 120, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Spiga, O.; Trezza, A.; Frosini, M.; Floriddia, E.; Teodori, E.; Dei, S.; Saponara, S. In vitro and in silico analysis of the vascular effects of asymmetrical N,N-bis(alkanol)amine aryl esters, novel multidrug resistance-reverting agents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1033–1043. [Google Scholar] [CrossRef]
- Fusi, F.; Manetti, F.; Durante, M.; Sgaragli, G.; Saponara, S. The vasodilator papaverine stimulates L-type Ca2+ current in rat tail artery myocytes via a PKA-dependent mechanism. Vasc. Pharmacol. 2016, 76, 53–61. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Chiricosta, S.; Puoci, F.; Altimari, I.; Picci, N. Antioxidant properties of extravirgin olive oil from cerasuola cv olive fruit: Effect of stone removal. Int. J. Food Sci. 2011, 23, 62–71. [Google Scholar]
- Cirillo, G.; Puoci, F.; Iemma, F.; Curcio, M.; Parisi, O.I.; Spizzirri, U.G.; Altimari, I.; Picci, N. Starch-quercetin conjugate by radical grafting: Synthesis and biological characterization. Pharm. Dev. Technol. 2012, 17, 466–476. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermos-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef]
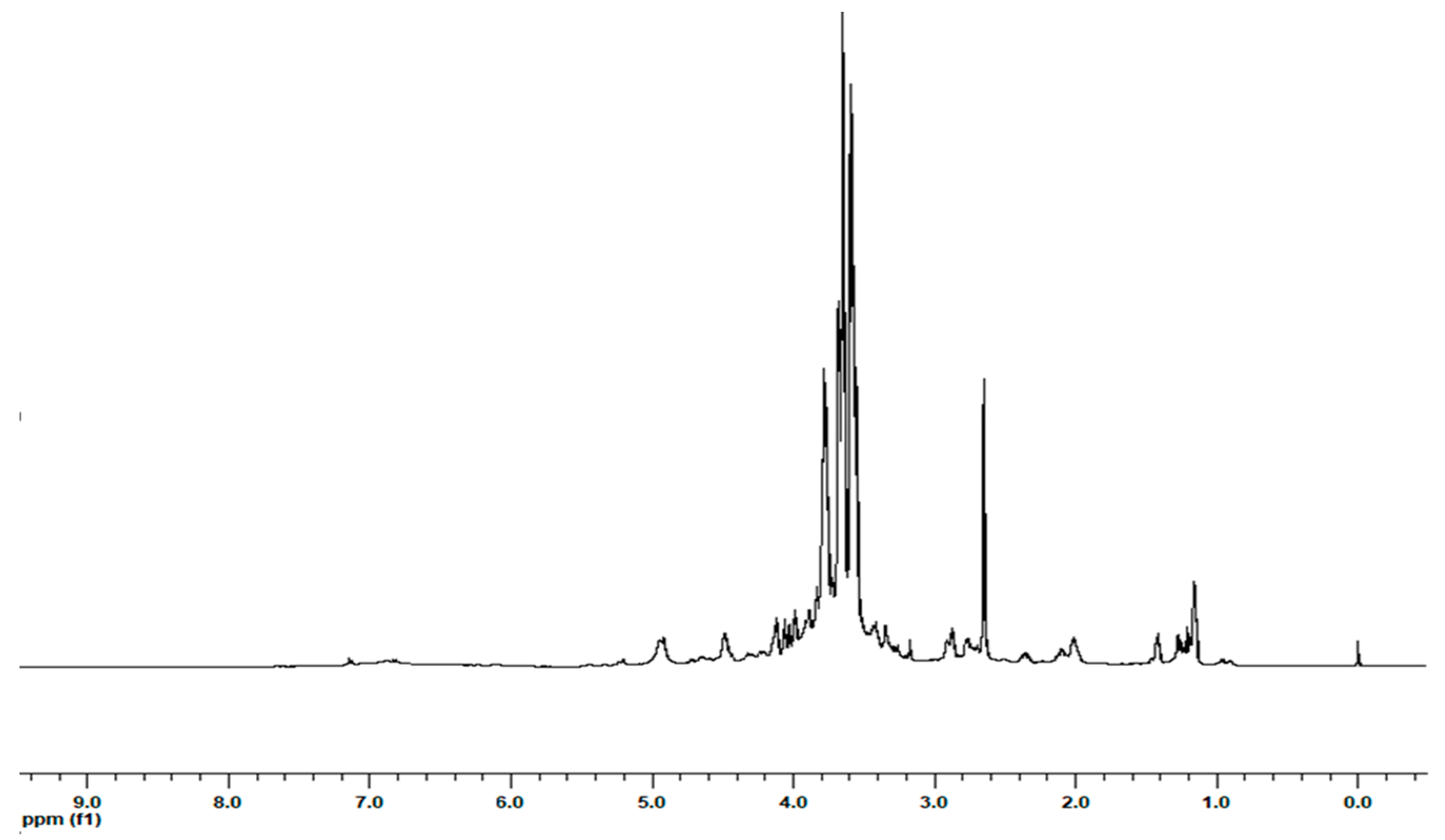
| Molecule | Amount (µmol/g) | ||||||
|---|---|---|---|---|---|---|---|
| LDC1 | LDC2 | LDC3 | LDC4 | LDC5 | LDC6 | ||
| Amino acids | Isoleucine | - | 1.28 ± 0.04 | - | 3.56 ± 0.11 | - | 7.85 ± 0.24 |
| Valine | - | 1.92 ± 0.06 | - | 5.63 ± 0.17 | - | 11.40 ± 0.34 | |
| Alanine | 22.92 ± 0.69 | 11.60 ± 0.35 | 30.90 ± 0.93 | 26.37 ± 0.79 | 30.11 ± 0.90 | 23.62 ± 0.71 | |
| GABA | 59.77 ± 1.79 | 3.84 ± 0.12 | 134.24 ± 4.03 | 21.33 ± 0.64 | - | - | |
| Glutamate | - | 15.12 ± 0.45 | - | - | - | - | |
| Glutamine | - | 17.04 ± 0.51 | - | - | - | - | |
| Tyrosine | - | - | - | 1.67 ± 0.05 | - | - | |
| Phenylalanine | - | 1.68 ± 0.05 | - | 2.67 ± 0.08 | - | 10.53 ± 0.32 | |
| Tryptophan | - | 1.92 ± 0.06 | - | 5.78 ± 0.17 | - | 9.51 ± 0.29 | |
| Organic acids | Lactic acid | - | 14.08 ± 0.42 | 42.51 ± 1.28 | 14.37 ± 0.43 | - | - |
| Quinic acid | 104.92 ± 3.15 | 66.00 ± 1.98 | 869.41 ± 26.08 | 285.33 ± 8.56 | - | - | |
| Malic acid | 369.92 ± 11.10 | 93.60 ± 2.81 | 896.94 ± 26.91 | 430.00 ± 12.90 | 309.38 ± 9.28 | 202.64 ± 6.08 | |
| Citric acid | - | 16.68 ± 0.50 | 603.18 ± 18.10 | 103.33 ± 3.10 | - | - | |
| Succinic acid | - | 59.76 ± 1.79 | 857.24 ± 25.72 | 18.28 ± 0.55 | - | 51.11 ± 1.53 | |
| Fumaric acid | 8.54 ± 0.26 | 2.76 ± 0.08 | - | - | - | - | |
| Formic acid | 1.38 ± 0.04 | 1.20 ± 0.04 | 3.76 ± 0.11 | 3.11 ± 0.09 | 5.45 ± 0.16 | 1.36 ± 0.04 | |
| Phenols | Gallic acid | 5.19 ± 0.16 | 3.72 ± 0.11 | 67.53 ± 2.03 | 2.56 ± 0.08 | 51.05 ± 1.53 | 91.81 ± 2.75 |
| p-Coumaric acid | - | 5.16 ± 0.15 | - | - | - | - | |
| Caffeic acid | - | 7.20 ± 0.22 | - | - | - | - | |
| Catechin | - | 3.72 ± 0.11 | 18.12 ± 0.54 | - | 283.64 ± 8.51 | 171.40 ± 5.14 | |
| Glycosylated flavonoids (eq Q3G) | 20.69 ± 0.62 | 9.84 ± 0.30 | 90.51 ± 2.72 | - | - | - | |
| Carbohydrates | Sucrose | 18.23 ± 0.55 | - | - | - | - | 190.42 ± 5.71 |
| Glucose | 3497 ± 104 | 123.36 ± 3.70 | 613.65 ± 18.41 | 11081 ± 332 | 1468.15 ± 44.04 | 309.74 ± 9.29 | |
| Fructose | - | 36.00 ± 1.08 | 597.65 ± 17.93 | - | 242.84 ± 7.29 | 234.34 ± 7.03 | |
| Miscellaneous | 2,3 Butanediol | - | 2.72 ± 0.08 | 205.41 ± 6.16 | - | - | 17.74 ± 0.53 |
| Nicotinamide | 0.46 ± 0.01 | 0.72 ± 0.02 | 7.29 ± 0.22 | 3.33 ± 0.10 | 5.24 ± 0.16 | 1.13 ± 0.03 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, G.; Ahmed, A.; Fusi, F.; Sciubba, F.; Di Cocco, M.E.; Restuccia, D.; Spizzirri, U.G.; Saponara, S.; Aiello, F. Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceuticals 2020, 13, 87. https://doi.org/10.3390/ph13050087
Carullo G, Ahmed A, Fusi F, Sciubba F, Di Cocco ME, Restuccia D, Spizzirri UG, Saponara S, Aiello F. Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceuticals. 2020; 13(5):87. https://doi.org/10.3390/ph13050087
Chicago/Turabian StyleCarullo, Gabriele, Amer Ahmed, Fabio Fusi, Fabio Sciubba, Maria Enrica Di Cocco, Donatella Restuccia, Umile Gianfranco Spizzirri, Simona Saponara, and Francesca Aiello. 2020. "Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv." Pharmaceuticals 13, no. 5: 87. https://doi.org/10.3390/ph13050087
APA StyleCarullo, G., Ahmed, A., Fusi, F., Sciubba, F., Di Cocco, M. E., Restuccia, D., Spizzirri, U. G., Saponara, S., & Aiello, F. (2020). Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceuticals, 13(5), 87. https://doi.org/10.3390/ph13050087