A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antioxidant Activities In Vitro
2.3. Acute Oral Toxicity Study
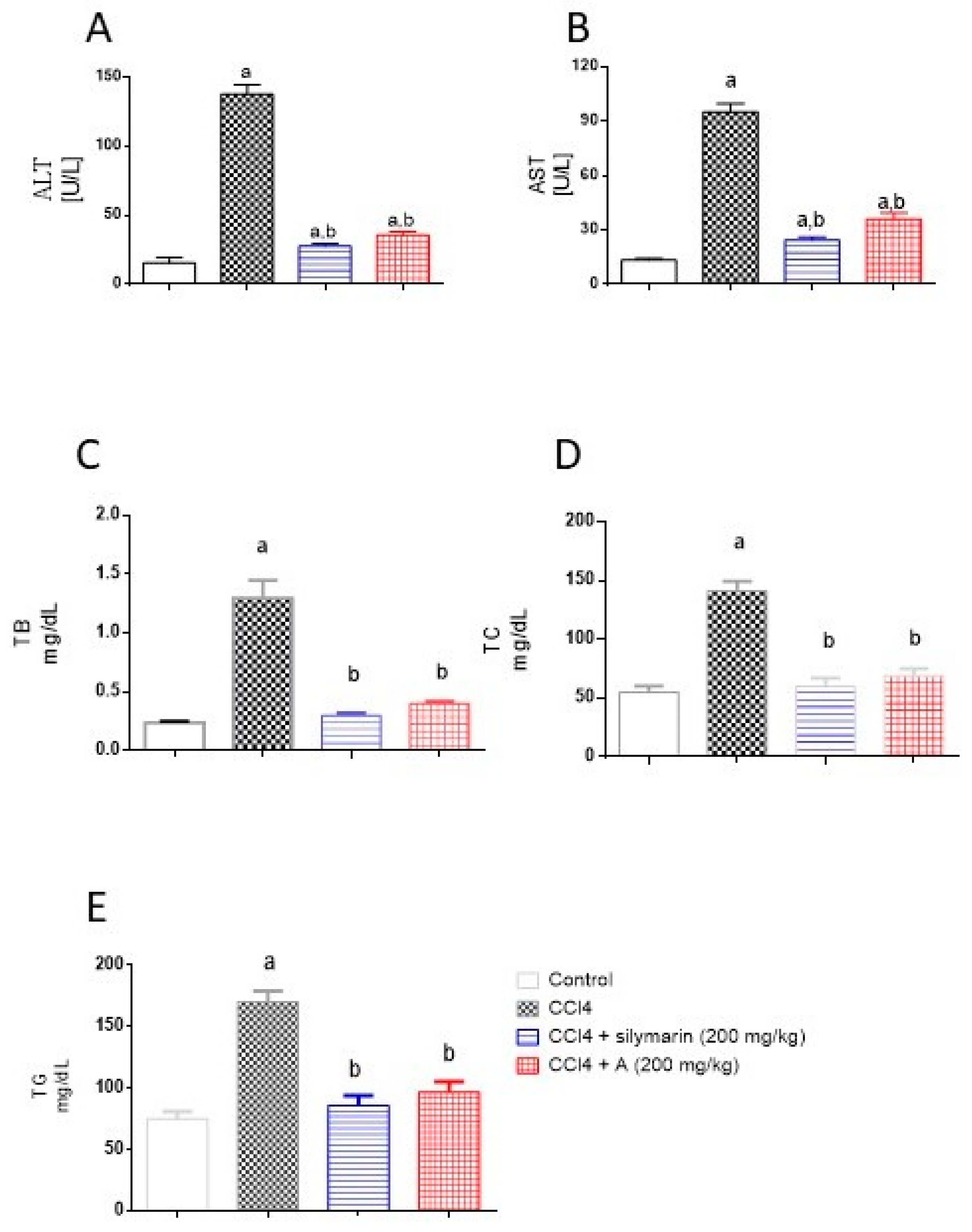
2.4. Effect of EAF on Serum Hepatotoxicity Markers
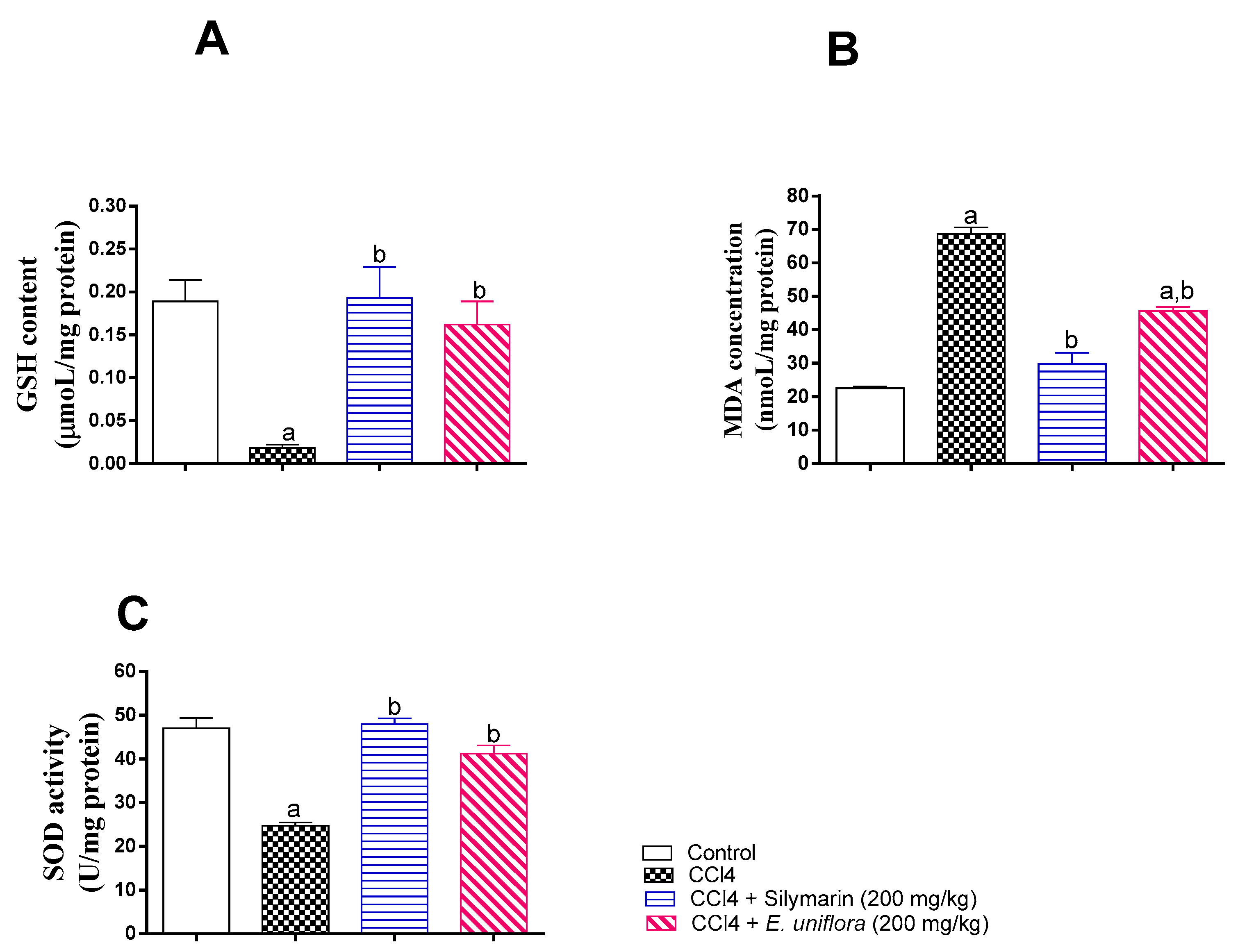
2.5. Effect of EAF on Liver Antioxidant Status
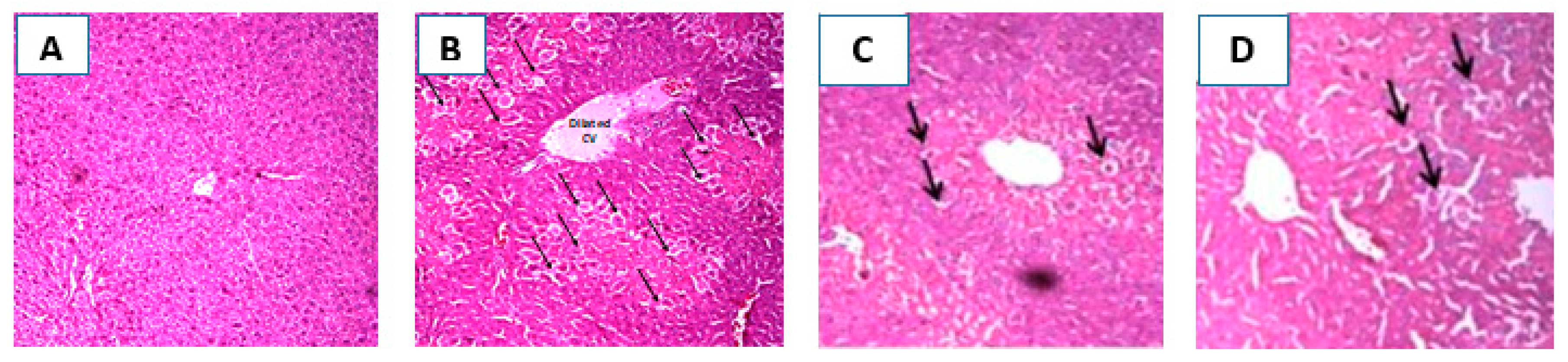
2.6. Histopathological Investigation of the Liver
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction and Fractionation
4.2. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
4.3. Antioxidant Activities In Vitro
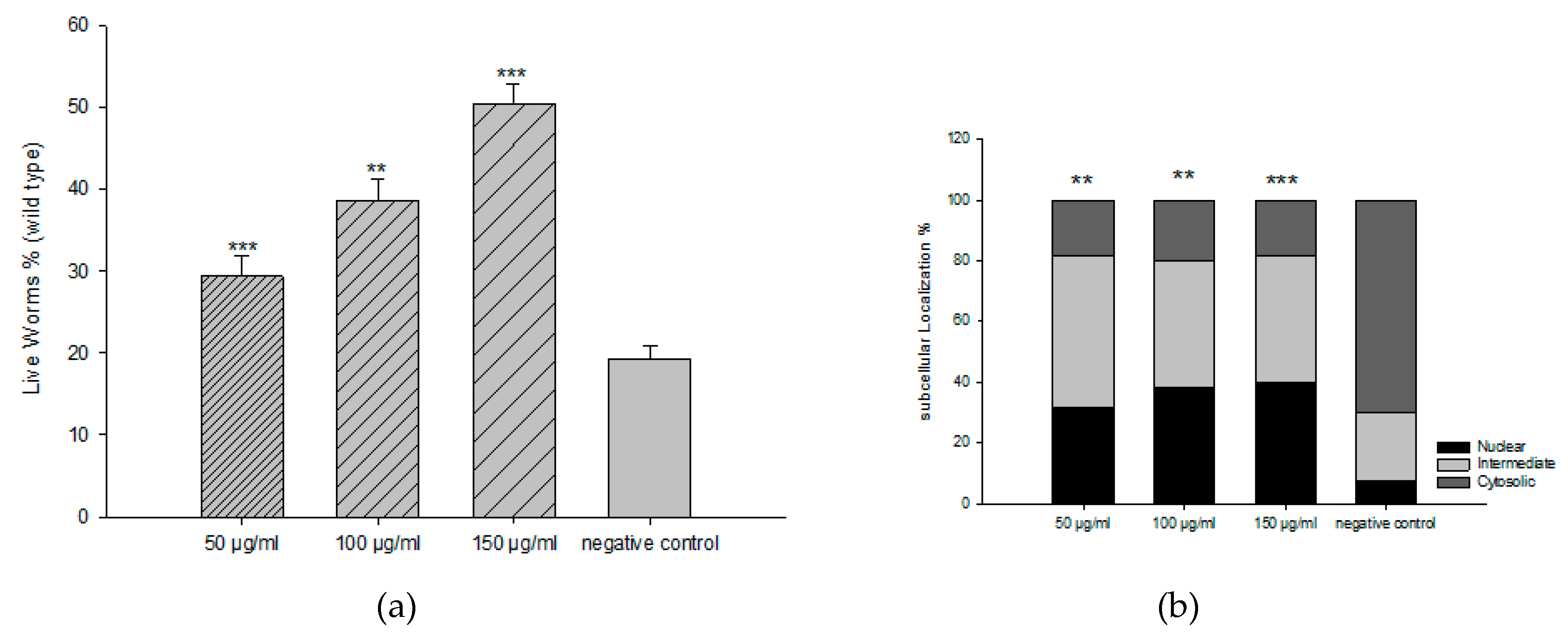
4.4. Antioxidant Activities in Caenorhabditis elegans
4.5. In Vivo Antioxidant and Hepatoprotective Assessment
4.5.1. Rat Experiments
4.5.2. Acute Oral Toxicity Study
4.5.3. Experimental Design
4.5.4. Blood Sampling and Tissue Preparation
4.5.5. Biochemical Analyses
4.5.6. Histopathological Examination
4.5.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, J.E.; Guyton, A.C. Textbook of Medical Physiology, 11th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 859–862. [Google Scholar]
- Sheth, K.; Bankey, P. The liver as an immune organ. Curr. Opin. Crit. Care 2001, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.O.; Beale, J.M.; Block, J.H. Wilson’s and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Beale, J.M., Block, J.H., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2011; pp. 43–104. [Google Scholar]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E.; Wink, M. Medicinal plants of the World, 2nd ed.; Briza: Pretoria, South Africa, 2017. [Google Scholar]
- Lim, T.K. Eugenia Uniflora. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 3, pp. 620–630. [Google Scholar]
- Lee, M.-H.; Chiou, J.-F.; Yen, K.-Y.; Yang, L.-L. EBV DNA polymerase inhibition of tannins from Eugenia uniflora. Cancer Lett. 2000, 154, 131–136. [Google Scholar] [CrossRef]
- Almeida, C.E.; Karnikowski, M.G.; Foleto, R.; Baldisserotto, B. Analysis of antidiarrhoeic effect of plants used in popular medicine. Rev. Saude Publica 1995, 29, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Arai, I.; Amagaya, S.; Komatsu, Y.; Okada, M.; Hayashi, T.; Kasai, M.; Arisawa, M.; Momose, Y. Improving effects of the extracts from Eugenia uniflora on hyperglycemia and hypertriglyceridemia in mice. J. Ethnopharmacol. 1999, 68, 307–314. [Google Scholar] [CrossRef]
- Sobeh, M.; Braun, M.S.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Wink, M. Chemical profiling of the essential oils of Syzygium aqueum, Syzygium samarangense and Eugenia uniflora and their discrimination using chemometric analysis. Chem. Biodiv. 2016, 13, 1537–1550. [Google Scholar] [CrossRef]
- Adewunmi, C.O.; Agbedahunsi, J.M.; Adebajo, A.C.; Aladesanmi, A.J.; Murphy, N.; Wando, J. Ethnoveterinary medicine: Screening of Nigerian medicinal plants for trypanocidal properties. J. Ethnopharmacol. 2001, 77, 19–24. [Google Scholar] [CrossRef]
- Amorim, A.C.L.; Lima, C.K.F.; Hovell, A.M.C.; Miranda, A.L.P.; Rezende, C.M. Antinociceptive and hypothermic evaluation of the leaf essential oil and isolated terpenoids from Eugenia uniflora L. (Brazilian Pitanga). Phytomedicine 2009, 16, 923–928. [Google Scholar] [CrossRef]
- Oliveira, M.D. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol. 2008, 371–376. [Google Scholar] [CrossRef]
- Sobeh, M.; El-Raey, M.; Rezq, S.; Abdelfattah, M.A.O.; Petruk, G.; Osman, S.; El-Shazly, A.M.; El-Beshbishy, H.A.; Mahmoud, M.F.; Wink, M. Chemical profiling of secondary metabolites of Eugenia uniflora and their antioxidant, anti-inflammatory, pain killing and anti-diabetic activities: A comprehensive approach. J. Ethnopharmacol. 2019, 240, 111939. [Google Scholar] [CrossRef] [PubMed]
- Brautbar, N.; Williams, J. 2nd, Industrial solvents and liver toxicity: Risk assessment, risk factors and mechanisms. Int. J. Hyg. Environ. Health 2002, 205, 479–491. [Google Scholar] [CrossRef]
- Maes, M.; Vinken, M.; Jaeschke, H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol. Appl. Pharmacol. 2016, 290, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gong, X.; Ai, Q.; Ge, P.; Lin, L.; Zhang, L. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside alleviated carbon tetrachloride-induced acute hepatitis in mice. Int. Immunopharmacol. 2015, 25, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Esmat, A.; Petruk, G.; Abdelfattah, M.A.O.; Dmirieh, M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Phenolic compounds from Syzygium jambos (Myrtaceae) exhibit distinct antioxidant and hepatoprotective activities in vivo. J. Funct. Foods 2018, 41, 223–231. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A Polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front Pharmacol 2018, 9, 566. [Google Scholar] [CrossRef]
- Jain, A.; Soni, M.; Deb, L.; Jain, A.; Rout, S.P.; Gupta, V.B.; Krishna, K.L. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J. Ethnopharmacol. 2008, 115, 61–66. [Google Scholar] [CrossRef]
- Cao, J.J.; Lv, Q.Q.; Zhang, B.; Chen, H.Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohydr. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Sánchez-Hernández, E.; R Romero, M.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Exploring target genes involved in the effect of quercetin on the response to oxidative stress in Caenorhabditis elegans. Antioxidants 2019, 8, 585. [Google Scholar] [CrossRef]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. IJMS 2013, 14, 11895–11914. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute oral toxicity-Acute Toxic Class Method; OECD: Paris, France, 2002. [Google Scholar]
- Falcao, T.R.; de Araujo, A.A.; Soares, L.A.L.; de Moraes Ramos, R.T.; Bezerra, I.C.F.; Ferreira, M.R.A.; de Souza Neto, M.A.; Melo, M.C.N.; de Araujo, R.F., Jr.; de Aguiar Guerra, A.C.V.; et al. Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complement Altern. Med. 2018, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Gan, R.-Y.; Li, H.-B. Natural Products for Prevention and Treatment of Chemical-Induced Liver Injuries. CRFSFS 2018, 17, 472–495. [Google Scholar] [CrossRef]
- Yan, J.Y.; Ai, G.; Zhang, X.J.; Xu, H.J.; Huang, Z.M. Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. J. Ethnopharmacol. 2015, 172, 202–213. [Google Scholar] [CrossRef]
- Velazquez, E.; Tournier, H.A.; Mordujovich de Buschiazzo, P.; Saavedra, G.; Schinella, G.R. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Davila, J.C.; Lenherr, A.; Acosta, D. Protective effect of flavonoids on drug-induced hepatotoxicity in vitro. Toxicology 1989, 57, 267–286. [Google Scholar] [CrossRef]
- Chen, J.H.; Tipoe, G.L.; Liong, E.C.; So, H.S.; Leung, K.M.; Tom, W.M.; Fung, P.C.; Nanji, A.A. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am. J. Clin. Nutr. 2004, 80, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Rattmann, Y.D.; de Souza, L.M.; Malquevicz-Paiva, S.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Analysis of flavonoids from Eugenia uniflora leaves and its protective effect against Murine Sepsis. Evid. Based Complement Altern. Med. 2012, 2012, 623940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, E.; Patten, C.J.; Chen, L.; Yang, C.S. Effects of flavonoids on cytochrome P450-dependent acetaminophen metabolism in rats and human liver microsomes. Drug Metab. Dispos. 1994, 22, 566–571. [Google Scholar] [PubMed]
- Sheweita, S.A.; El-Gabar, M.A.; Bastawy, M. Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: Role of antioxidants. Toxicology 2001, 169, 83–92. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.; Sabry, O.M.; Ghareeb, M.A.; El-Shazly, A.M.; Wink, M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci. Rep. 2018, 19, 1–6. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Cheng, H.; El-Shazly, A.M.; Wink, M. Senna singueana: Antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 2015, 49 (Suppl. 1), 4–11. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Yahya, F.; Mamat, S.S.; Mahmood, N.D.; Mohtarrudin, N.; Taher, M.; Hamid, S.S.; Teh, L.K.; Salleh, M.Z. Hepatoprotective action of various partitions of methanol extract of Bauhinia purpurea leaves against paracetamol-induced liver toxicity: Involvement of the antioxidant mechanisms. BMC Complement Altern. Med. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
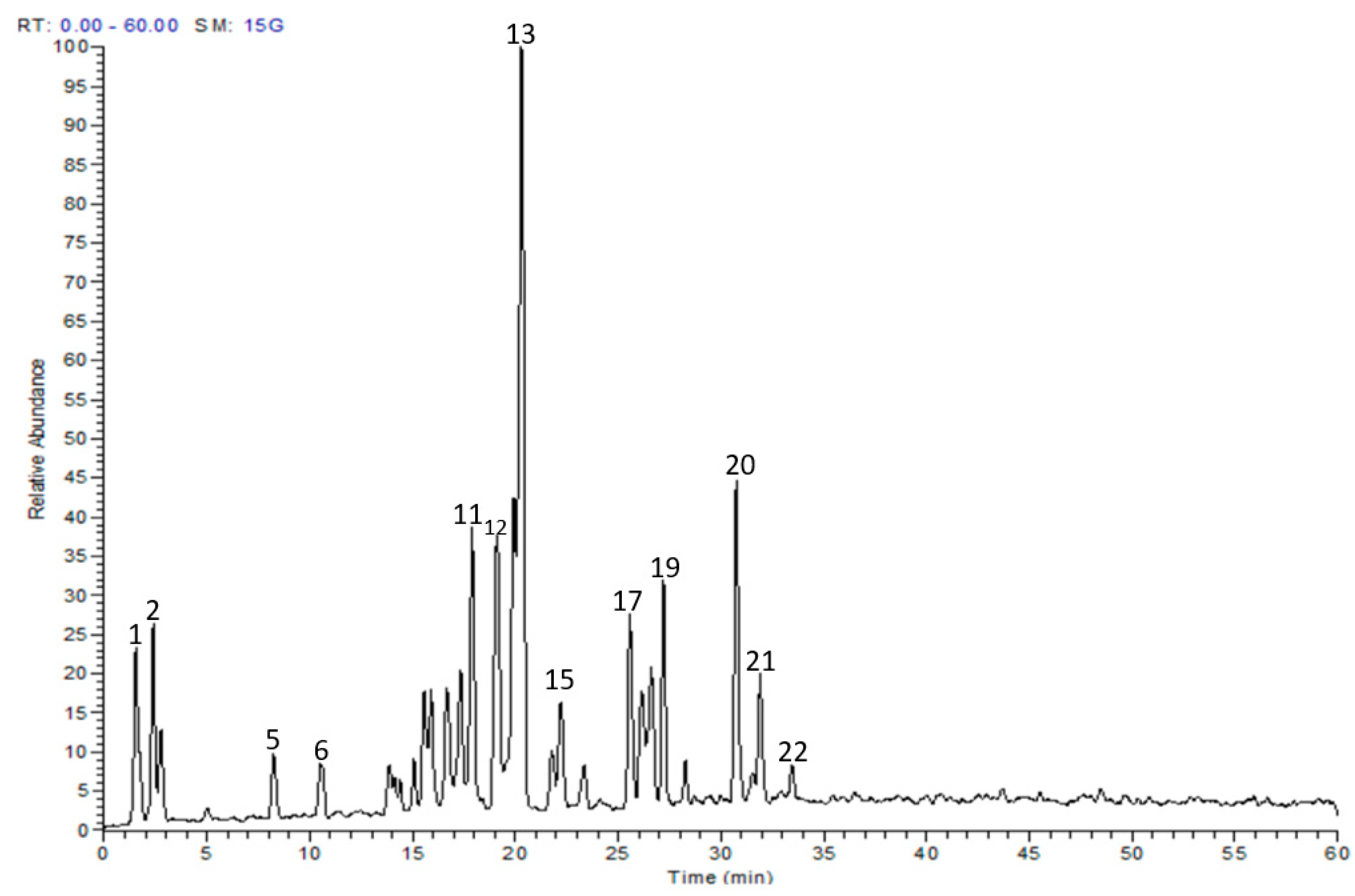
| No. | tR (min) | [M-H]− (m/z) | MS/MS | Proposed Compound | Content (%) |
|---|---|---|---|---|---|
| 1 | 1.53 | 191 | 111, 127 | Quinic acid | 4.74 |
| 2 | 2.37 | 169 | 125 | Gallic acid * | 4.07 |
| 3 | 2.89 | 483 | 169 | Digalloyl-hexoside | 2.18 |
| 4 | 5.02 | 633 | 301 | Galloyl-HHDP-hexoside | 0.40 |
| 5 | 8.25 | 453 | 169, 285, 313 | Pyrogallol-O-methylgalloyl glucose | 1.61 |
| 6 | 10.50 | 337 | 191 | p-coumarolylquinic acid | 1.80 |
| 7 | 13.83 | 275 | 275, 257 | 3,4,8,9,10-pentahydroxy-6-oxobenzo[c]chromene | 2.43 |
| 8 | 15.07 | 479 | 151, 317 | Myricetin-3-O-β-D-glucoside * | 1.03 |
| 9 | 15.51 | 479 | 151, 317 | Myricetin-3-O-β-D-galactoside * | 2.86 |
| 10 | 17.13 | 449 | 151, 179, 317 | Myricetin pentoside | 3.75 |
| 11 | 17.90 | 449 | 151, 179, 317 | Myricetin pentoside | 6.74 |
| 12 | 19.07 | 463 | 151, 179, 317 | Myricetin rhamnoside | 7.65 |
| 13 | 20.30 | 463 | 151, 179, 317 | Myricetin-3-O-rhamnoside * | 27.89 |
| 14 | 21.71 | 463 | 151, 179, 301 | Quercetin glucoside | 1.60 |
| 15 | 22.23 | 433 | 151, 179, 301 | Quercetin pentoside | 3.21 |
| 16 | 23.36 | 433 | 151, 179, 301 | Quercetin pentoside | 1.55 |
| 17 | 25.59 | 447 | 151, 179, 301 | Quercetin rhamnoside | 4.88 |
| 18 | 26.62 | 447 | 151, 179, 301 | Quercetin rhamnoside | 4.56 |
| 19 | 27.16 | 431 | 269 | Apigenin glucoside | 4.73 |
| 20 | 30.75 | 615 | 179, 317, 463 | Myricetin galloyl-rhamonside | 7.21 |
| 21 | 32.01 | 431 | 285 | Kaempferol rhamnoside | 4.07 |
| 22 | 33.42 | 521 | 179, 317, 479 | Myricetin-3-O-[6′’-O-β-D-acetyl galactoside] * | 1.03 |
| Extract or Fraction | DPPH |
|---|---|
| (IC50 µg/mL) | |
| N-Hexane | >200 |
| Ethyl acetate | 3.35 |
| N-Butanol | 8.8 |
| The rest | 97.32 |
| Methanol extract | 7.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobeh, M.; Hamza, M.S.; Ashour, M.L.; Elkhatieb, M.; El Raey, M.A.; Abdel-Naim, A.B.; Wink, M. A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals 2020, 13, 84. https://doi.org/10.3390/ph13050084
Sobeh M, Hamza MS, Ashour ML, Elkhatieb M, El Raey MA, Abdel-Naim AB, Wink M. A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals. 2020; 13(5):84. https://doi.org/10.3390/ph13050084
Chicago/Turabian StyleSobeh, Mansour, Marwa S. Hamza, Mohamed L. Ashour, Mona Elkhatieb, Mohamed A El Raey, Ashraf B. Abdel-Naim, and Michael Wink. 2020. "A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo" Pharmaceuticals 13, no. 5: 84. https://doi.org/10.3390/ph13050084
APA StyleSobeh, M., Hamza, M. S., Ashour, M. L., Elkhatieb, M., El Raey, M. A., Abdel-Naim, A. B., & Wink, M. (2020). A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals, 13(5), 84. https://doi.org/10.3390/ph13050084







