A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep
Abstract
1. Introduction
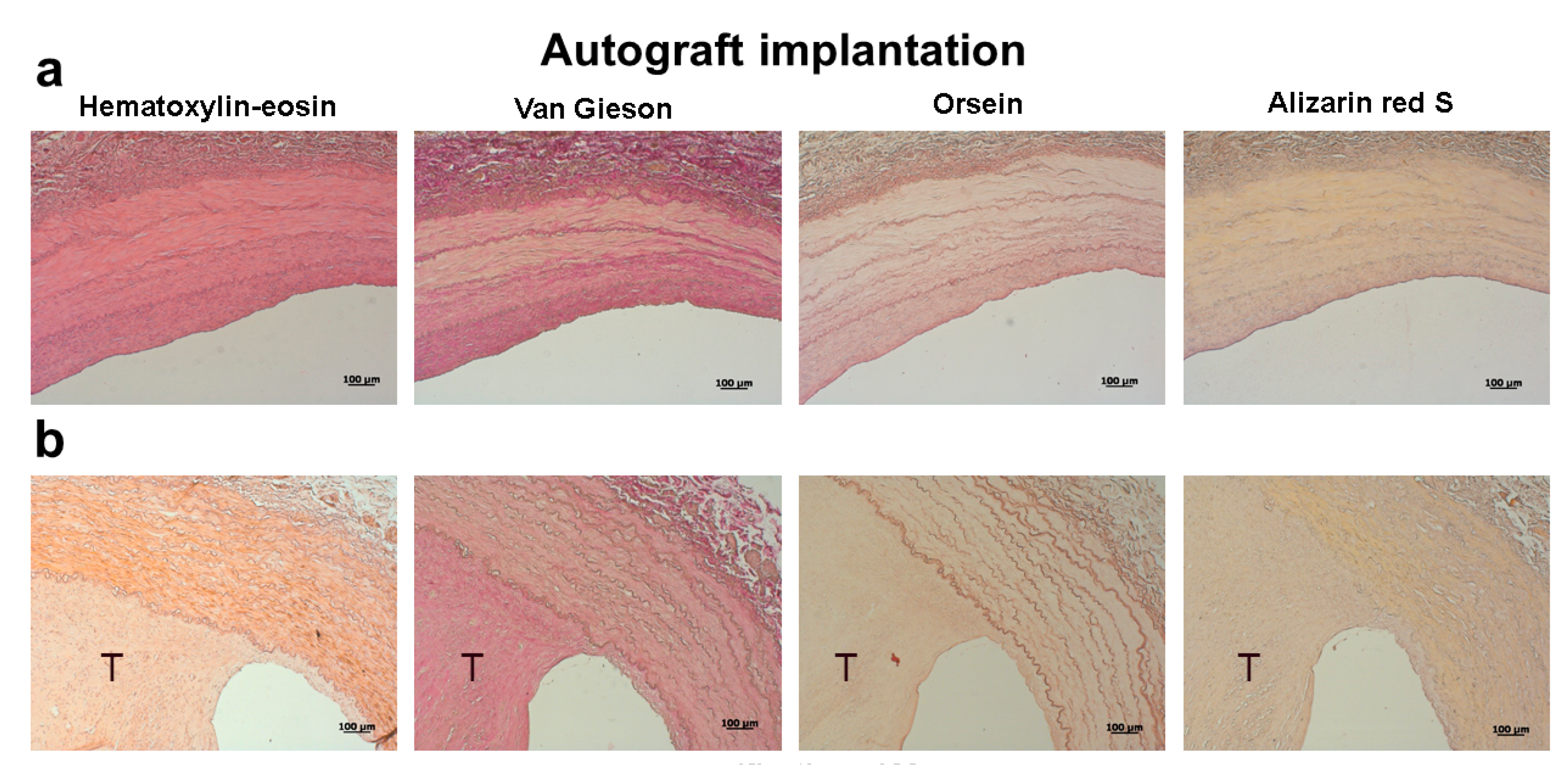
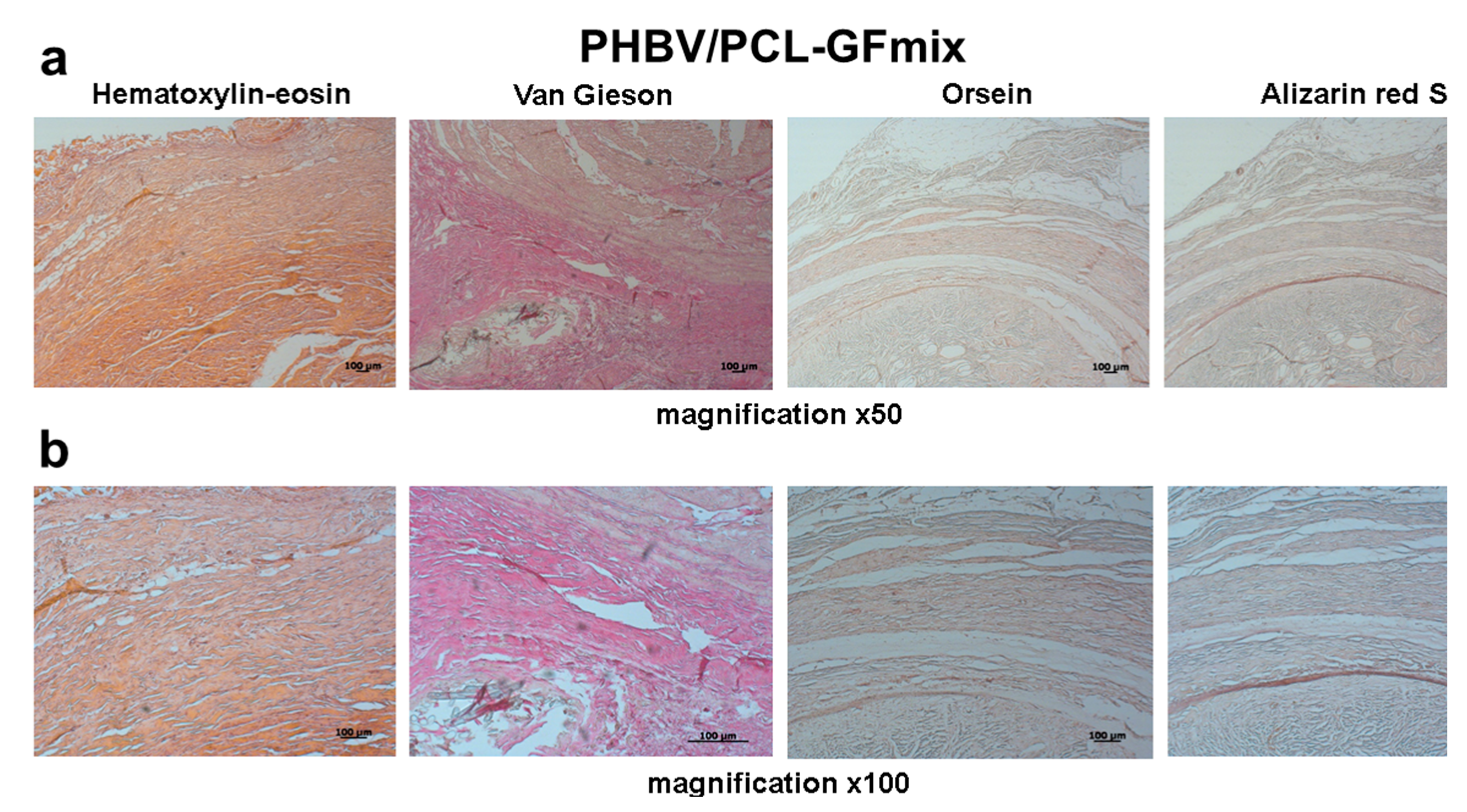
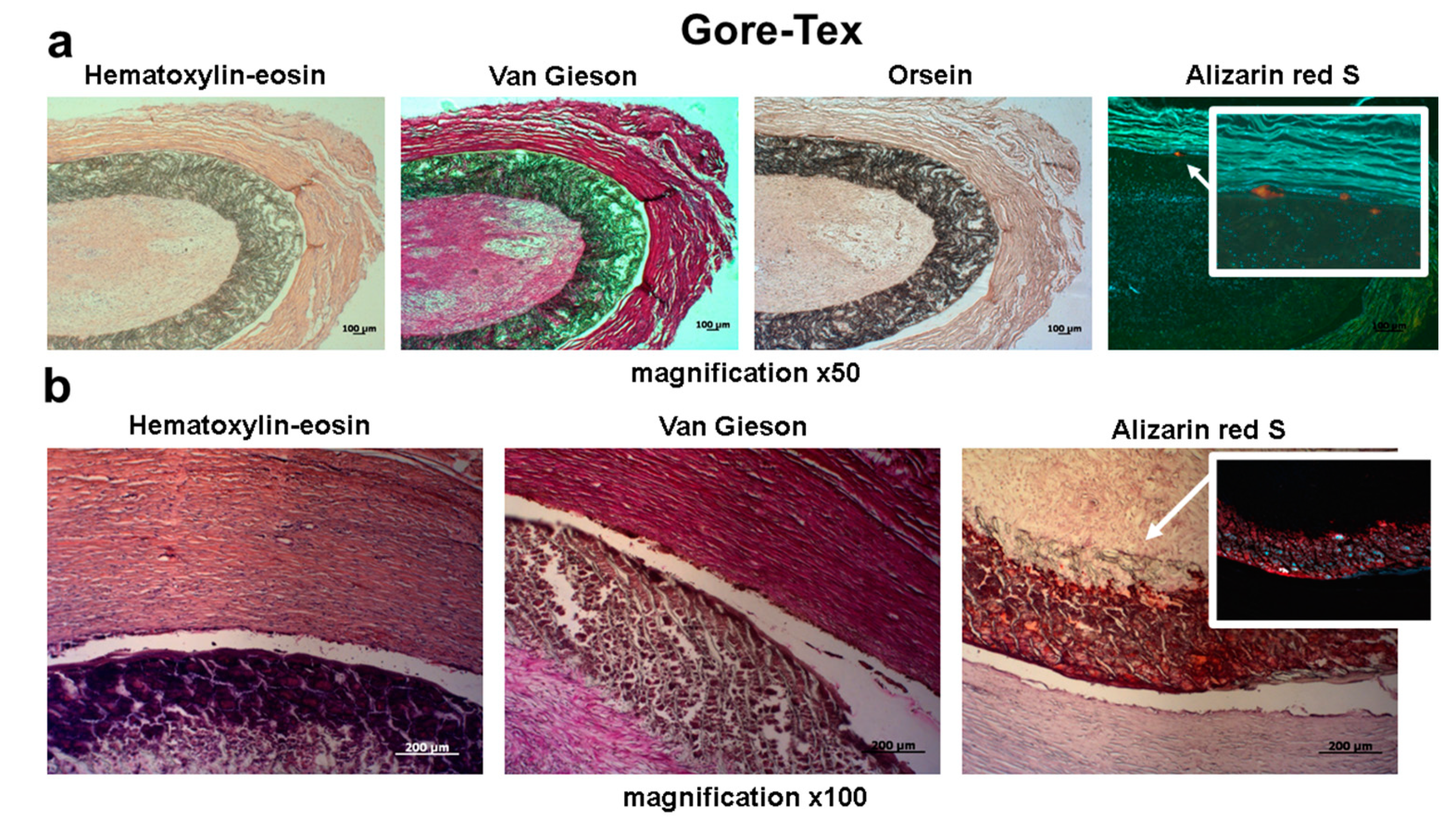
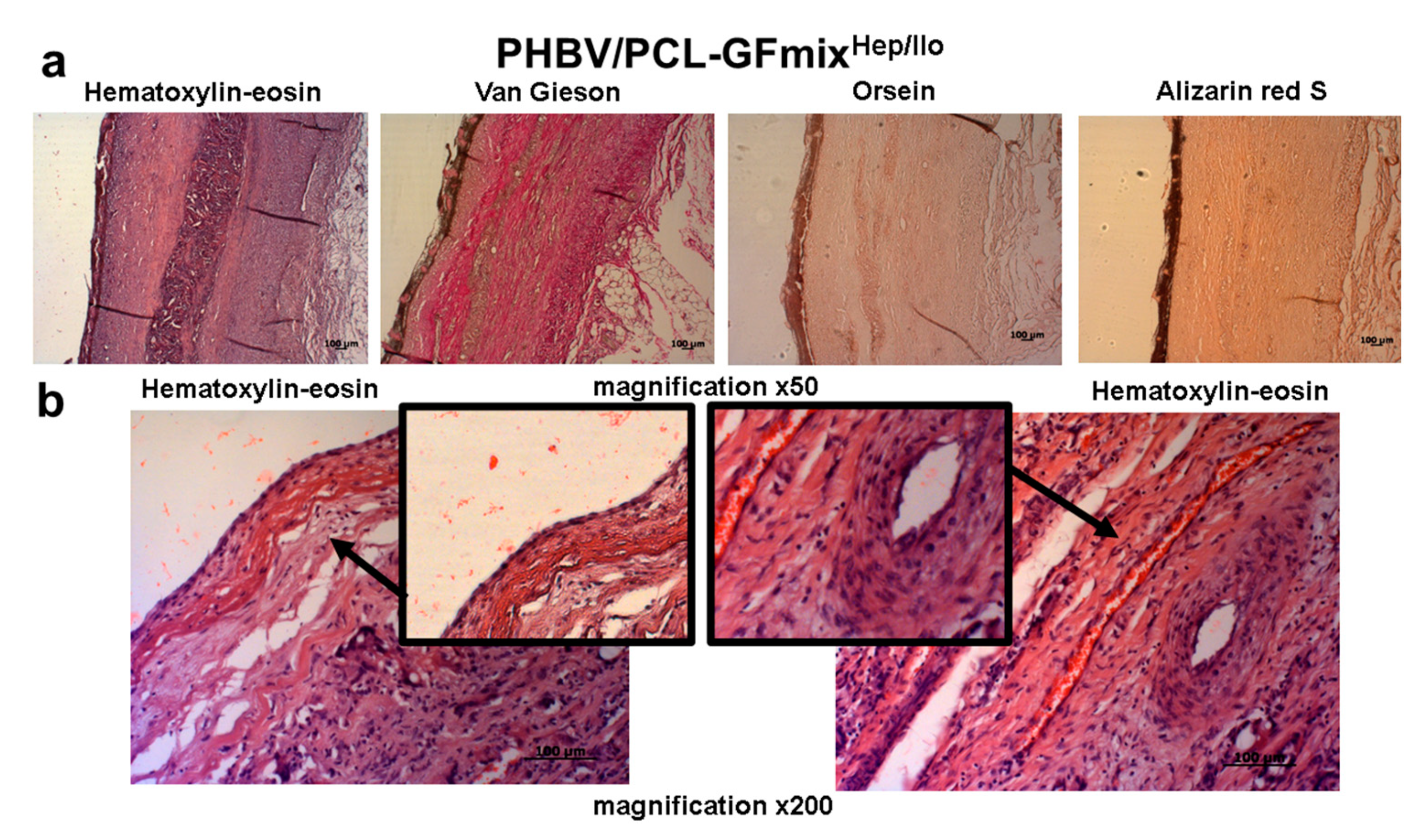
2. Results
3. Discussion
4. Materials and Methods
4.1. Graft Fabrication
4.2. Graft Implantation into the Carotid Artery of the Sheep
4.3. Histological Examination
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Winkler, B. Current Trends in Selection of Conduits for Coronary Artery Bypass Grafting. Gen Thorac. Cardiovasc. Surg. 2017, 65, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P. Current status of arterial grafts for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 427–430. [Google Scholar] [PubMed]
- Wang, X.; Lin, P.; Yao, Q.; Chen, C. Development of small-diameter vasculargrafts. World J. Surg. 2007, 31, 682–689. [Google Scholar] [CrossRef]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Chong, D.S.; Lindsey, B.; Dalby, M.J.; Gadegaard, N.; Seifalian, A.M.; Hamilton, G. Luminal surface engineering, ‘micro and nanopatterning’: Potential for self endothelialising vascular grafts? Eur. J. Vasc. Endovasc. Surg. 2014, 47, 566–576. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef]
- Niu, G.; Sapoznik, E.; Soker, S. Bioengineered blood vessels. Expert Opin. Biol. Ther. 2014, 14, 403–410. [Google Scholar] [CrossRef]
- Greenwald, S.E.; Berry, C.L. Improving vascular grafts: The importance of mechanical and haemodynamic properties. J. Pathol. 2000, 190, 292–299. [Google Scholar] [CrossRef]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Williams, S.K.; Kleinert, L.B.; Patula-Steinbrenner, V. Accelerated Neovascularization and Endothelialization of Vascular Grafts Promoted by Covalently Bound Laminin Type 1. J. Biomed. Mater Res. Part A 2011, 99, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sevostyanova, V.V.; Antonova, L.V.; Velikanova, E.A.; Matveeva, V.G.; Krivkina, E.O.; Glushkova, T.V.; Mironov, A.V.; Burago, A.Y.; Barbarash, L.S. Endothelialization of Polycaprolactone Vascular Graft under the Action of Locally Applied Vascular Endothelial Growth Factor. Bull Exp. Biol. Med. 2018, 165, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mironov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular Endothelial Growth Factor Improves Physico-Mechanical Properties and Enhances Endothelialization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(ε-caprolactone) Small-Diameter Vascular Grafts In vivo. Front Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; Mironov, A.V.; Krivkina, A.V.; Barbarash, O.L.; Barbarash, L.S. Influence Of bFGF, SDF-1α, or VEGF incorporated into tubular polymer scaffolds on the formation of small-diameter tissue-engineered blood vessel in vivo. Vestnik Transplantologii i Iskusstvennykh Organov 2018, 20, 96–109. [Google Scholar] [CrossRef]
- Antonova, L.V.; Sevostyanova, V.V.; Mironov, A.V.; Krivkina, E.O.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; Elgudin, Y.L.; Barbarash, L.S. In situ vascular tissue remodeling using biodegradable tubular scaffolds with incorporated growth factors and chemoattractant molecules. Complex Issues Cardiovasc. Dis. 2018, 7, 25–36. [Google Scholar] [CrossRef]
- Swartz, D.D.; Andreadis, S.T. Animal models for vascular tissue-engineering. Curr. Opin. Biotechnol. 2013, 24, 916–925. [Google Scholar] [CrossRef]
- Ahmed, M.; Hamilton, G.; Seifalian, A.M. The performance of a small-calibre graft for vascular reconstructions in a senescent sheep model. Biomaterials 2014, 35, 9033–9040. [Google Scholar] [CrossRef]
- Thomas, L.V.; Lekshmi, V.; Nair, P.D. Tissue engineered vascular grafts--preclinical aspects. Int. J. Cardiol. 2013, 167, 1091–1100. [Google Scholar] [CrossRef]
- Jaspan, V.N.; Hines, G.L. The current status of tissue-engineered vascular grafts. Cardiol. Rev. 2015, 23, 236–239. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Lewellis, S.W.; Knaut, H. Attractive guidance: How the chemokine SDF1/CXCL12 guides different cells to different locations. Semin. Cell Dev. Biol. 2012, 23, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater Sci. 2013, 34, 123–140. [Google Scholar]
- Liu, H.; Pancholi, M.; Stubbs, J., III; Raghavan, D. Influence of Hydroxyvalerate Composition of Polyhydroxy Butyrate Valerate (PHBV) Copolymer on Bone Cell Viability and In Vitro Degradation. J. Appl. Polym. Sci. 2010, 116, 3225–3231. [Google Scholar]
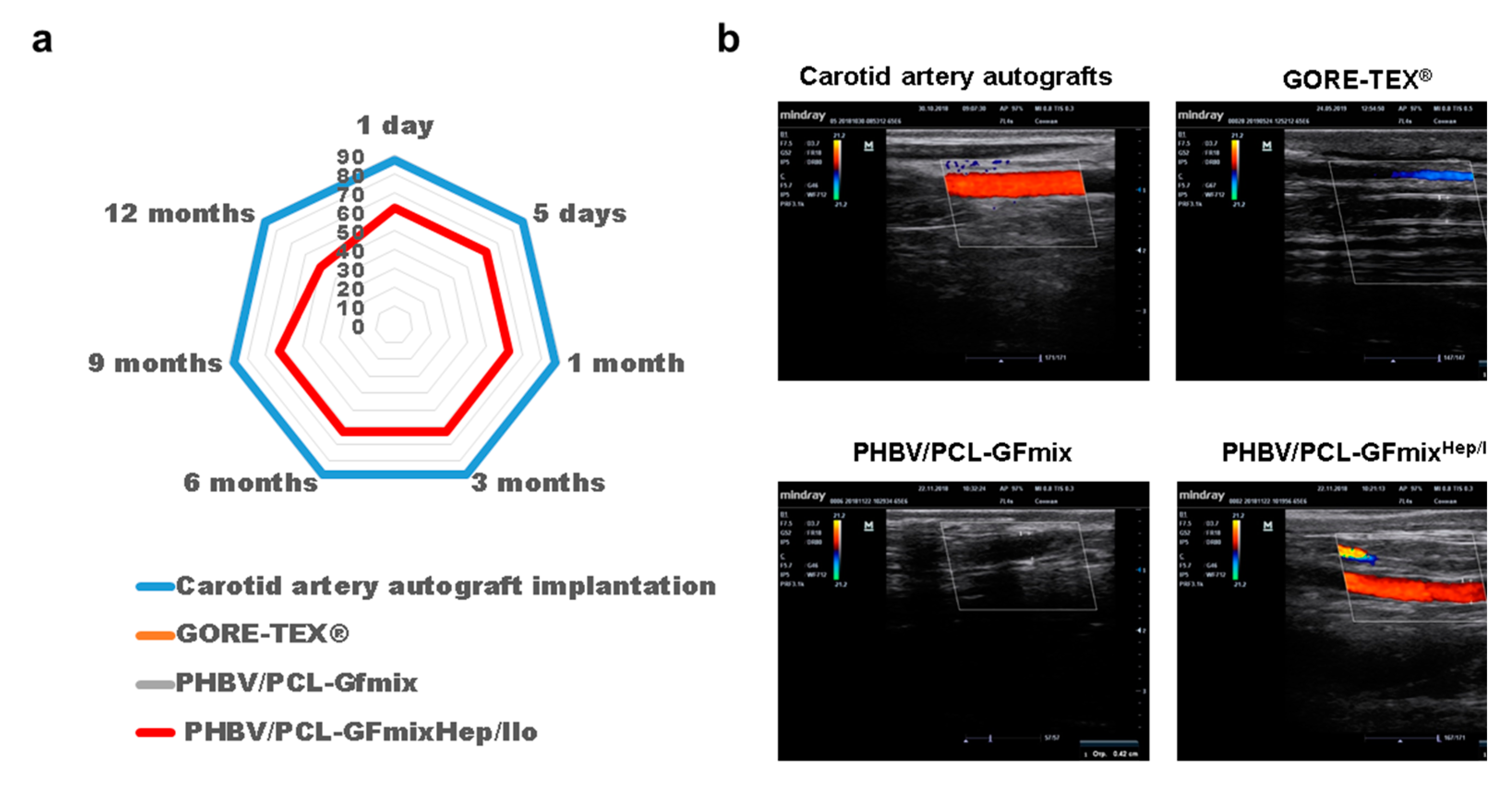
| Study Group | Primary Patency | Thrombosis | Calcium Deposition | |||||
|---|---|---|---|---|---|---|---|---|
| Timepoint | 1 Day | 6 Months | 12 Months | 1 Day | 6 Months | 12 Months | 6 Months | 12 Months |
| Carotid artery autograft implantation | 7/8 | 7/8 | 7/8 | 1/8 | 1/8 | 1/8 | 0/8 | 0/8 |
| GORE-TEX® | 0/5 | 0/5 | - | 5/5 | 5/5 | - | 3/5 | - |
| PHBV/PCL-GFmix | 0/8 | 0/8 | 0/8 | 8/8 | 8/8 | 8/8 | 0/8 | 0/8 |
| PHBV/PCL-GFmixHep/Ilo | 5/8 | 5/8 | 4/8 | 3/8 | 3/8 | 3/8 | 0/8 | 0/8 |
| Study Group | Carotid Artery Autograft Implantation | GORE-TEX® | PHBV/PCL-GFmix | PHBV/PCL-GFmixHep/Ilo |
|---|---|---|---|---|
| VEGF | N/A | - | + | + |
| bFGF | N/A | - | + | + |
| SDF-1α | N/A | - | + | + |
| heparin | N/A | - | - | + |
| iloprost | N/A | - | - | + |
| n | 8 | 5 | 8 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Krivkina, E.O.; Shabaev, A.R.; Rezvova, M.A.; Tkachenko, V.O.; Khanova, M.Y.; Sergeeva, T.Y.; Krutitskiy, S.S.; et al. A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals 2020, 13, 101. https://doi.org/10.3390/ph13050101
Antonova LV, Mironov AV, Yuzhalin AE, Krivkina EO, Shabaev AR, Rezvova MA, Tkachenko VO, Khanova MY, Sergeeva TY, Krutitskiy SS, et al. A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals. 2020; 13(5):101. https://doi.org/10.3390/ph13050101
Chicago/Turabian StyleAntonova, Larisa V., Andrey V. Mironov, Arseniy E. Yuzhalin, Evgeniya O. Krivkina, Amin R. Shabaev, Maria A. Rezvova, Vadim O. Tkachenko, Mariam Yu. Khanova, Tatiana Yu. Sergeeva, Sergei S. Krutitskiy, and et al. 2020. "A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep" Pharmaceuticals 13, no. 5: 101. https://doi.org/10.3390/ph13050101
APA StyleAntonova, L. V., Mironov, A. V., Yuzhalin, A. E., Krivkina, E. O., Shabaev, A. R., Rezvova, M. A., Tkachenko, V. O., Khanova, M. Y., Sergeeva, T. Y., Krutitskiy, S. S., & Barbarash, L. S. (2020). A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals, 13(5), 101. https://doi.org/10.3390/ph13050101






