Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation
Abstract
1. Introduction
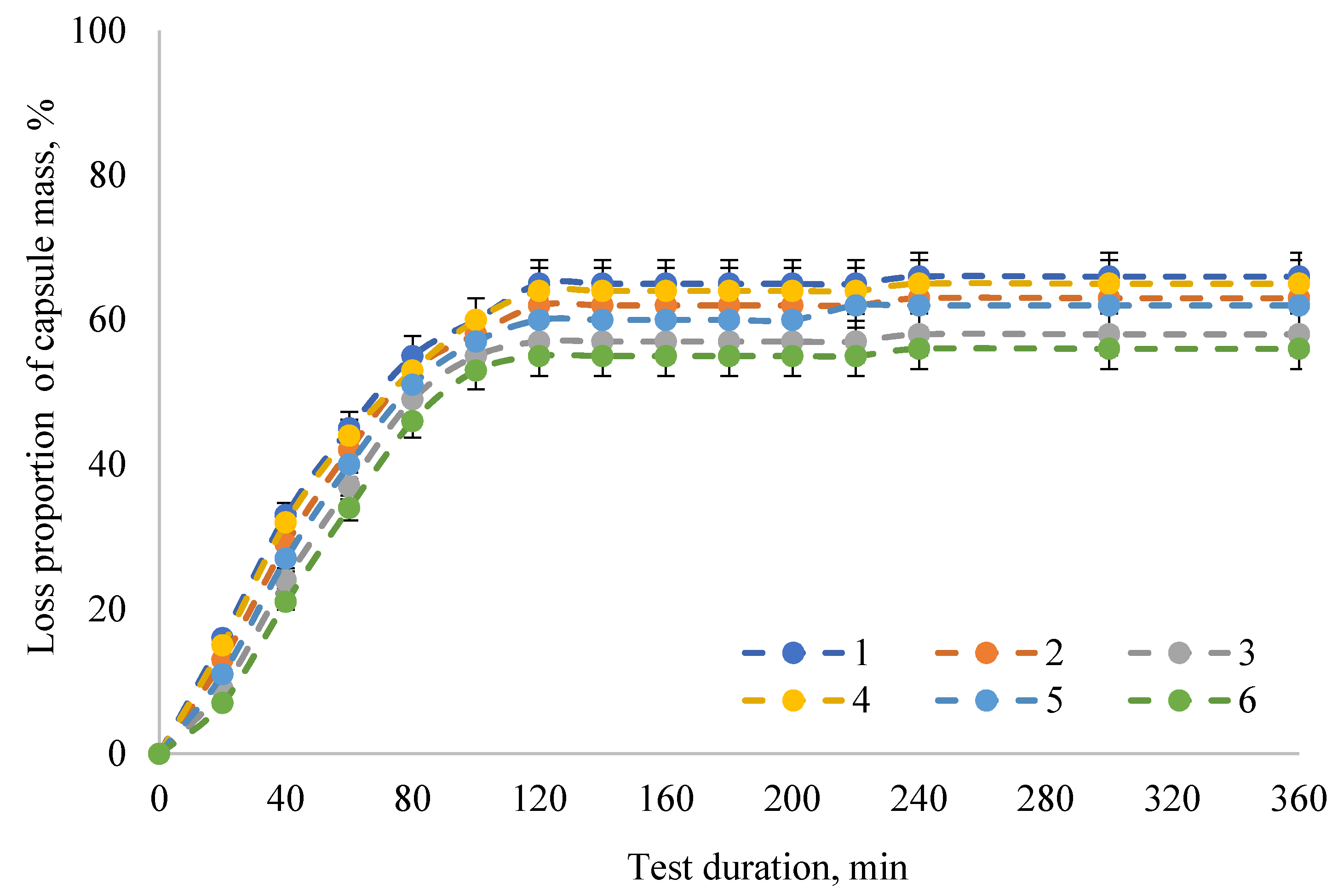
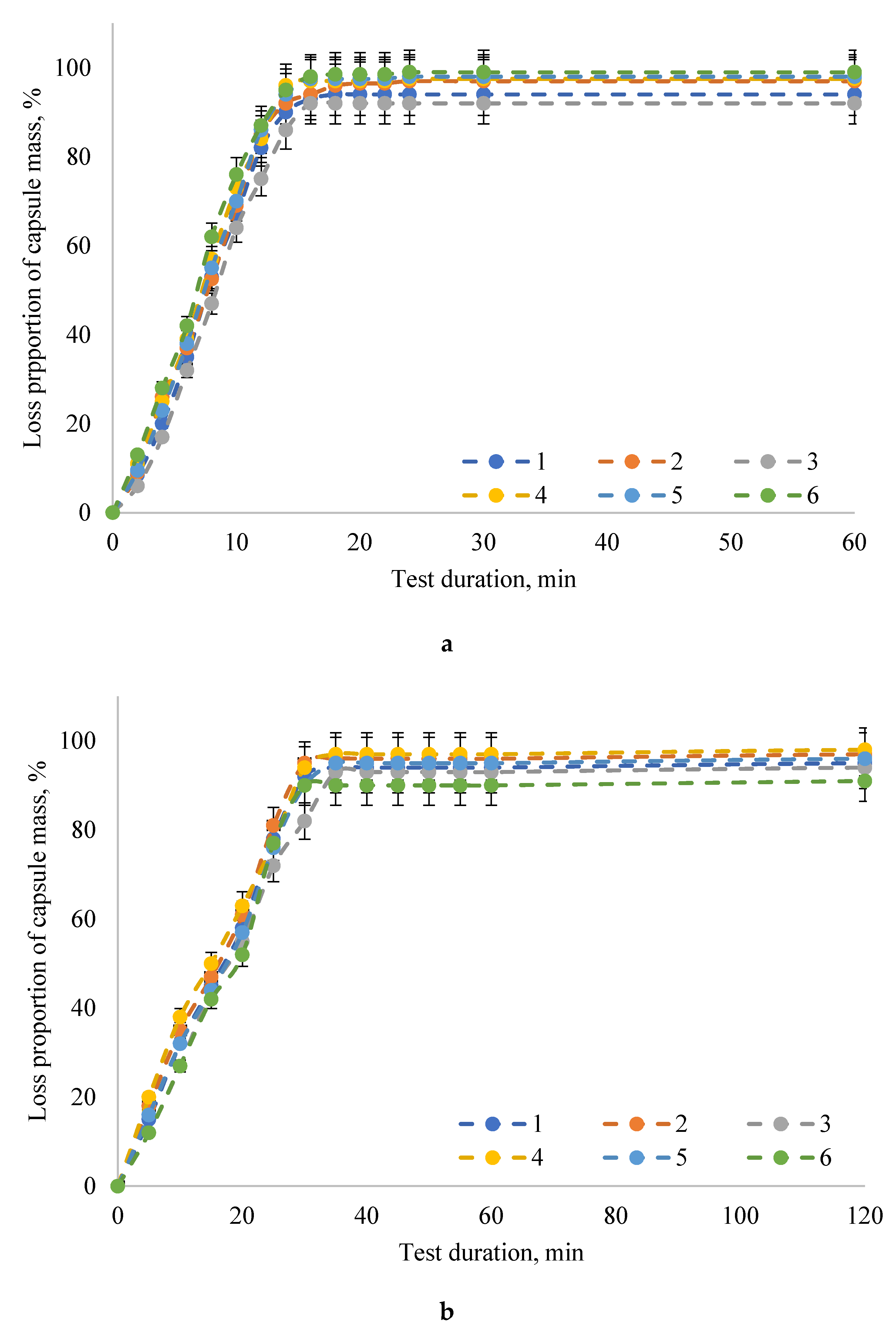
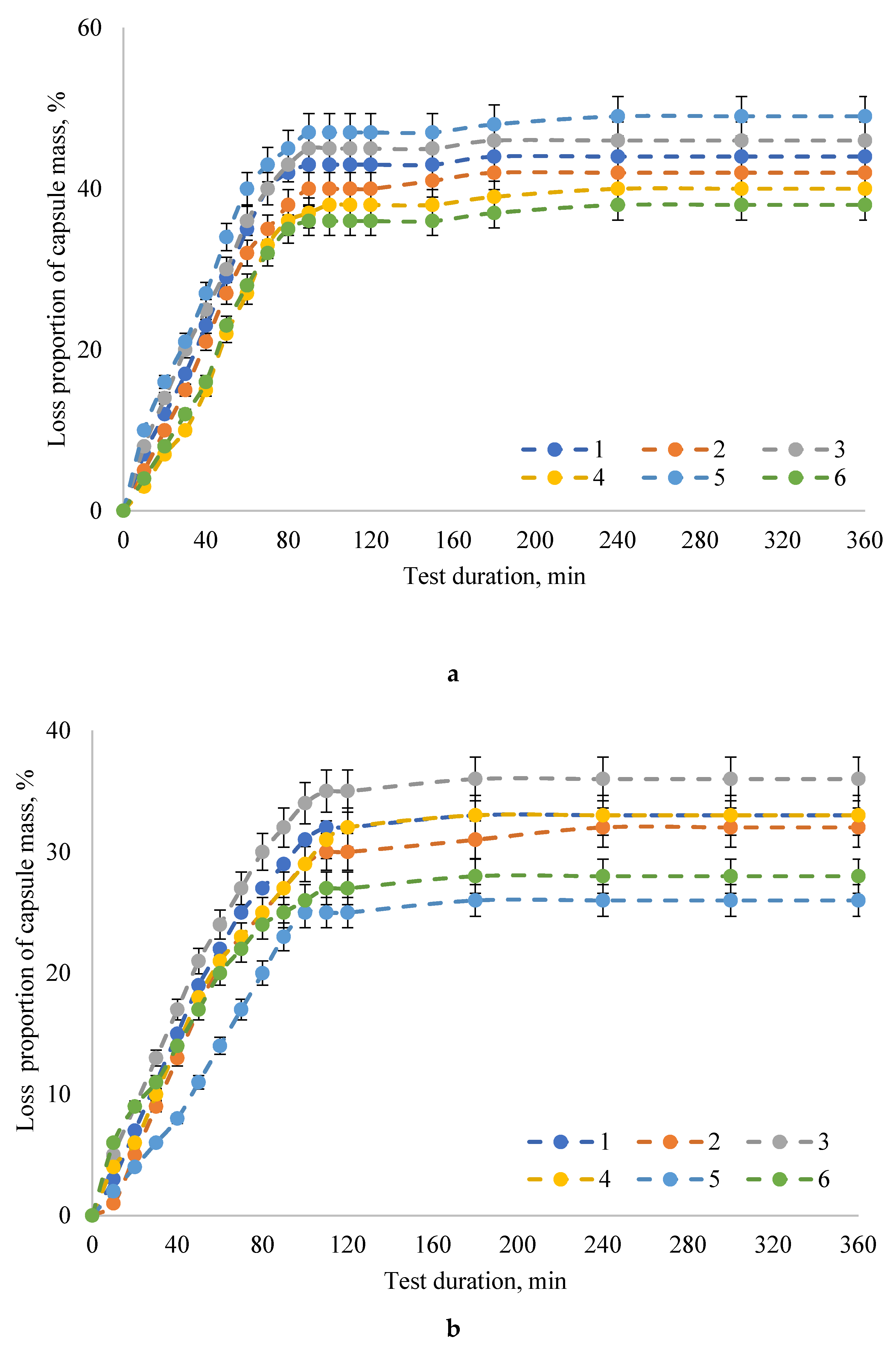
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Capsule Preparation
4.3. Capsule Quality Analysis
4.4. Capsule Destruction
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lichter-Konecki, U.; Vockley, J. Phenylketonuria: Current treatments and future developments. Drugs 2019, 79, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Bélanger-Quintana, A.; Burlina, A.; Harding, C.O.; Muntau, A.C. Up to date knowledge on different treatment strategies for phenylketonuria. Mol. Genet. Metab. 2011, 104, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.A.; Jack, G.; Peiris, R.J.D.; Starr, D.J.T.; Wade, H.E.; Wright, E.C.; Stern, J. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet 1980, 315, 392–394. [Google Scholar] [CrossRef]
- Acosta, P.B.; Yannicelli, S.; Singh, R.; Eisas, L.J., II; Kennedy, M.J.; Bernstein, L.; Rohr, F.; Trahms, C.; Koch, R.; Breck, J. Intake and blood levels of fatty acids in treated patients with phenylketonuria. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 253–259. [Google Scholar] [CrossRef]
- Acosta, P.B.; Yannicelli, S.; Singh, R.; Mofidi, S.; Steiner, R.; Devincentis, E.; Jurecki, E.; Bernstein, L.; Gleason, S.; Chetty, M.; et al. Nutrient intakes and physical growth of children with phenylketonuria undergoing nutrition therapy. J. Am. Diet. Assoc. 2003, 103, 1167–1173. [Google Scholar] [CrossRef]
- Yannicelli, S.; Singh, R.H.; Elsas, L.J., II; Mofidi, S.; Steiner, R.D. Iron status of children with phenylketonuria undergoing nutrition therapy assessed by transferrin receptors. Genet. Med. 2004, 6, 96–101. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Vered, I.; Schwartz, G.; Anikster, Y.; Abraham, S.; Segev, R.; Efrati, O. Peak bone mass in patients with phenylketonuria. J. Inherit. Metab. Dis. 2007, 30, 202–208. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Babich, O.O.; Prosekov, A.Y.; Dyshlyuk, L.S.; Ivanova, S.A. Investigation of kinetic aspects of L-phenylanine ammonia-lyase production in pigmental yeast. Chim. Oggi Chem. Today 2015, 33, 16–20. [Google Scholar]
- Babich, O.O.; Pokrovsky, V.S.; Anisimova, N.Y.; Sokolov, N.N.; Prosekov, A.Y. Recombinant l-phenylalanine ammonia lyase from Rhodosporidium toruloides as a potential anticancer agent. Biotechnol. Appl. Biochem. 2013, 60, 316–322. [Google Scholar] [CrossRef]
- Babich, O.O. Screening and identification of pigmental yeast producing L-phenylalanine ammonia-lyase and their physiological and biochemical characteristics. Foods Raw Mater. 2014, 2, 75–87. [Google Scholar] [CrossRef]
- Babich, O.O.; Dyshlyuk, L.S.; Milent’eva, I.S. Expression of recombinant L-phenylalanine ammonia-lyase in Escherichia coli. Foods Raw Mater. 2013, 1, 48–53. [Google Scholar] [CrossRef]
- Levy, H.L.; Sarkissian, C.N.; Scriver, C.R. Phenylalanine ammonia lyase (PAL): From discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metab. 2018, 124, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sarkissian, C.N.; Shao, Z.; Blain, F.; Peevers, R.; Su, H.; Helf, R.; Chang, T.M.S.; Scriver, C.R. A different approach to treatment of phenylketonuria: Phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc. Natl. Acad. Sci. USA 1999, 96, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, H.; Hough, E.; Fusetti, F.; Stevens, R.C.; Fusetti, F.; Stevens, R.C.; Martinez, A.; Flatmark, T.; Fusetti, F. Crystal structure of the catalytic domain of human phenylalanine hydroxylase reveals the structural basis for phenylketonuria. Nat. Struct. Biol. 1997, 4, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Harding, C.O.; Burton, B.K.; Grange, D.K.; Vockley, J.; Wasserstein, M.; Rice, G.M.; Dorenbaum, A.; Neuenburg, J.K.; Musson, D.G.; et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: An open-label, multicentre, phase 1 dose-escalation trial. Lancet 2014, 384, 37–44. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Pavsky, V.A.; Poplavskaya, M.A.; Novoselova, M.V. Studying the biokinetics of pigmented yeast by stochastic methods. Foods Raw Mater. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Babich, O.O.; Prosekov, A. Optimization of L-phenylalanine-ammonia-lyase lyophilization. Biomeditsinskaya Khimiya 2013, 59, 682–692. [Google Scholar] [CrossRef][Green Version]
- Orndorff, S.A.; Durham, D.R. Phenylalanine Ammonia Lyase-Producing Strains. U.S. Patent No. 4757015, 12 July 1988. [Google Scholar]
- McGuire, J.C.; Montgomery, J.P.; Yang, H.H. Phenylalanine Ammonia Lyase-Producing Microbial Cells. U.S. Patent No. 4636466, 13 January 1987. [Google Scholar]
- Babich, O.O.; Ulrikh, E.V. Study of physicochemical and thermal properties of l-phenylalanine ammonia-lyase. Foods Raw Mater. 2013, 1, 50–56. [Google Scholar] [CrossRef]
- Li, Q.; Kämpe, O. Recombinant Phe-Free Proteins for Use in the Treatment of Phenylketonuria. U.S. Patent No. 15512974, 18 January 2018. [Google Scholar]
- Babich, O.O.; Dyshlyuk, L.S.; Prosekov, A.Y. Effect of lyophilization conditions of recombinant L-phenylalanine-ammonia-lyase on enzyme properties. Middle East J. Sci. Res. 2013, 15, 1455–1459. [Google Scholar] [CrossRef]
- Eigtved, P.; Clausen, I.G. Stabilised Phenylalanine Ammonia Lyase. U.S. Patent No. 5753487, 19 May 1998. [Google Scholar]
- Kishore, G.M. Stabilization of L-Phenylalanine Ammonia-Lyase Enzyme. U.S. Patent No. 4562151, 31 December 1985. [Google Scholar]
- Vellard, M.C.; Fitzpatrick, P.A.; Kakkis, E.D. Compositions of prokaryotic phenylalanine ammonia-lyase and methods of using compositions thereof. U.S. Patent No. 7531341, 12 May 2009. [Google Scholar]
- Gilbert, H.J.; Jack, G.W. The effect of proteinases on phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis. Biochem. J. 1981, 199, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.J.; Tully, M. Protection of phenylalanine ammonia-lyase from proteolytic attack. Biochem. Biophys. Res. Commun. 1985, 131, 557–563. [Google Scholar] [CrossRef]
- Sarkissian, C.N.; Gámez, A.; Wang, L.; Charbonneau, M.; Fitzpatrick, P.; Lemontt, J.F.; Zhao, B.; Vellard, M.; Bell, S.M.; Henschell, C.; et al. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc. Natl. Acad. Sci. USA 2008, 105, 20894–20899. [Google Scholar] [CrossRef] [PubMed]
- Galle, M.; Gregory, P.-C.; Potthoff, A.; Hennines, F. Microbial Enzyme Mixtures Useful to Treat Digestive Disorders. U.S. Patent Application No. 20040057944, 25 March 2004. [Google Scholar]
- Sipos, T. High Buffer-Containing Enteric Coating Digestive Enzyme Bile Acid Compositions and Method of Treating Digestive Disorders Therewith. U.S. Patent No. 5,750,104, 12 May 1998. [Google Scholar]
- Sipos, T. Preparation of Enteric Coated Digestive Enzyme Compositions. U.S. Patent Application No. 4379125, 14 March 1978. [Google Scholar]
- Sipos, T. Compositions of Digestive Enzymes and Salts of Bile Acids and Process For Preparation Thereof. U.S. Patent No. 5,324,514, 28 June 1994. [Google Scholar]
- Bilton, G.L. Enzyme-Containing Digestive Aid Compostions. U.S. Patent No. 4,447,412, 8 May 1984. [Google Scholar]
- Dai, Y.; Sun, H.; Pal, S.; Zhang, Y.; Park, S.; Kabb, C.P.; Wei, W.D.; Sumerlin, B.S. Near-IR-induced dissociation of thermally-sensitive star polymers. Chem. Sci. 2017, 8, 1815–1821. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; et al. Self-Assembled Aptamer-Grafted Hyperbranched Polymer Nanocarrier for Targeted and Photoresponsive Drug Deliver. Angew. Chem. Int. Ed. 2018, 57, 17048–17052. [Google Scholar] [CrossRef]
- Sun, H.; Choi, W.; Zang, N.; Battistella, C.; Thompson, M.P.; Cao, W.; Zhou, X.; Forman, C.; Gianneschi, N.C. Bioactive Peptide Brush Polymers via Photoinduced Reversible-Deactivation Radical Polymerization. Angew. Chem. Int. Ed. 2019, 58, 17359–17364. [Google Scholar] [CrossRef]
- Dai, C.; Wang, B.; Zhao, H. Microencapsulation peptide and protein drugs delivery system. Colloids Surf. B Biointerfaces 2005, 41, 117–120. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, Á.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xue, J.; Zhu, G.; Liu, H.; Xia, Y. Encapsulation of a Phase-Change Material in Nanocapsules with a Well-Defined Hole in the Wall for the Controlled Release of Drugs. Angew. Chem. Int. Ed. 2019, 58, 10606–10611. [Google Scholar] [CrossRef]
- Vidovič, S.; Horvat, M.; Bizjak, A.; Planinsek, O.; Petek, B.; Burjak, M.; Peternel, L.; Parojčić, J.; Duris, J.; Ibric, S.; et al. Elucidating molecular properties of kappa-carrageenan as critical material attributes contributing to drug dissolution from pellets with a multivariate approach. Int. J. Pharm. 2019, 566, 662–673. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Smith, A.M.; Morris, G.A. The parallel lives of polysaccharides in food and pharmaceutical formulations. Curr. Opin. Food Sci. 2015, 4, 13–18. [Google Scholar] [CrossRef]
- Pereira de Sousa, I.; Bernkop-Schnürch, A. Pre-systemic metabolism of orally administered drugs and strategies to overcome it. J. Control Release 2014, 192, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Sousa, I.; Gourmel, C.; Berkovska, O.; Berger, M.; Leroux, J.-C. A microparticulate based formulation to protect therapeutic enzymes from proteolytic digestion: Phenylalanine ammonia lyase as case study. Sci. Rep. 2020, 10, 3651. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. Recent progress in preparation and agricultural application of microcapsules. J. Biomed. Mater. Res. A 2019, 107, 2371–2385. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Noskova, S.; Prosekov, A.; Ivanova, S.; Pavsky, V. The effectiveness of plant hydrocolloids at maintaining the quality characteristics of the encapsulated form of L-phenylalanine-ammonia-lyase. Heliyon 2020, 6, e03096. [Google Scholar] [CrossRef]
- OFS.1.2.4.0002.15. Mikrobiologicheskaya Chistota [OFS.1.2.4.0002.15 Microbiological Purity]; Ministry of Health of the Russian Federation: Moscow, Russia, 2016. (In Russian)
- Trusek-Holownia, A.; Noworyta, A. Efficient utilisation of hydrogel preparations with encapsulated enzymes–a case study on catalase and hydrogen peroxide degradation. Biotechnol. Rep. 2015, 6, 13–19. [Google Scholar] [CrossRef]
- Grimm, M.; Ball, K.; Scholz, E.; Schneider, F.; Sivert, A.; Benameur, H.; Kromrey, M.-L.; Kuhn, J.-P.; Weitschies, W. Characterization of the gastrointestinal transit and disintegration behavior of floating and sinking acid-resistant capsules using a novel MRI labeling technique. Eur. J. Pharm. Sci. 2019, 129, 163–172. [Google Scholar] [CrossRef]
- Weitschies, W.; Blume, H.; Mönnikes, H. Magnetic marker monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef]
- Khatri, P.; Desai, D.; Shelke, N.; Minko, T. Role of plasticizer in membrane coated extended release oral drug delivery system. J. Drug Deliv. Sci. Technol. 2018, 44, 231–243. [Google Scholar] [CrossRef]
- Hoag, S.W. Capsules dosage form: Formulation and manufacturing considerations. In Developing Solid Oral Dosage Forms. Pharmaceutical Theory and Practice; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 723–747. [Google Scholar] [CrossRef]
- Prosekov, A.; Ulrikh, E.; Dishluk, L.; Babich, O. The Chemical Resistance of Capsule Shells Based on Vegetable Analog of Pharmaceutical Gelatin. Biol. Med. 2015, 7, 2. [Google Scholar]
- Dyshlyuk, L.S.; Asyakina, L.K.; Babich, O.O. Studying of structure and properties of sodium alginate for obtaining biodegraded polymer materials. In Proceedings of the 3rd European Conference on Biology and Medical Sciences, Vienna, Austrian, 28 October 2014; East West Association for Advanced Studies and Higher Education GmbH: Vienna, Austrian, 2014; pp. 162–165. [Google Scholar]
- Prosekov, A.; Petrov, A.; Ulrich, E.; Dyshlyuk, L.; Kozlova, O. Physical and Chemical Properties of Capsule Shell Based on Plant Analogues of Pharmaceutical Gelatin. Biol. Med. 2015, 7, 2. [Google Scholar]
| Formulation No. | The Amount of Component, Mass. % | ||||
|---|---|---|---|---|---|
| Carrageenan | Agar-agar | CMC* | Glycerol | Water | |
| 1 | 10.0 | ̶ | 5.0 | 5.0 | 80.0 |
| 2 | 5.0 | ̶ | 10.0 | 5.0 | 80.0 |
| 3 | ̶ | 10.0 | 5.0 | 5.0 | 80.0 |
| 4 | 5.0 | 5.0 | ̶ | 5.0 | 85.0 |
| 5 | 10.0 | 10.0 | ̶ | 5.0 | 75.0 |
| 6 | 10.0 | 10.0 | 10.0 | 5.0 | 65.0 |
| Indicator | Capsule Samples | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Capsule diameter, mm | 6.5 ± 0.3a | 6.2 ± 0.3a | 6.0 ± 0.3a | 6.7 ± 0.3a | 6.9 ± 0.3a | 6.4 ± 0.3a |
| Capsule length, mm | 14.8 ± 0.7a | 15.0 ± 0.8a | 14.7 ± 0.7a | 14.5 ± 0.7a | 14.4 ± 0.7a | 14.6 ± 0.7a |
| Useful volume, ml | 0.25 ± 0.01a | 0.24 ± 0.01a | 0.27 ± 0.01a | 0.26 ± 0.01a | 0.23 ± 0.01a | 0.24 ± 0.01a |
| Capsule weight, mg | 42.0 ± 2.1a | 40.0 ± 2.0a | 45.0 ± 2.3a | 43.0 ± 2.2a | 44.0 ± 2.2a | 41.0 ± 2.1a |
| Moisture content, % | 12.8 ± 0.6a | 13.5 ± 0.7a | 12.5 ± 0.6a | 13.0 ± 0.7a | 14.2 ± 0.7b | 13.7 ± 0.7a |
| Disintegration of the capsule, min | 16.0 ± 0.8b | 15.5 ± 0.8a | 14.0 ± 0.7a | 15.0 ± 0.7a | 17.0 ± 0.9b | 15.0 ± 0.7a |
| Flowability, g/s | 8.5 ± 0.4b | 7.7 ± 0.4a | 8.0 ± 0.4a | 8.4 ± 0.4b | 7.2 ± 0.4a | 7.0 ± 0.4a |
| Indicator | Capsule Samples | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| QMAFAnM, CFU/g | 0.9·101 | 0.5·101 | 0.2·102 | 1.0·101 | 1.2·101 | 0.7·101 |
| Yeast and mold, cfu/g | - | - | - | - | - | - |
| Bacteria of the E. coli group, cells in 1 g | - | - | - | - | - | - |
| Medium Designation | Medium Composition |
|---|---|
| Intestinal | |
| SIF w/o pancreatin (pH 7.5) | Potassium dihydrogen phosphate—50 mmole Sodium hydroxide—up to pH 7.5 |
| FaSSIF (pH 6.5) | Sodium taurocholate—3 mmole Lecithin—0.2 mmole Maleic acid—19.12 mmole Sodium hydroxide—34.8 mmole Sodium chloride—68.62 mmole |
| Gastric | |
| SGF w/o pepsin (pH 1.2) | Sodium chloride—30 mmole Hydrochloric acid—up to pH 1.2 |
| FaSSGF (pH 1.6) | Sodium taurocholate—0.08 mmole Lecithin—0.02 mmole Pepsin with an activity of at least 600 U/mg—0.1 mg/mL Sodium chloride—34.2 mmole Hydrochloric acid—up to pH 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babich, O.; Dyshlyuk, L.; Prosekov, A.; Noskova, S.; Ivina, O.; Pavsky, V.; Ivanova, S.; Bulgakova, O. Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation. Pharmaceuticals 2020, 13, 63. https://doi.org/10.3390/ph13040063
Babich O, Dyshlyuk L, Prosekov A, Noskova S, Ivina O, Pavsky V, Ivanova S, Bulgakova O. Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation. Pharmaceuticals. 2020; 13(4):63. https://doi.org/10.3390/ph13040063
Chicago/Turabian StyleBabich, Olga, Lyubov Dyshlyuk, Alexander Prosekov, Svetlana Noskova, Oksana Ivina, Valery Pavsky, Svetlana Ivanova, and Olga Bulgakova. 2020. "Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation" Pharmaceuticals 13, no. 4: 63. https://doi.org/10.3390/ph13040063
APA StyleBabich, O., Dyshlyuk, L., Prosekov, A., Noskova, S., Ivina, O., Pavsky, V., Ivanova, S., & Bulgakova, O. (2020). Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation. Pharmaceuticals, 13(4), 63. https://doi.org/10.3390/ph13040063








