Preparation, Characterization, and Evaluation of Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved In Vitro and In Vivo Anticancer Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Nanoparticles
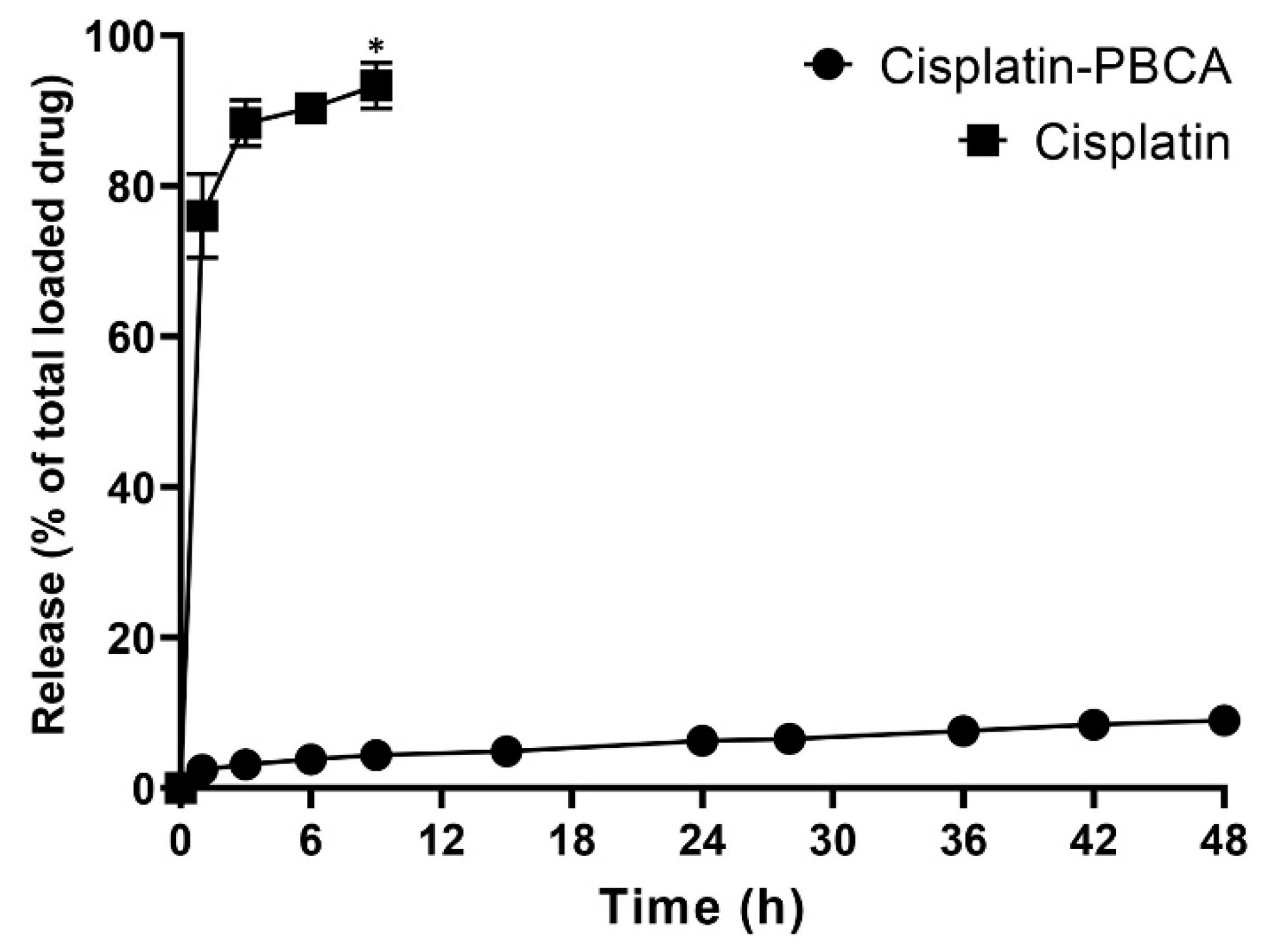
2.2. Drug Release Study
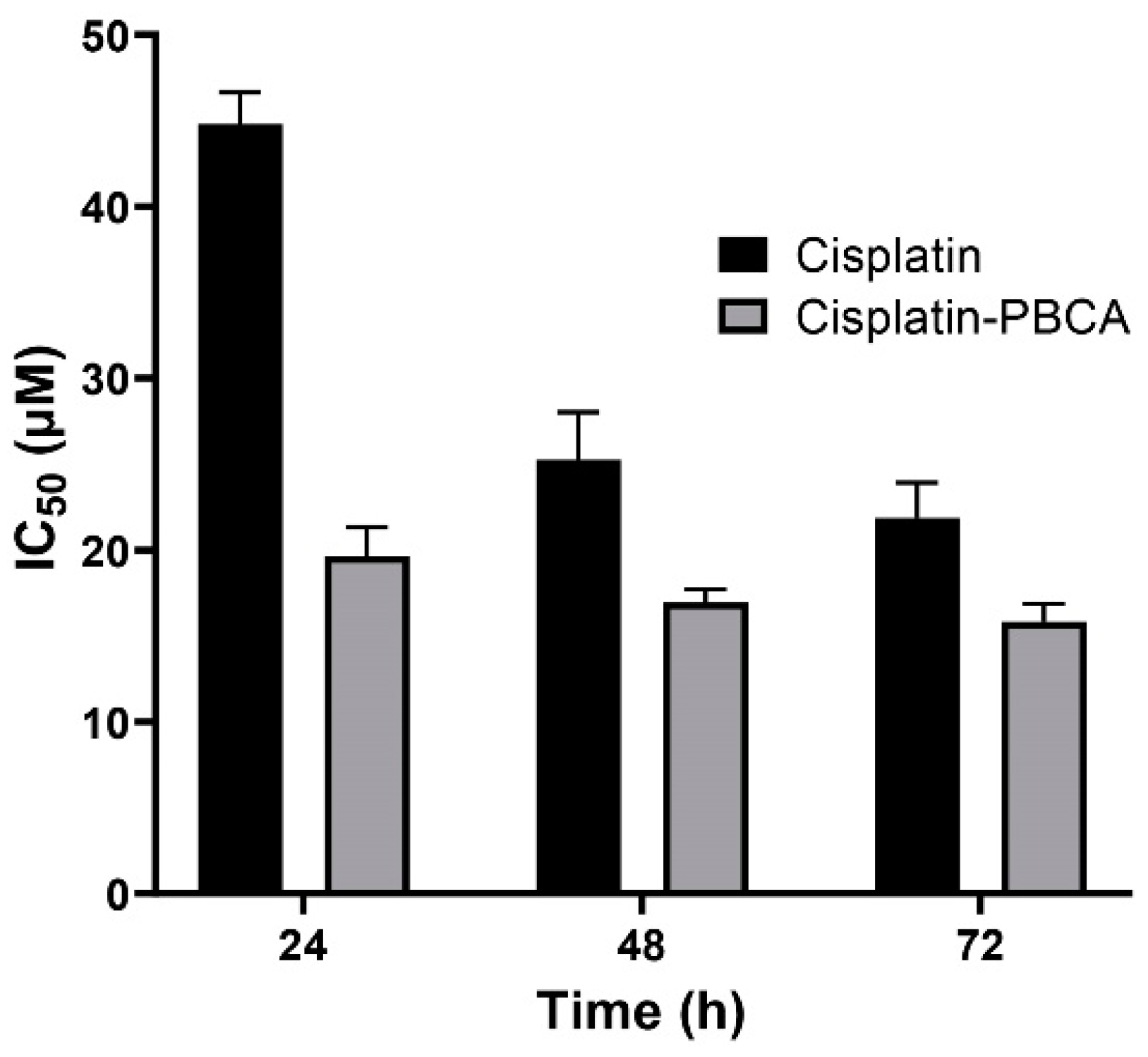
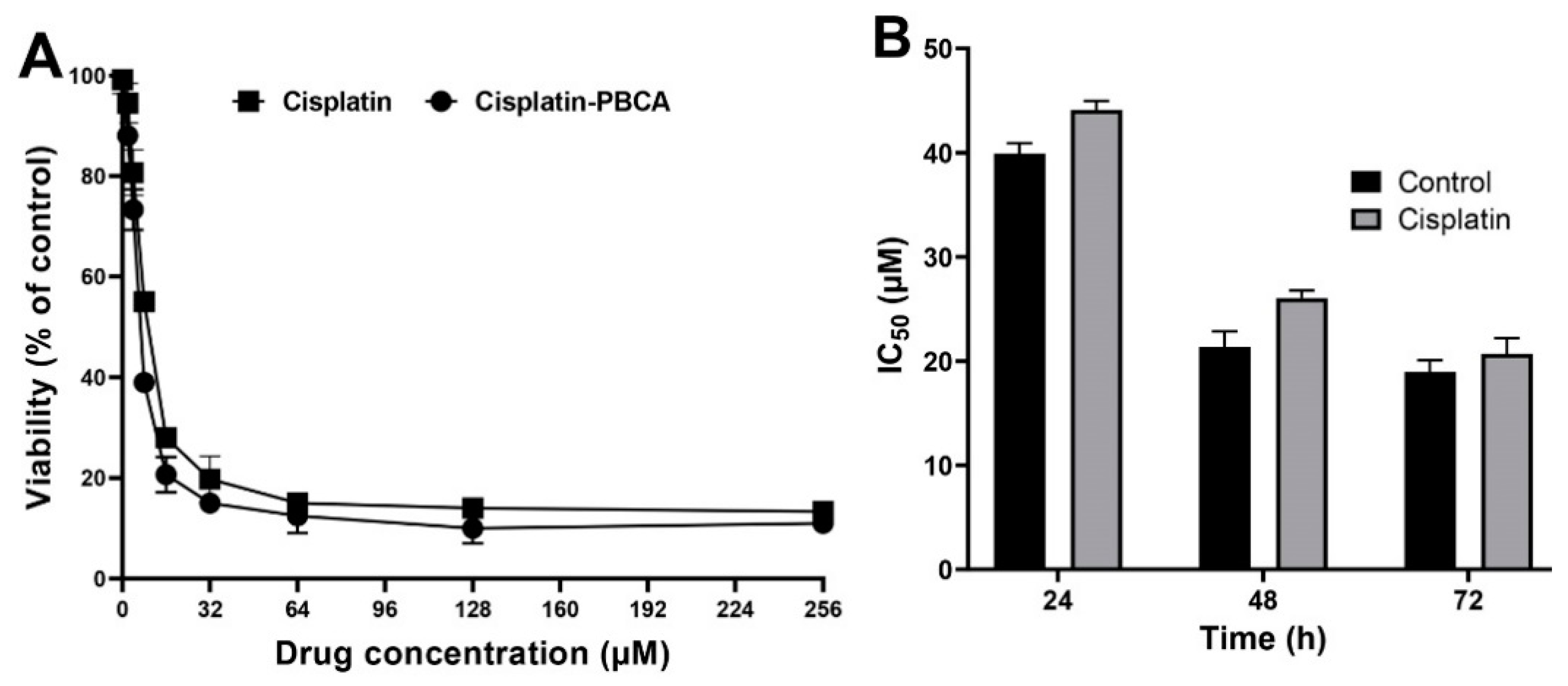
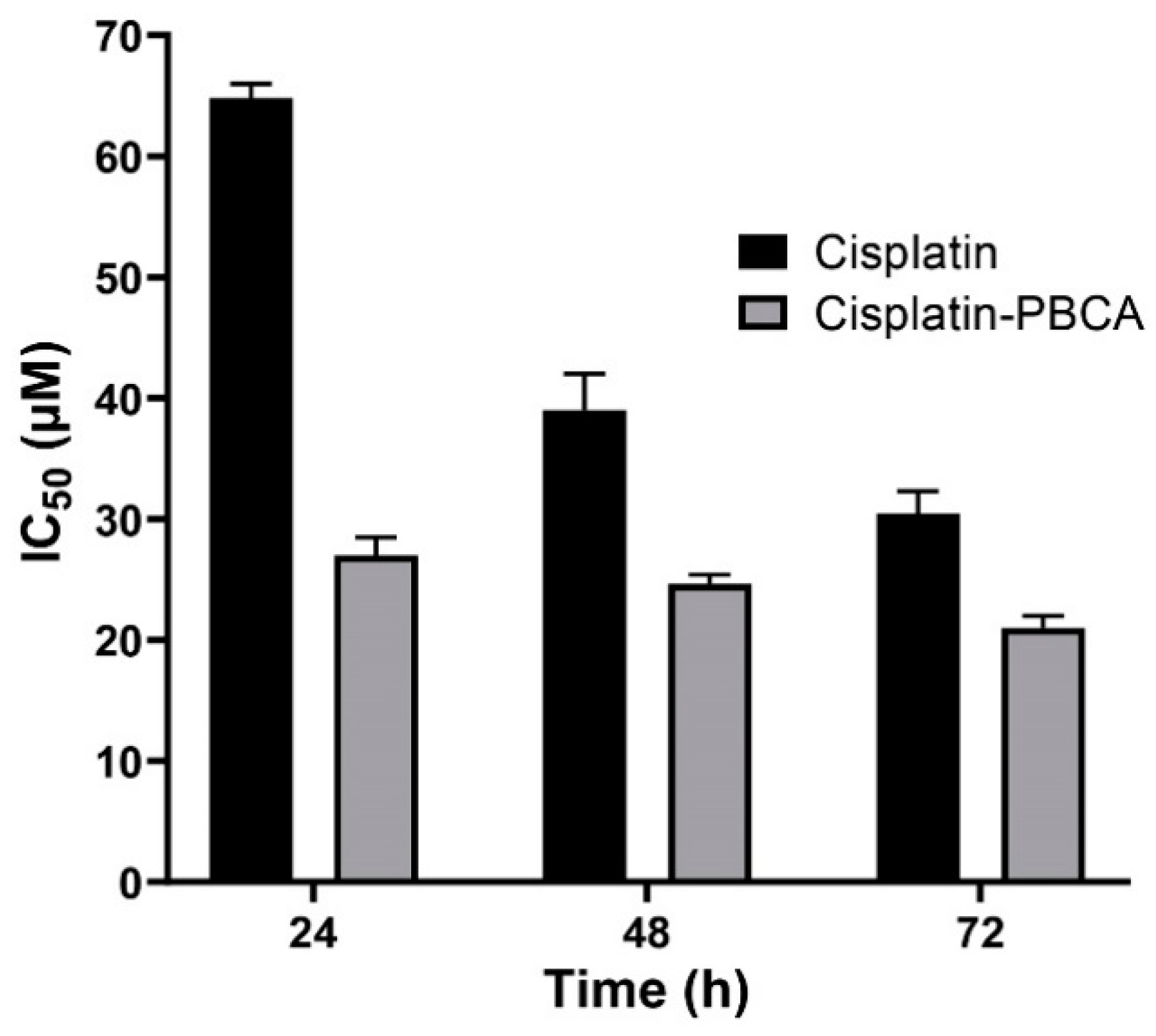
2.3. In Vitro Cytotoxicity Effects
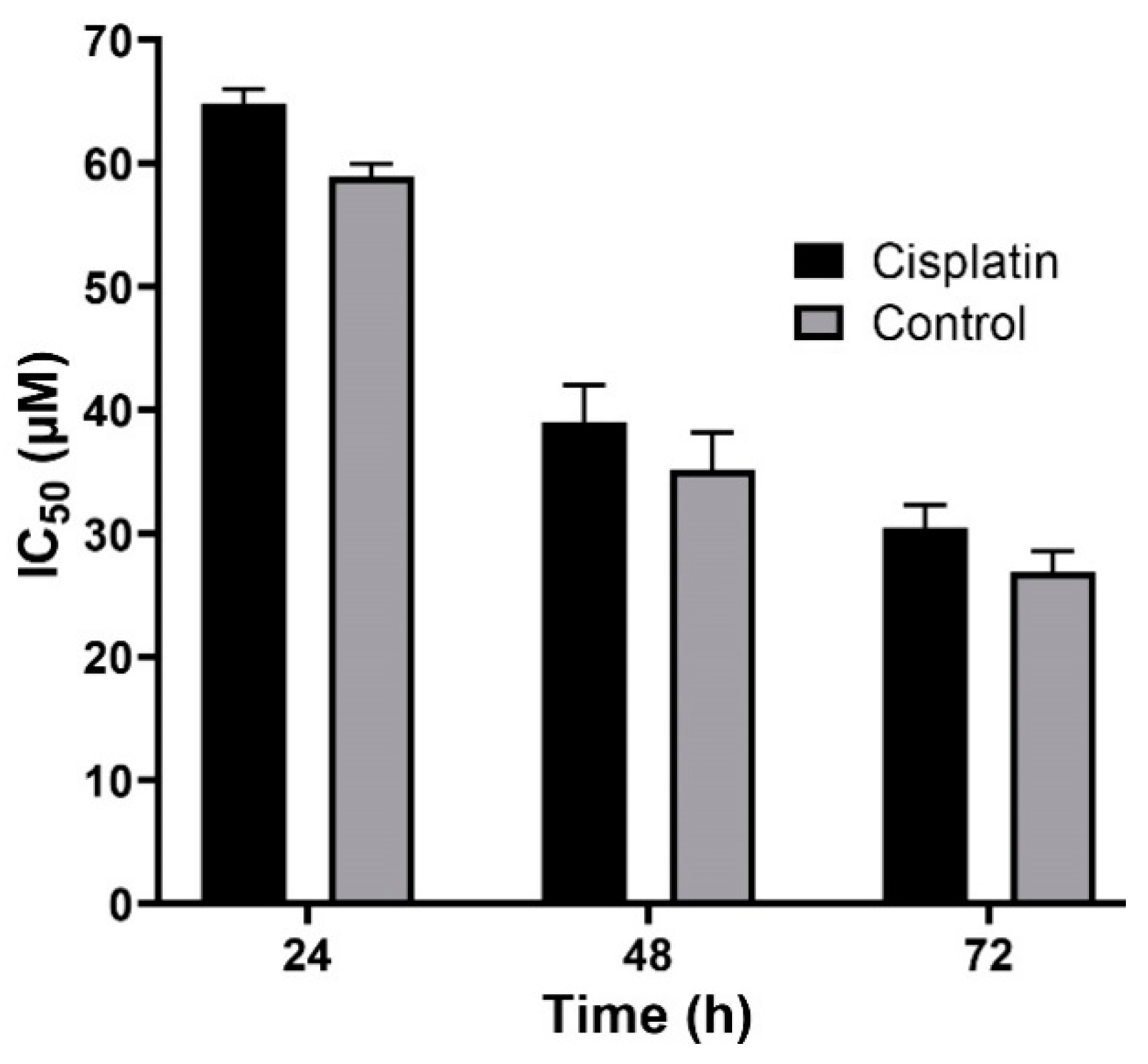
2.4. In Vitro Stability
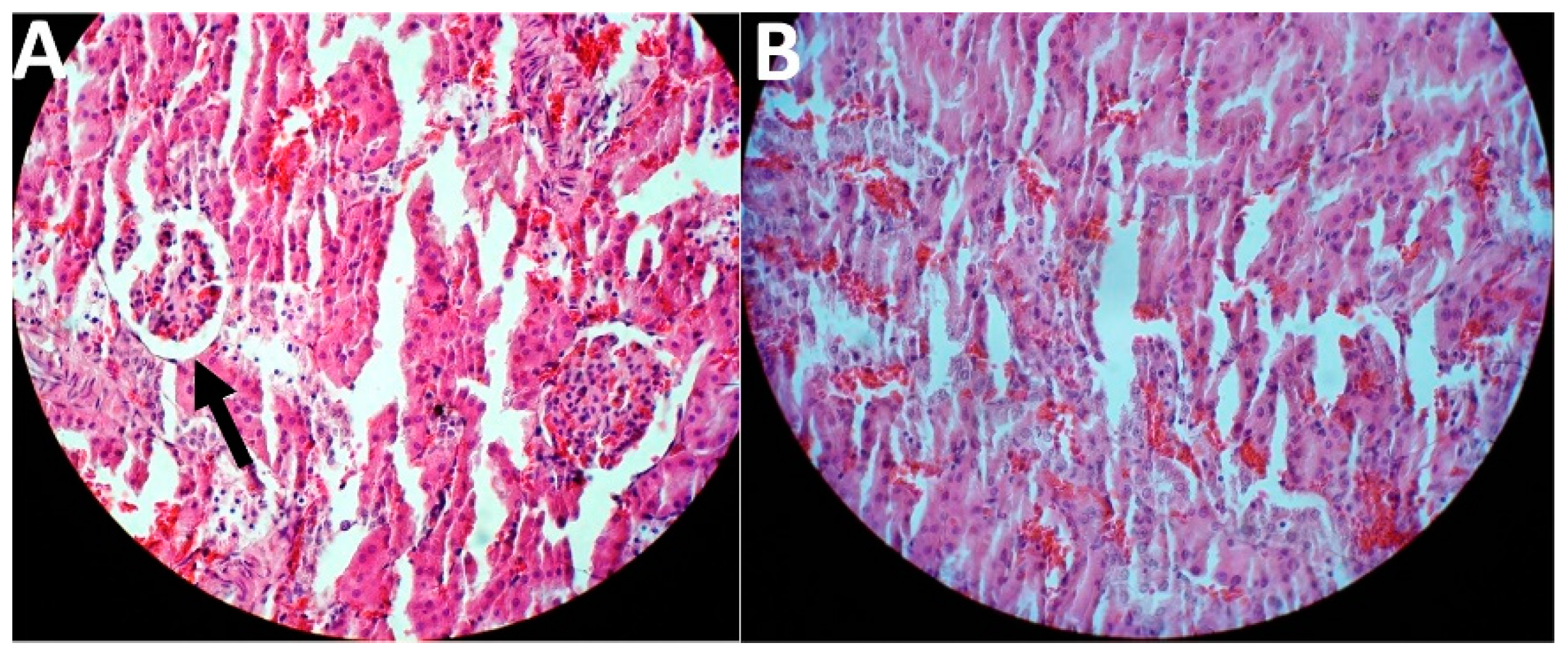
2.5. In Vivo Toxicity and Therapeutic Effects
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cisplatin-Loaded PBCA Nanoparticles
3.3. Characterization of Nanoparticles
3.3.1. Size, Size Distribution, and Zeta Potential
3.3.2. Drug Loading and Encapsulation Efficiencies
3.3.3. Morphology
3.3.4. Chemical Structure
3.4. Drug Release Study
3.5. In Vitro Cytotoxicity Effects
3.6. In Vitro Toxicity and Therapeutic Effects
3.6.1. In Vivo Kidney Tumor Establishment
3.6.2. In Vivo Anticancer and Toxicity Effects of the Formulations
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Cai, S.; Cai, A.; Zhao, Y.; Xu, T.; Ma, Y.; Xu, Y.; Wang, Y.; Wang, H.; Hu, Y. The improved antitumor efficacy of continuous intratumoral chemotherapy with cisplatin-loaded implants for the treatment of sarcoma 180 tumor-bearing mice. Drug Deliv. 2019, 26, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Gobe, G.; Johnson, D.W.; Healy, H. Inhibition of nuclear factor kappa B transcription activity drives a synergistic effect of pyrrolidine dithiocarbamate and cisplatin for treatment of renal cell carcinoma. Apoptosis 2010, 15, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Boonkaew, B.; Arora, J.; Mandava, S.H.; Maddox, M.M.; Chava, S.; Callaghan, C.; He, J.; Dash, S.; John, V.T. Comparison of sorafenib-loaded poly (lactic/glycolic) acid and DPPC liposome nanoparticles in the in vitro treatment of renal cell carcinoma. J. Pharm. Sci. 2015, 104, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Jahan, S.; Najar, L.; Hassan, S.; Mohammad, M. Metastatic clear cell variant of renal cell carcinoma of the mandible: Review and case report. Ann. Maxillofac. Surg. 2016, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jin, H.; Sun, S.; Chen, C.; Zhang, J.; Guo, Z.; Liu, X. Effects of gallic acid biofabricated rGO nanosheets combined with radiofrequency radiation for the treatment of renal cell carcinoma. Mater. Sci. Eng. C 2018, 93, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, G.; Di Corato, R.; Benedetti, M.; De Castro, F.; Fanizzi, F.P.; Rinaldi, R. Design and application of cisplatin-loaded magnetic nanoparticle clusters for smart chemotherapy. ACS Appl. Mater. Interfaces 2018, 11, 1864–1875. [Google Scholar] [CrossRef]
- Ahmad, Z.; Majeed, S.; Shah, A. In vitro release and cytotoxicity of cisplatin loaded methoxy poly (ethylene glycol)-block-poly (glutamic acid) nanoparticles against human breast cancer cell lines. J. Drug Deliv. Sci. Technol. 2018, 43, 85–93. [Google Scholar] [CrossRef]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Shahbazian, S.; Ebrahimi Shahmabadi, H. Drug delivery of cisplatin to breast cancer by polybutylcyanoacrylate nanoparticles. Adv. Polym. Technol. 2018, 37, 674–678. [Google Scholar] [CrossRef]
- Behera, A.; Patil, S.; Sahoo, S.; Sahoo, S. Nanosizing of drugs: A promising approach for drug delivery. Pharm. Sin. 2010, 1, 20–28. [Google Scholar]
- Ghaferi, M.; Asadollahzadeh, M.J.; Akbarzadeh, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Enhanced efficacy of PEGylated liposomal cisplatin: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2020, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Cabot, P.J.; Moyle, P.M. Glucagon-like peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019, 16, 2278–2295. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Esfahani, M.K.M.; Ghassemi, S.; Akbarzadeh, A.; Hassanshahi, G. In vitro evaluation of the efficacy of liposomal and pegylated liposomal hydroxyurea. Indian J. Clin. Biochem. 2014, 29, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Mansouri, H.; Esfahani, M.K.M.; Movahedi, F.; Akbarzadeh, A.; Chiani, M. Archaeosome: As new drug carrier for delivery of paclitaxel to breast cancer. Indian J. Clin. Biochem. 2014, 29, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.K.M.; Alavi, S.E.; Movahedi, F.; Alavi, F.; Akbarzadeh, A. Cytotoxicity of liposomal Paclitaxel in breast cancer cell line mcf-7. Indian J. Clin. Biochem. 2013, 28, 358–360. [Google Scholar] [CrossRef]
- Esfahani, M.K.M.; Alavi, S.E.; Akbarzadeh, A.; Ghassemi, S.; Saffari, Z.; Farahnak, M.; Chiani, M. Pegylation of nanoliposomal paclitaxel enhances its efficacy in breast cancer. Trop. J. Pharm. Res. 2014, 13, 1195–1198. [Google Scholar] [CrossRef]
- Alavi, S.E.; Muflih Al Harthi, S.; Ebrahimi Shahmabadi, H.; Akbarzadeh, A. Cisplatin-loaded polybutylcyanoacrylate nanoparticles with improved properties as an anticancer agent. Int. J. Mol. Sci. 2019, 20, 1531. [Google Scholar] [CrossRef]
- Raza, A.; Sime, F.B.; Cabot, P.J.; Maqbool, F.; Roberts, J.A.; Falconer, J.R. Solid nanoparticles for oral antimicrobial drug delivery: A review. Drug Discov.Today 2019, 24, 858–866. [Google Scholar] [CrossRef]
- Alsaab, H.O. Tumor Multicomponent Targeting Polymer-Lipid Hybrid Nanoparticles to Overcome Drug Resistance in Renal Cell Carcinoma. Ph.D. Thesis, Wayne State University, Detroit, MI, USA, 2018. [Google Scholar]
- Liu, J.; Abshire, C.; Carry, C.; Sholl, A.B.; Mandava, S.H.; Datta, A.; Ranjan, M.; Callaghan, C.; Peralta, D.V.; Williams, K.S. Nanotechnology combined therapy: Tyrosine kinase-bound gold nanorod and laser thermal ablation produce a synergistic higher treatment response of renal cell carcinoma in a murine model. BJU Int. 2017, 119, 342–348. [Google Scholar] [CrossRef]
- Raza, A.; Sun, H.; Bano, S.; Zhao, Y.; Xu, X.; Tang, J. Preparation, characterization, and in vitro anti-inflammatory evaluation of novel water soluble kamebakaurin/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2017, 1130, 319–326. [Google Scholar] [CrossRef]
- Fatemeh, D.R.A.; Shahmabadi, H.E.; Abedi, A.; Alavi, S.E.; Movahedi, F.; Esfahani, M.K.M.; Mehrizi, T.Z.; Akbarzadeh, A. Polybutylcyanoacrylate nanoparticles and drugs of the platinum family: Last status. Indian J. Clin. Biochem. 2014, 29, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour Doun, S.K.; Alavi, S.E.; Koohi Moftakhari Esfahani, M.; Ebrahimi Shahmabadi, H.; Alavi, F.; Hamzei, S. Efficacy of Cisplatin-loaded poly butyl cyanoacrylate nanoparticles on the ovarian cancer: An in vitro study. Tumor Biol. 2014, 35, 7491–7497. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Asghar, S.; Zhu, X.; Chen, Z.; Liu, J.; Li, Y.; Li, H.; Ping, Q.; Xiao, Y. Enhanced oral bioavailability of 10-hydroxycamptothecin through the use of poly (n-butyl cyanoacrylate) nanospheres. Drug Dev. Ind. Pharm. 2017, 43, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chen, C.-M.; Lee, Y.-D. Synthesis of high loading and encapsulation efficient paclitaxel-loaded poly (n-butyl cyanoacrylate) nanoparticles via miniemulsion. Int. J. Pharm. 2007, 338, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Shahmabadi, H.; Movahedi, F.; Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Eslamifar, A.; Mohammadi Anaraki, G.; Akbarzadeh, A. Efficacy of Cisplatin-loaded polybutyl cyanoacrylate nanoparticles on the glioblastoma. Tumor Biol. 2014, 35, 4799–4806. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Chen, R.; Wang, Y.; Sun, Y.; Jiang, Y.; Li, G. Paclitaxel-loaded poly (n-butylcyanoacrylate) nanoparticle delivery system to overcome multidrug resistance in ovarian cancer. Pharm. Res. 2011, 28, 897–906. [Google Scholar] [CrossRef]
- Petri, B.; Bootz, A.; Khalansky, A.; Hekmatara, T.; Müller, R.; Uhl, R.; Kreuter, J.; Gelperina, S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly (butyl cyanoacrylate) nanoparticles: Revisiting the role of surfactants. J. Controll. Release 2007, 117, 51–58. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Arias, J.L.; Prados, J.; Martínez, M.A.R.; Entrena, J.M.; Luque, R.; Melguizo, C. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly (butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 2015, 10, 1291. [Google Scholar]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: New York, NY, USA, 2019; pp. 59–100. [Google Scholar]
- Koutelidakis, A.E.; Argyri, K.; Sevastou, Z.; Lamprinaki, D.; Panagopoulou, E.; Paximada, E.; Sali, A.; Papalazarou, V.; Mallouchos, A.; Evageliou, V. Bioactivity of epigallocatechin gallate nanoemulsions evaluated in mice model. J. Med. Food 2017, 20, 923–931. [Google Scholar] [CrossRef]
- Palanisamy, K.; Devabharathi, V.; Sundaram, N.M. Antibacterial study of olive oil stabilized superparamagnetic iron oxide nanoparticles. Nano Vis. 2013, 3, 145–152. [Google Scholar]
- THOMAS, D.; Latha, M.; THOMAS, K.K. Alginate/Chitosan nanoparticles for improved oral delivery of rifampicin: Optimization, characterization and in vitro evaluation. Asian J. Chem. 2018, 30, 736–740. [Google Scholar] [CrossRef]
- Pal, C.; Sosa-Vargas, L.; Ojeda, J.J.; Sharma, A.K.; Cammidge, A.N.; Cook, M.J.; Ray, A.K. Charge transport in lead sulfide quantum dots/phthalocyanines hybrid nanocomposites. Org. Electron. 2017, 44, 132–143. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Zhan, Y.; Zheng, C.; Wang, G. A novel and simple one-step solid-state reaction for the synthesis of PbS nanoparticles in the presence of a suitable surfactant. Mater. Res. Bull. 2001, 36, 1977–1984. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, H.-L.; Li, J.; Qian, F.-C.; Li, L.-Q.; Niu, P.-P.; Dai, L.-C. Development of hybrid-type modified chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells. Hepatobil. Pancreat. Dis. Int. 2015, 14, 82–89. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Yang, B.; Deng, F.; Ji, J.; Yang, Y.; Huang, Z.; Zhang, X.; Wei, Y. Luminescence tunable fluorescent organic nanoparticles from polyethyleneimine and maltose: Facile preparation and bioimaging applications. RSC Adv. 2014, 4, 22294–22298. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pang, X.; Yu, K.; Liu, L.; Zhou, J. Preparation, characterization and in vivo distribution of solid lipid nanoparticles loaded with cisplatin. Pharm. Int. J. Pharm. Sci. 2008, 63, 593–597. [Google Scholar]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, S. Self-assembled nanomaterials for synergistic antitumour therapy. J. Mater. Chem. B 2018, 6, 6685–6704. [Google Scholar] [CrossRef]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Bagherpour Doun, S.K.; Halal Khor, S.; Qujeq, D.; Ebrahimi Shahmabadi, H.; Alavi, S.E.; Movahedi, F.; Akbarzadeh, A. Effect of sodium chloride on efficiency of cisplatinum dissolved in dimethyl sulfoxide: An in vitro study. Indian J. Clin. Biochem. 2014, 29, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Cauchois, O. Shape-controlled nanoparticles for drug delivery and targeting applications. In Polymer Nanoparticles for Nanomedicines; Springer: Cham, Switzerland, 2016; pp. 159–184. [Google Scholar]
- McDaid, H.M.; Bhattacharya, S.K.; Chen, X.-T.; He, L.; Shen, H.-J.; Gutteridge, C.E.; Horwitz, S.B.; Danishefsky, S.J. Structure-activity profiles of eleutherobin analogs and their cross-resistance in Taxol-resistant cell lines. Cancer Chemother. Pharmacol. 1999, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Luechtefeld, T.; Marsh, D.; Rowlands, C.; Hartung, T. Machine learning of toxicological big data enables read-across structure activity relationships (RASAR) outperforming animal test reproducibility. Toxicol. Sci. 2018, 165, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Munaweera, I.; Shi, Y.; Koneru, B.; Patel, A.; Dang, M.H.; Di Pasqua, A.J.; Balkus Jr, K.J. Nitric oxide-and cisplatin-releasing silica nanoparticles for use against non-small cell lung cancer. J. Inorg. Biochem. 2015, 153, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, Y.; Chen, Y.; Shukla, A.J. Application of artificial neural networks in the design of controlled release drug delivery systems. Adv. Drug Deliv. Rev. 2003, 55, 1201–1215. [Google Scholar] [CrossRef]
- Tian, X.-H.; Lin, X.-N.; Wei, F.; Feng, W.; Huang, Z.-C.; Wang, P.; Ren, L.; Diao, Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011, 6, 445. [Google Scholar]
- Zhang, Q.; Shen, Z.; Nagai, T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int. J. Pharm. 2001, 218, 75–80. [Google Scholar] [CrossRef]
- Singh, R.; Lillard Jr, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Misra, C.; Gaur, M.; Gupta, L.N. Nanotechnology: Emerging platform for drug based delivery system in cancer. J. Drug Deliv. Ther. 2019, 9, 744–749. [Google Scholar]
- Ou, H.; Cheng, T.; Zhang, Y.; Liu, J.; Ding, Y.; Zhen, J.; Shen, W.; Xu, Y.; Yang, W.; Niu, P. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018, 65, 339–348. [Google Scholar] [CrossRef]
- Katstra, W.E. Fabrication of Complex Oral Drug Delivery Forms by Three Dimensional Printing (tm). Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2001. [Google Scholar]
- Chauhan, M.; Suthar, S.; Shah, A.; Polara, M.; Patel, M.; Patel, J. Bilayer tablet: Immediate release and sustain release: A review. Res. J. Pharm. Technol. 2012, 5, 716. [Google Scholar]
- Yordanov, G.; Evangelatov, A.; Skrobanska, R. Epirubicin loaded to pre-polymerized poly (butyl cyanoacrylate) nanoparticles: Preparation and in vitro evaluation in human lung adenocarcinoma cells. Coll. Surf. B Biointerfaces 2013, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Chacko, R.T.; Jiwpanich, S.; Bickerton, S.; Babu, R.P.; Thayumanavan, S. Self-cross-linked polymer nanogels: A versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010, 132, 17227–17235. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.; dos Santos, C.; dos Santos, E.P.; Mansur, C.R. Nanosystems in photoprotection. J. Nanosci. Nanotechnol. 2015, 15, 9679–9688. [Google Scholar] [CrossRef]
- Kimoto, T.; Koya, S.; Hino, K.; Yamamoto, Y.; Nomura, Y.; Micallef, M.J.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Renal carcinogenesis induced by ferric nitrilotriacetate in mice, and protection from it by Brazilian propolis and artepillin C. Pathol. Int. 2000, 50, 679–689. [Google Scholar] [CrossRef]
- Rahmawati, O.; Pratami, D.K.; Raffiudin, R.; Sahlan, M. Alpha-glucosidase inhibitory activity of stingless bee honey from Tetragonula biroi and Tetragonula laeviceps. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2019; p. 030001. [Google Scholar]
- Ariza-Ortega, J.A.; Robles-López, M.R.; del Socorro Cruz-Cansino, N.; Betanzos-Cabrera, G.; de Jesús Saucedo-Molina, T.; Robles-De-la-Torre, R.R. Preliminary study on the application of an electric field as a method of preservation for virgin olive oil. Acta Sci. Technol. 2016, 38, 391–394. [Google Scholar] [CrossRef]
- Pina, M.F.; Lau, W.; Scherer, K.; Parhizkar, M.; Edirisinghe, M.; Craig, D. The generation of compartmentalized nanoparticles containing siRNA and cisplatin using a multi-needle electrohydrodynamic strategy. Nanoscale 2017, 9, 5975–5985. [Google Scholar] [CrossRef]
- Carland, M.; Tan, K.J.; White, J.M.; Stephenson, J.; Murray, V.; Denny, W.A.; McFadyen, W.D. Syntheses, crystal structure and cytotoxicity of diamine platinum (II) complexes containing maltol. J. Inorg. Biochem. 2005, 99, 1738–1743. [Google Scholar] [CrossRef]
- Awai, M.; Narasaki, M.; Yamanoi, Y.; Seno, S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am. J. Pathol. 1979, 95, 663. [Google Scholar]
- Vargas-Olvera, C.Y.; Sánchez-González, D.J.; Solano, J.D.; Aguilar-Alonso, F.A.; Montalvo-Muñoz, F.; Martínez-Martínez, C.M.; Medina-Campos, O.N.; Ibarra-Rubio, M.E. Characterization of N-diethylnitrosamine-initiated and ferric nitrilotriacetate-promoted renal cell carcinoma experimental model and effect of a tamarind seed extract against acute nephrotoxicity and carcinogenesis. Mol. Cell. Biochem. 2012, 369, 105–117. [Google Scholar] [CrossRef]
- Sethi, M.; Sukumar, R.; Karve, S.; Werner, M.E.; Wang, E.C.; Moore, D.T.; Kowalczyk, S.R.; Zhang, L.; Wang, A.Z. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale 2014, 6, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
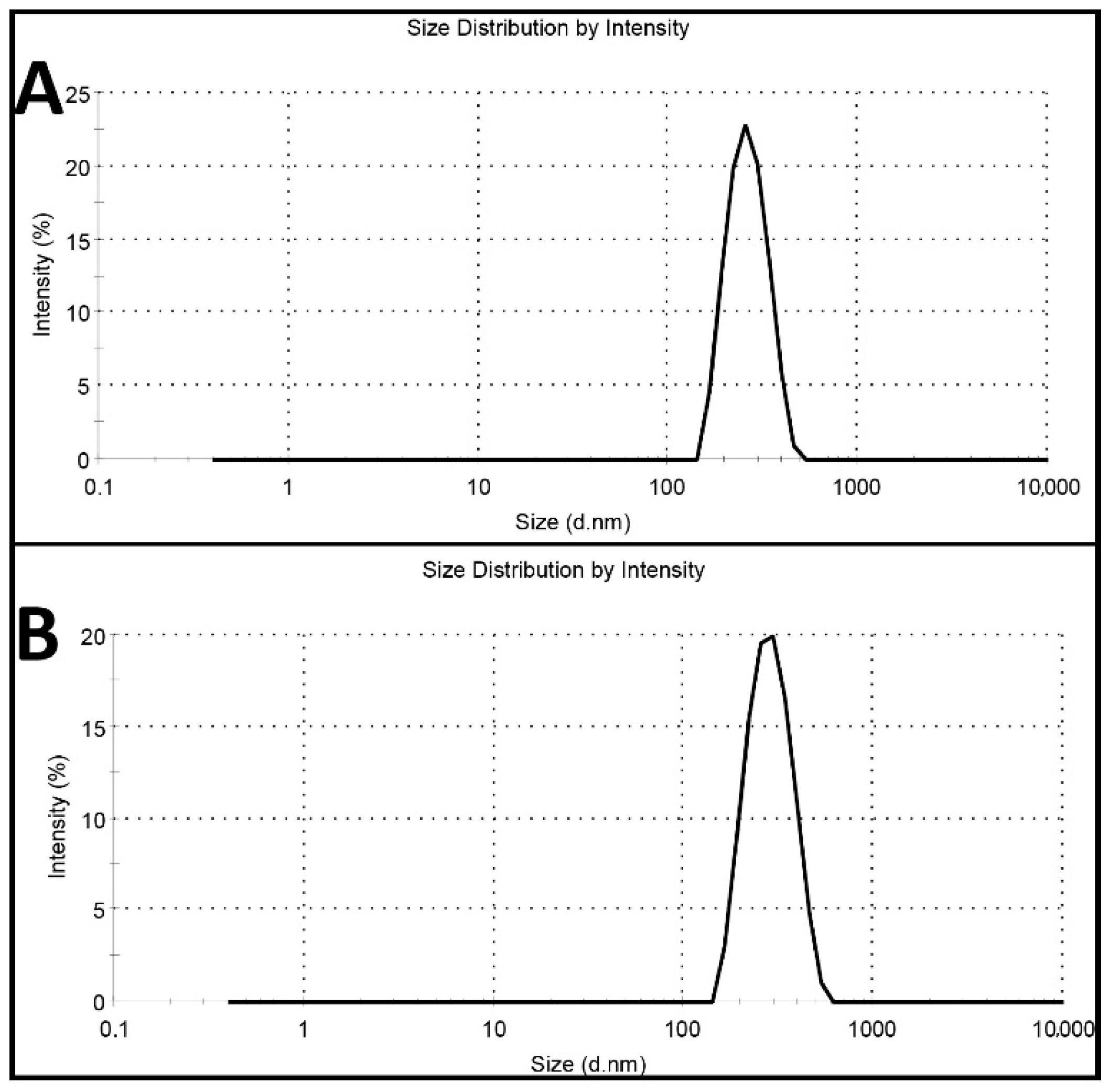
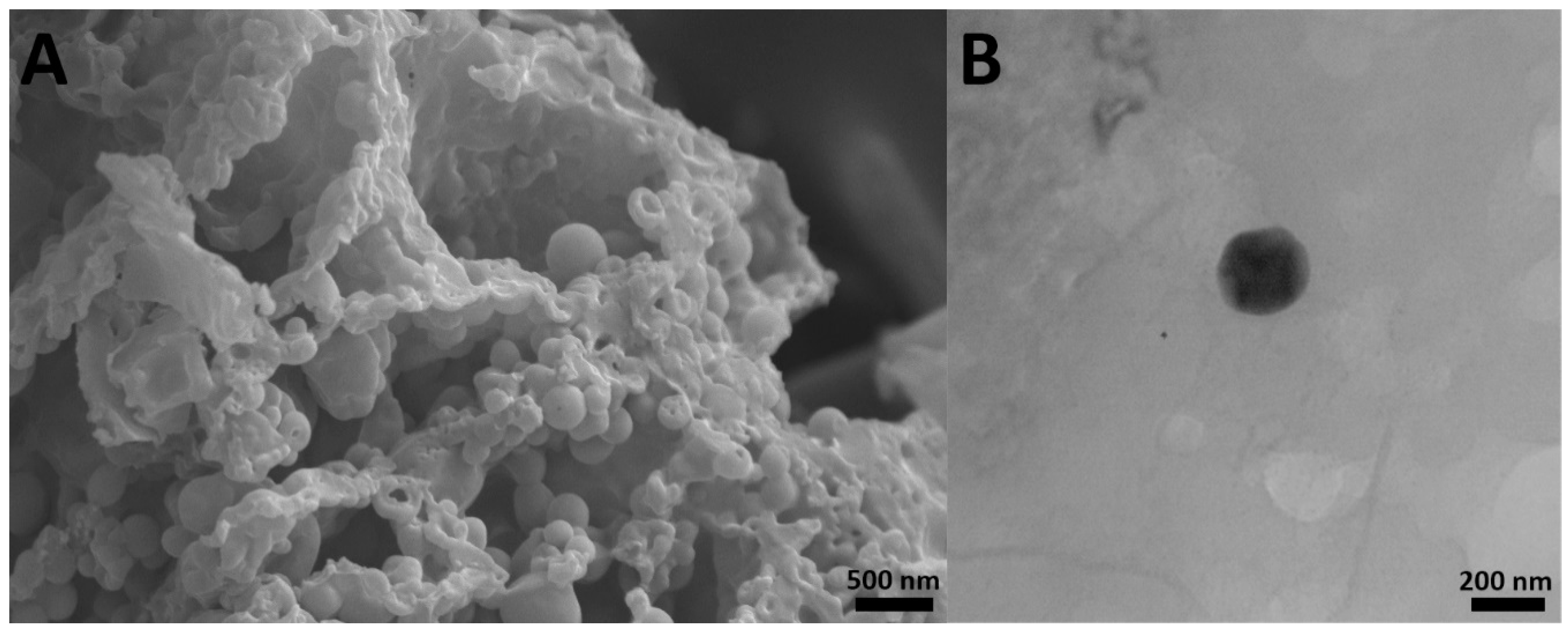
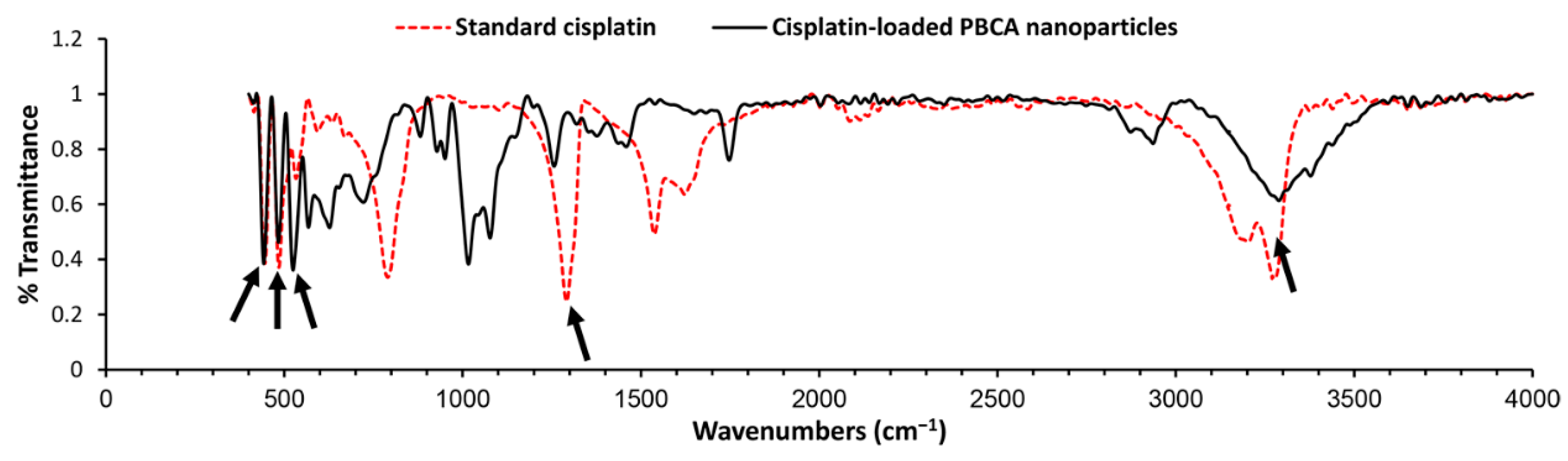

| Formulations | Size (nm) | Size Distribution | Zeta Potential (mV) | Encapsulation Efficiency (%) | Loading Efficiency (%) |
|---|---|---|---|---|---|
| PBCA nanoparticles | 251 ± 12.3 | 0.058 ± 0.002 | −9 ± 0.42 | - | - |
| Cisplatin-loaded PBCA nanoparticles | 274 ± 6.7 | 0.046 ± 0.002 | −7 ± 0.30 | 23 | 4.5 |
| Animal Groups | Healthy | PBS | Cisplatin | Cisplatin-Loaded PBCA | |
|---|---|---|---|---|---|
| Blood Factors | |||||
| BUN (mg/dL) | 16 ± 0.8 | 208 ± 12 | 172 ± 8.8 | 110 ± 7 | |
| Creatinine (mg/dL) | 0.9 ± 0.04 | 12.6 ± 0.6 | 9 ± 0.4 | 6 ± 0.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaferi, M.; Amari, S.; Vivek Mohrir, B.; Raza, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Preparation, Characterization, and Evaluation of Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved In Vitro and In Vivo Anticancer Activities. Pharmaceuticals 2020, 13, 44. https://doi.org/10.3390/ph13030044
Ghaferi M, Amari S, Vivek Mohrir B, Raza A, Ebrahimi Shahmabadi H, Alavi SE. Preparation, Characterization, and Evaluation of Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved In Vitro and In Vivo Anticancer Activities. Pharmaceuticals. 2020; 13(3):44. https://doi.org/10.3390/ph13030044
Chicago/Turabian StyleGhaferi, Mohsen, Samar Amari, Bhalchandra Vivek Mohrir, Aun Raza, Hasan Ebrahimi Shahmabadi, and Seyed Ebrahim Alavi. 2020. "Preparation, Characterization, and Evaluation of Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved In Vitro and In Vivo Anticancer Activities" Pharmaceuticals 13, no. 3: 44. https://doi.org/10.3390/ph13030044
APA StyleGhaferi, M., Amari, S., Vivek Mohrir, B., Raza, A., Ebrahimi Shahmabadi, H., & Alavi, S. E. (2020). Preparation, Characterization, and Evaluation of Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved In Vitro and In Vivo Anticancer Activities. Pharmaceuticals, 13(3), 44. https://doi.org/10.3390/ph13030044






