Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells
Abstract
1. Introduction
2. Results
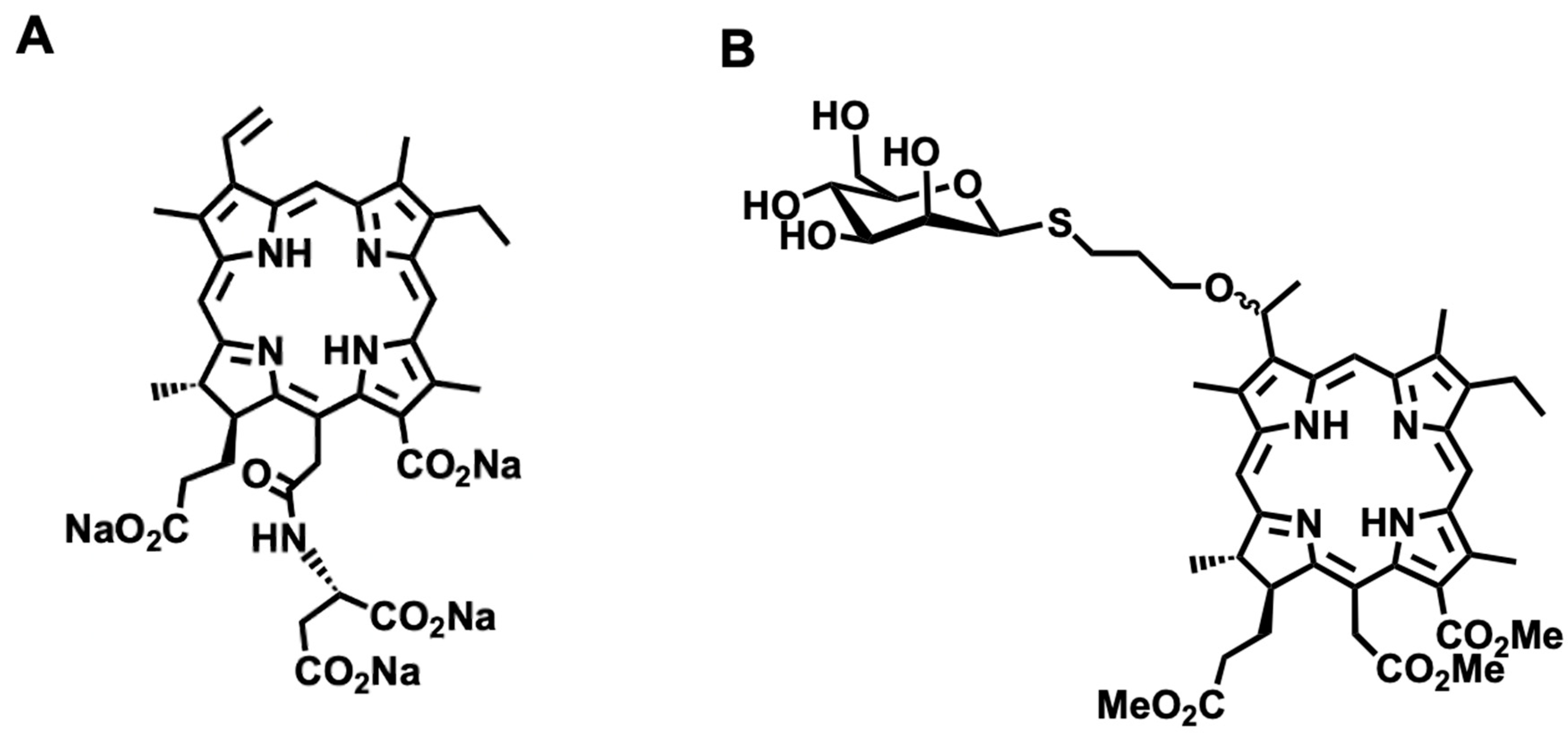
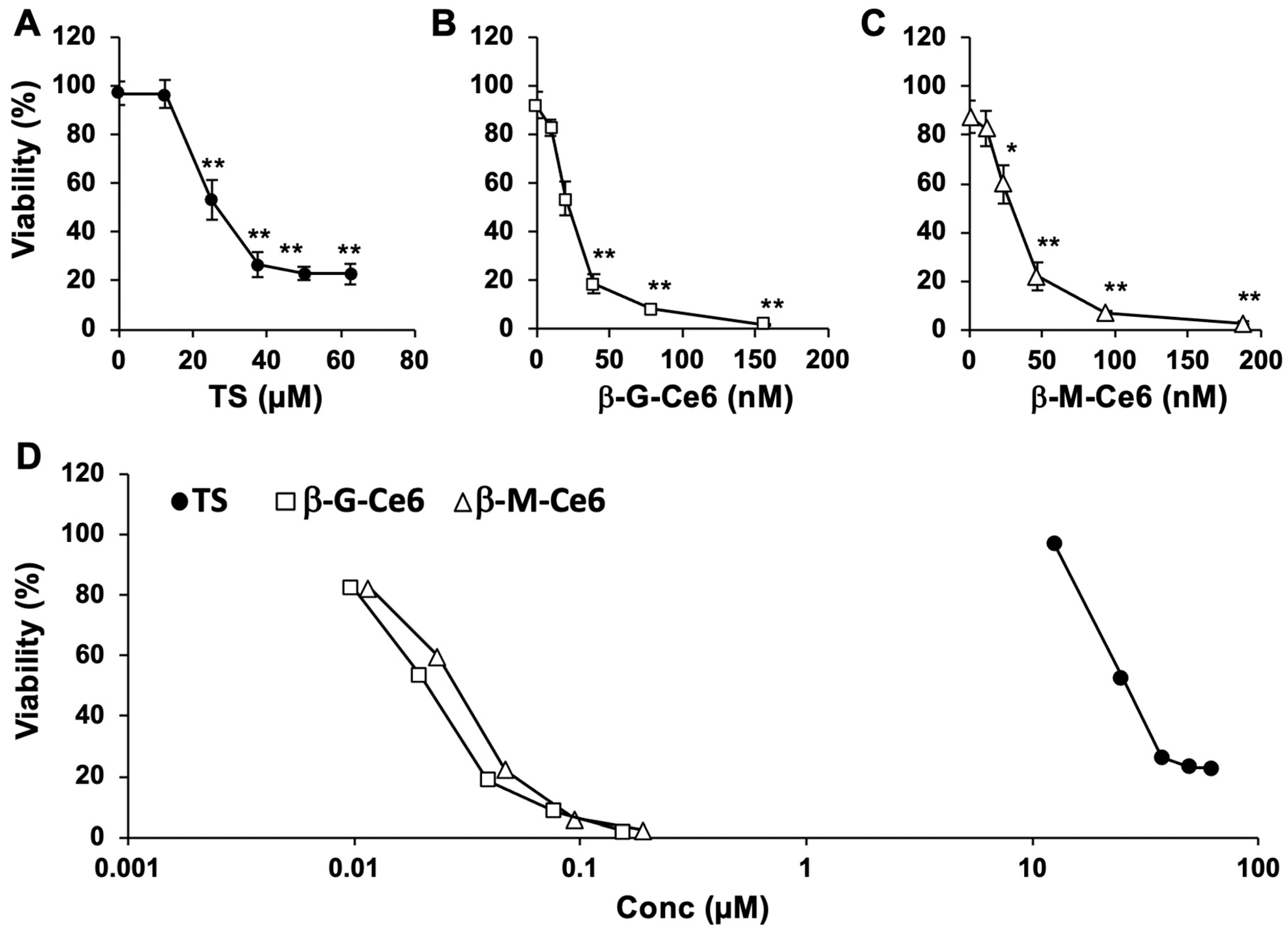
2.1. Potent Anti-Cancer Effects of β-M-Ce6 Compared with TS
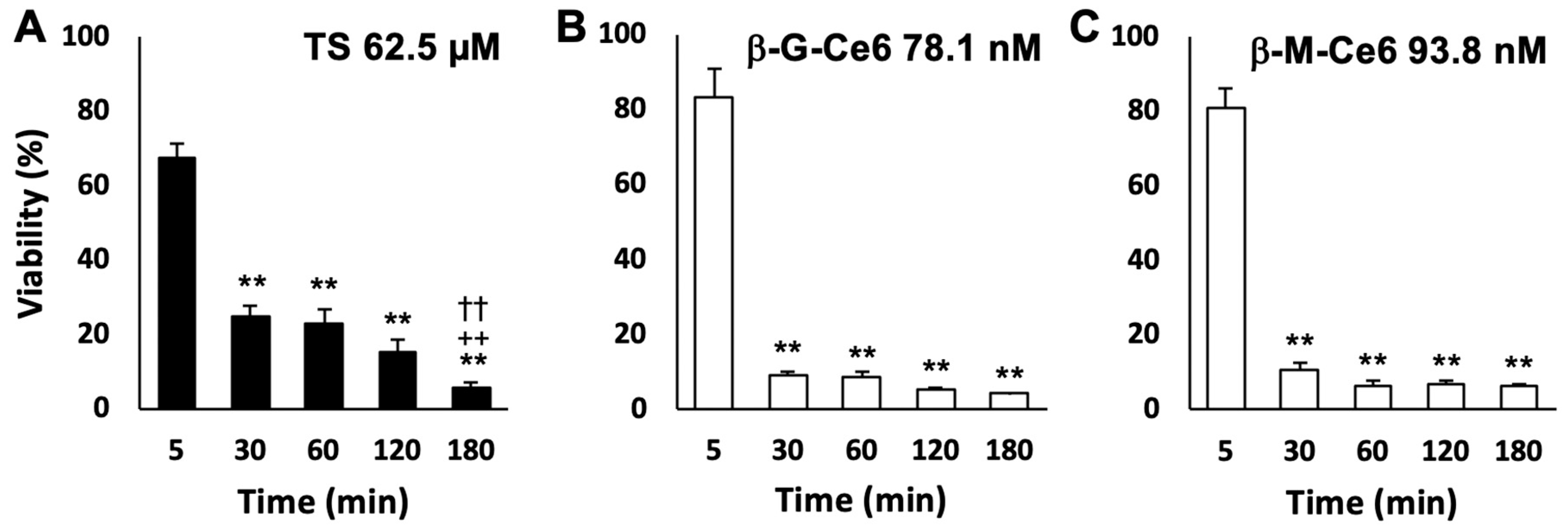
2.2. Fast Cellular Accumulation Property of β-M-Ce6
2.3. Fast Cellular Accumulation of β-M-Ce6 is Mainly Caused by Biological Machinery
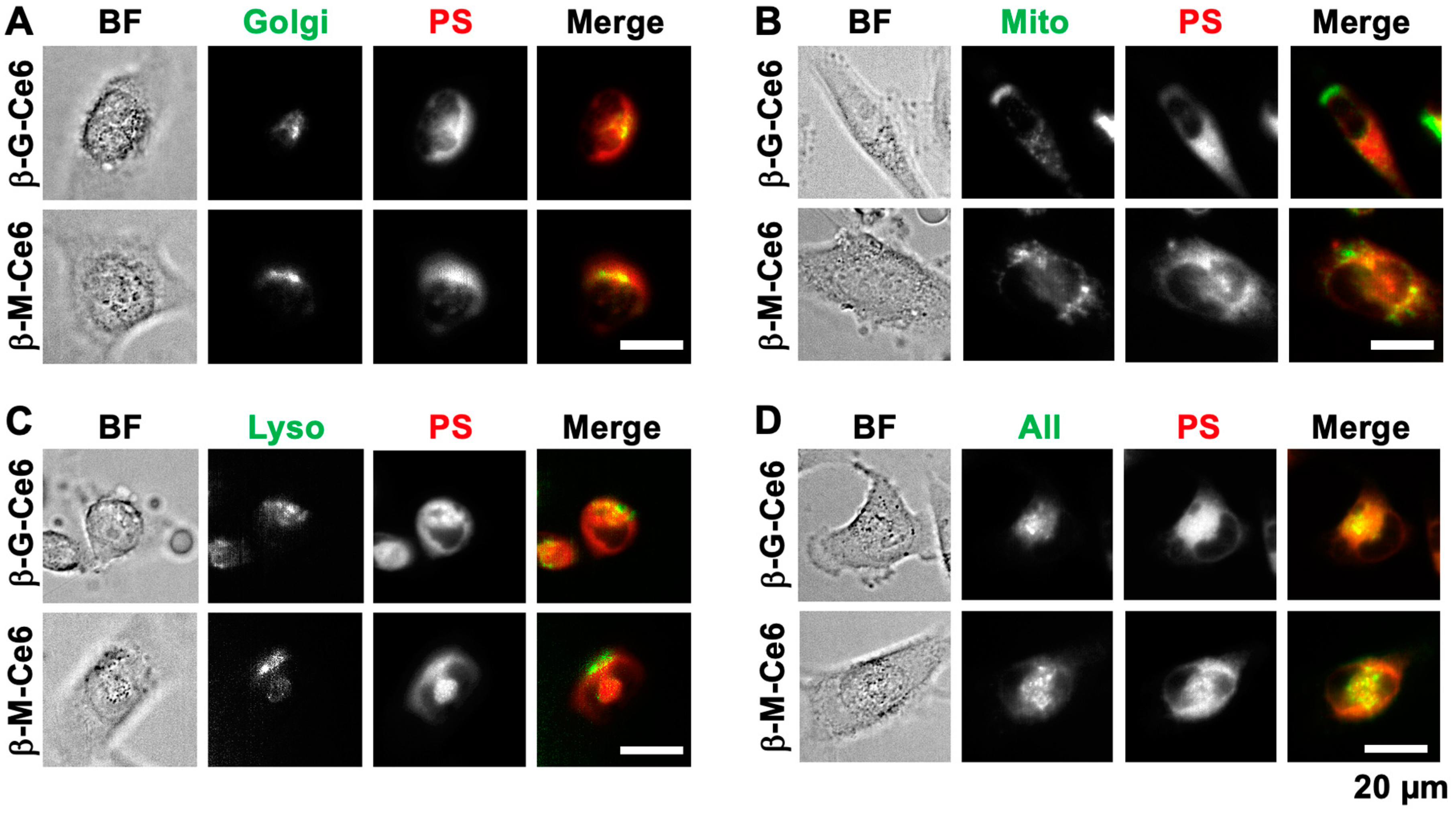
2.4. β-M-Ce6 Accumulates in Lipid-Containing Organelles Such as the Golgi Apparatus, Mitochondria, and Lysosomes
3. Discussion
4. Materials and Methods
4.1. Synthesis of β-M-Ce6
4.2. Cell Culture
4.3. PDT
4.4. Cell Viability Assay
4.5. Measurement of the Partition Coefficient
4.6. Photosensitizer Transport Assay
4.7. Photosensitizer Distribution Assay
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaen, P.; Sanz-Rodriguez, F.; Cuevas, J.; Gonzalez, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Colombeau, L.; Acherar, S.; Baros, F.; Arnoux, P.; Gazzali, A.M.; Zaghdoudi, K.; Toussaint, M.; Vanderesse, R.; Frochot, C. Inorganic Nanoparticles for Photodynamic Therapy. Top. Curr. Chem. 2016, 370, 113–134. [Google Scholar]
- Savoia, P.; Deboli, T.; Previgliano, A.; Broganelli, P. Usefulness of Photodynamic Therapy as a Possible Therapeutic Alternative in the Treatment of Basal Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 23300–23317. [Google Scholar] [CrossRef] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Braz. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Wang, S.; Bromley, E.; Xu, L.; Chen, J.C.; Keltner, L. Talaporfin sodium. Expert Opin. Pharmacother. 2010, 11, 133–140. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic Therapy for Malignant Brain Tumors. Neurol. Med. Chir. 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef]
- Usuda, J.; Tsutsui, H.; Honda, H.; Ichinose, S.; Ishizumi, T.; Hirata, T.; Inoue, T.; Ohtani, K.; Maehara, S.; Imai, K.; et al. Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer 2007, 58, 317–323. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Spikes, J.D.; Bommer, J.C. Photosensitizing properties of mono-L-aspartyl chlorin e6 (NPe6): A candidate sensitizer for the photodynamic therapy of tumors. J. Photochem. Photobiol. B 1993, 17, 135–143. [Google Scholar] [CrossRef]
- Gomer, C.J.; Ferrario, A. Tissue distribution and photosensitizing properties of mono-L-aspartyl chlorin e6 in a mouse tumor model. Cancer Res. 1990, 50, 3985–3990. [Google Scholar] [PubMed]
- Nishie, H.; Kataoka, H.; Yano, S.; Yamaguchi, H.; Nomoto, A.; Tanaka, M.; Kato, A.; Shimura, T.; Mizoshita, T.; Kubota, E.; et al. Excellent antitumor effects for gastrointestinal cancers using photodynamic therapy with a novel glucose conjugated chlorin e6. Biochem. Biophys. Res. Commun. 2018, 496, 1204–1209. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef]
- Laviron, M.; Boissonnas, A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019, 10, 1799. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Katara, G.K.; Jaiswal, M.K.; Kulshrestha, A.; Kolli, B.; Gilman-Sachs, A.; Beaman, K.D. Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene 2014, 33, 5649–5654. [Google Scholar] [CrossRef]
- Hayashi, N.; Kataoka, H.; Yano, S.; Tanaka, M.; Moriwaki, K.; Akashi, H.; Suzuki, S.; Mori, Y.; Kubota, E.; Tanida, S.; et al. A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol. Cancer Ther. 2015, 14, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Takahashi, T.; Akimoto, J.; Ichikawa, M.; Yamazaki, H.; Narumi, A.; Yano, S.; Fujiwara, Y. Comparative photodynamic therapy cytotoxicity of mannose-conjugated chlorin and talaporfin sodium in cultured human and rat cells. J. Toxicol. Sci. 2017, 42, 111–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirohara, S.; Obata, M.; Alitomo, H.; Sharyo, K.; Ando, T.; Tanihara, M.; Yano, S. Synthesis, photophysical properties and sugar-dependent in vitro photocytotoxicity of pyrrolidine-fused chlorins bearing S-glycosides. J. Photochem. Photobiol. B 2009, 97, 22–33. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Tal, A.O.; Scholz, A.; De Palma, M.; Patel, S.; Urbich, C.; Biswas, S.K.; Murdoch, C.; Plate, K.H.; Reiss, Y.; et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010, 70, 5270–5280. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Movahedi, K.; Van Ginderachter, J.A. The Ontogeny and Microenvironmental Regulation of Tumor-Associated Macrophages. Antioxid. Redox Signal 2016, 25, 775–791. [Google Scholar] [CrossRef]
- Dalle Vedove, E.; Costabile, G.; Merkel, O.M. Mannose and Mannose-6-Phosphate Receptor-Targeted Drug Delivery Systems and Their Application in Cancer Therapy. Adv. Healthc. Mater. 2018, 7, e1701398. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.B.; Figueroa, A.; Pulido, E.G.; Campelo, R.G.; Aparicio, L.A. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int. J. Endocrinol. 2010, 2010, 205357. [Google Scholar] [CrossRef]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. Glucose transport families SLC5 and SLC50. Mol. Aspects Med. 2013, 34, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Summerfield, J.A. Mammalian mannose-binding proteins. Clin. Sci. 1986, 70, 539–546. [Google Scholar] [CrossRef]
- Turner, M.W. Mannose binding protein. Biochem. Soc. Trans. 1994, 22, 88–94. [Google Scholar] [CrossRef]
- Bensoussan, C.; Rival, N.; Hanquet, G.; Colobert, F.; Reymond, S. Iron-catalyzed cross-coupling between C-bromo mannopyranoside derivatives and a vinyl Grignard reagent: Toward the synthesis of the C31–C52 fragment of amphidinol 3. Tetrahedron 2013, 69, 7759–7770. [Google Scholar] [CrossRef]
- Shu, P.; Zeng, J.; Tao, J.; Zhao, Y.; Yao, G. Selective S-deacetylation inspired by native chemical ligation: Practical syntheses of glycosyl thiols and drug mercapto-analogues. Green Chem. 2015, 17, 2545–2551. [Google Scholar] [CrossRef]
- Hargus, J.A.; Fronczek, F.R.; Vicente, G.H.; Smith, K.M. Mono-(L)-aspartylchlorin-e6. Photochem. Photobiol. 2007, 83, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Aoki, K.; Shinkai, A.; Seki, K.; Takahashi, T.; Tsuneoka, Y.; Akimoto, J.; Fujiwara, Y. Synergistic effect of dichloroacetate on talaporfin sodium-based photodynamic therapy on U251 human astrocytoma cells. Photodiagn. Photodyn. Ther. 2020, 31, 101850. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Misawa, S.; Suzuki, S.; Saeki, N.; Shinoda, Y.; Tsuneoka, Y.; Akimoto, J.; Fujiwara, Y. Possible mechanism of heme oxygenase-1 expression in rat malignant meningioma KMY-J cells subjected to talaporfin sodium-mediated photodynamic therapy. Photodiagn. Photodyn. Ther. 2020, 32, 102009. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, S.; Misawa, S.; Akimoto, J.; Shinoda, Y.; Fujiwara, Y. Photodynamic therapy using talaporfin sodium induces heme oxygenase-1 expression in rat malignant meningioma KMY-J cells. J. Toxicol. Sci. 2018, 43, 353–358. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinoda, Y.; Kujirai, K.; Aoki, K.; Morita, M.; Masuda, M.; Zhang, L.; Kaixin, Z.; Nomoto, A.; Takahashi, T.; Tsuneoka, Y.; et al. Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells. Pharmaceuticals 2020, 13, 316. https://doi.org/10.3390/ph13100316
Shinoda Y, Kujirai K, Aoki K, Morita M, Masuda M, Zhang L, Kaixin Z, Nomoto A, Takahashi T, Tsuneoka Y, et al. Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells. Pharmaceuticals. 2020; 13(10):316. https://doi.org/10.3390/ph13100316
Chicago/Turabian StyleShinoda, Yo, Kohei Kujirai, Kohei Aoki, Mai Morita, Masato Masuda, Lihao Zhang, Zhou Kaixin, Akihiro Nomoto, Tsutomu Takahashi, Yayoi Tsuneoka, and et al. 2020. "Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells" Pharmaceuticals 13, no. 10: 316. https://doi.org/10.3390/ph13100316
APA StyleShinoda, Y., Kujirai, K., Aoki, K., Morita, M., Masuda, M., Zhang, L., Kaixin, Z., Nomoto, A., Takahashi, T., Tsuneoka, Y., Akimoto, J., Kataoka, H., Rachi, R., Narumi, A., Yoshimura, T., Yano, S., & Fujiwara, Y. (2020). Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells. Pharmaceuticals, 13(10), 316. https://doi.org/10.3390/ph13100316







