Enhancement of α-Mangostin Wound Healing Ability by Complexation with 2-Hydroxypropyl-β-Cyclodextrin in Hydrogel Formulation
Abstract
1. Introduction
2. Results
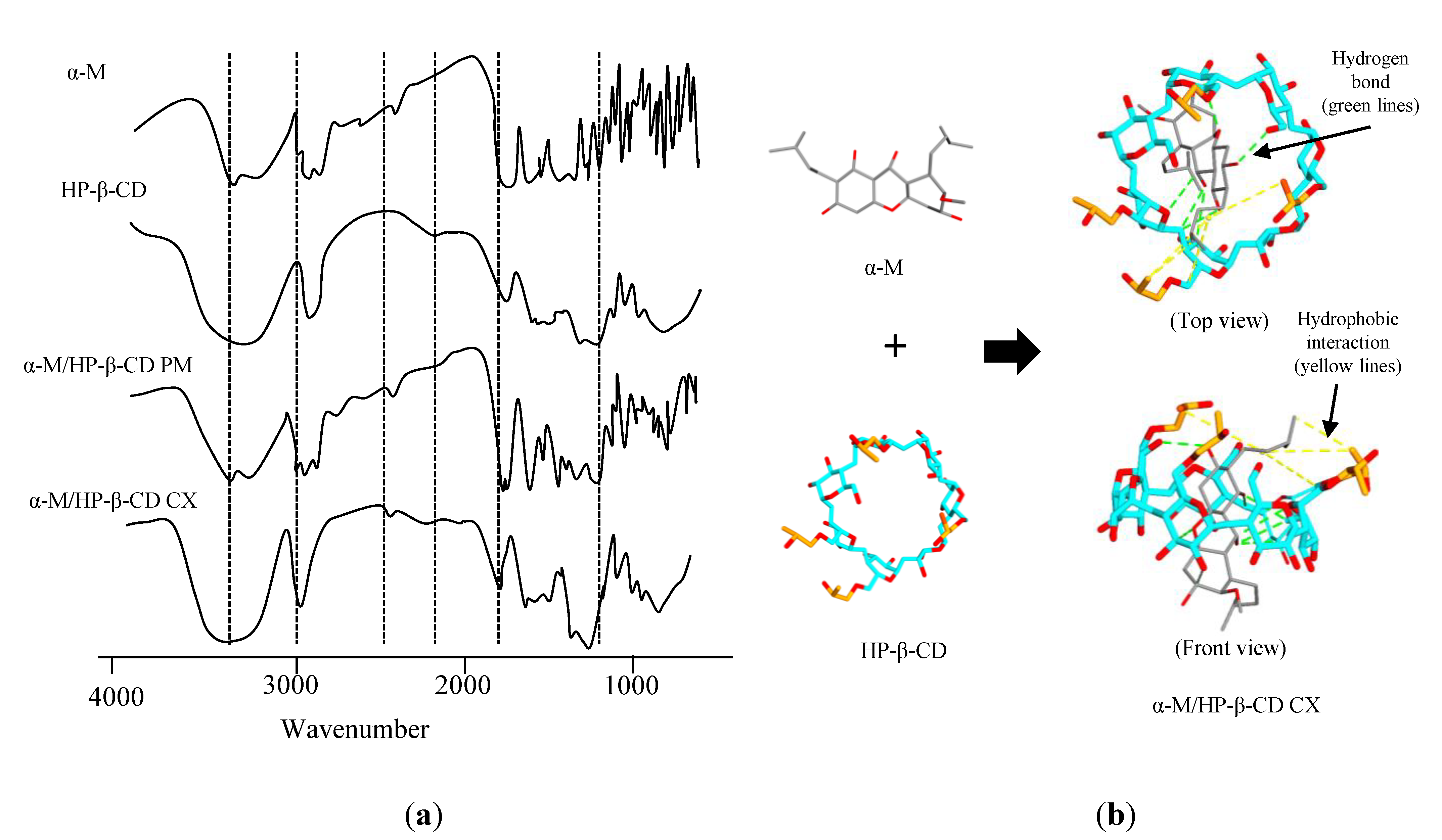
2.1. Characterization of α-M/HP-β-CD CX
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.2. Powder X-ray Diffraction (PXRD)
2.1.3. Solubility of α-M/HP-β-CD CX
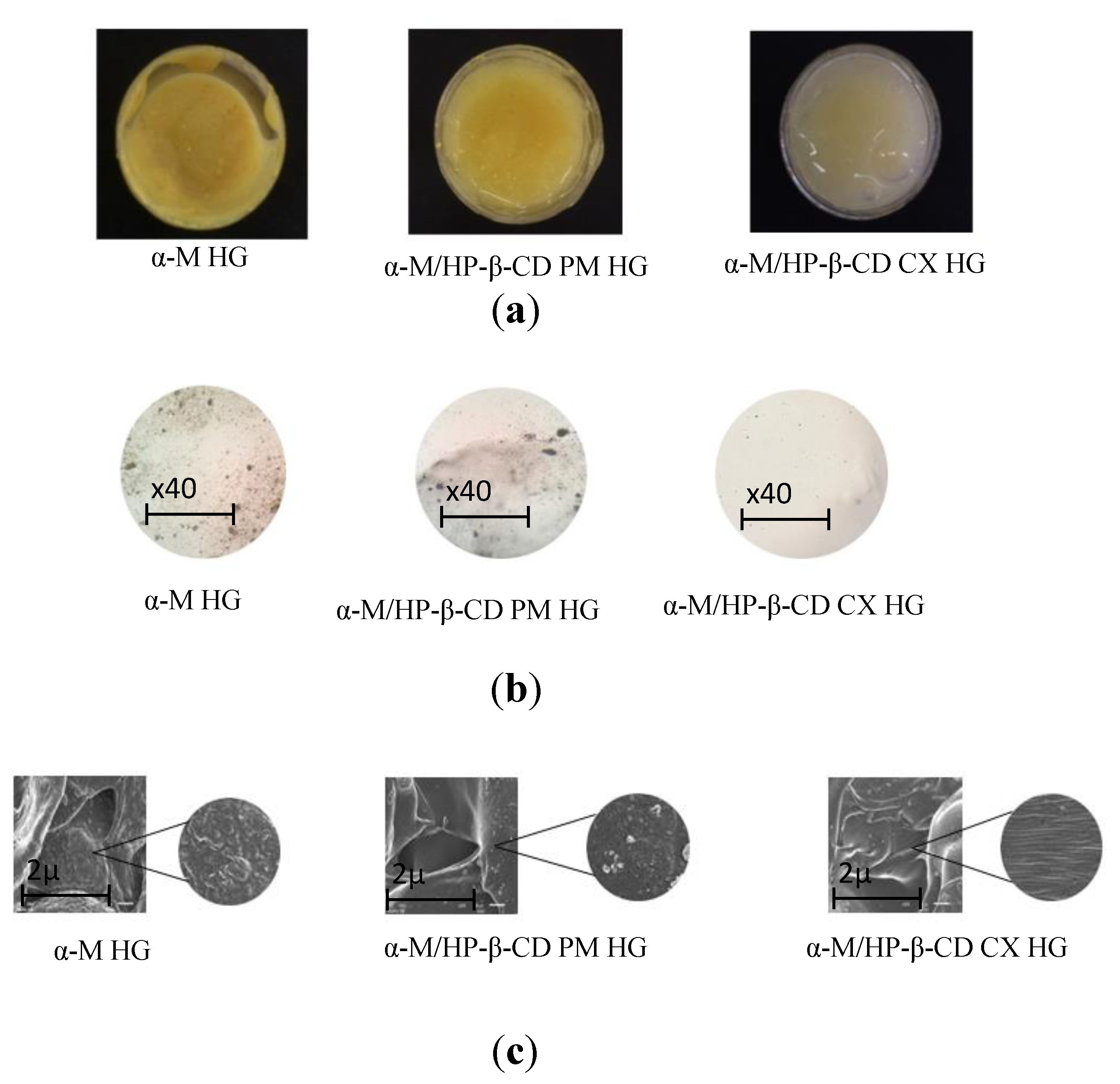
2.2. Preparation and Characterization of α-M/HP-β-CD CX HG
2.3. In Vitro Drug Release
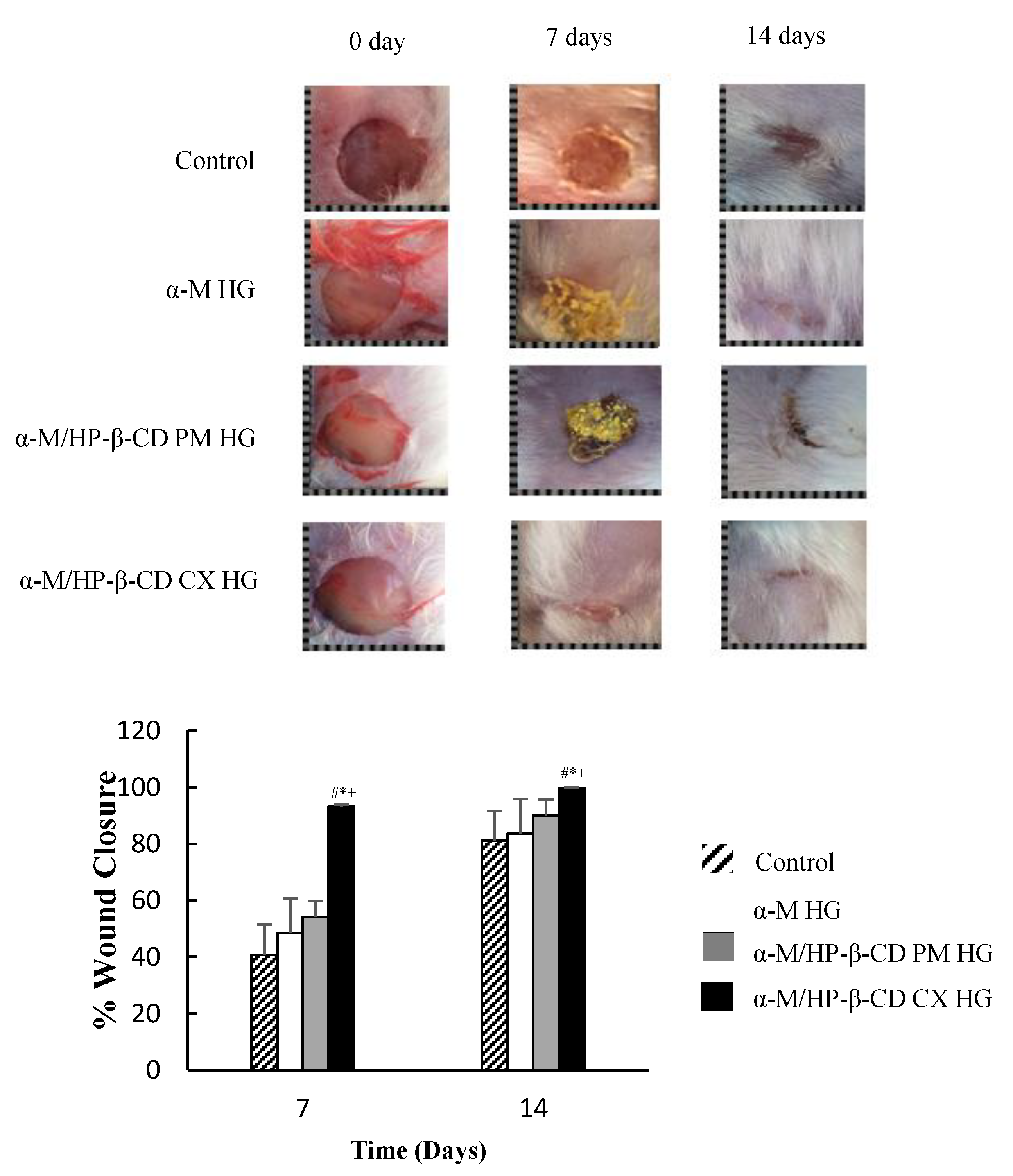
2.4. In Vivo Wound Healing Activity
3. Discussion
3.1. Characterization of α-M/HP-β-CD CX
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.2. Powder X-ray Diffraction (PXRD)
3.1.3. Solubility of α-M/HP-β-CD CX
3.2. Preparation and Characterization of α-M/HP-β-CD CX HG
3.3. In Vitro Drug Release
3.4. In Vivo Wound Healing Activity
4. Materials and Methods
4.1. Material
4.2. Methods
4.2.1. Preparation of α-M/HP-β-CD CX
4.2.2. Evaluation of α-M/HP-β-CD CX
Fourier Transform Infrared Spectroscopy (FTIR) and Molecular Docking
Powder X-ray Diffractometry (PXRD)
Solubility Study
4.2.3. Preparation of Na-CMC HG
4.2.4. Evaluation of α-M/HP-β-CD CX HG
Organoleptic Test
pH Evaluation
Homogeneity Test
Spreadability
Scanning Electron Microscopy (SEM)
Swelling Ratio
Consistency
In Vitro Drug Release
In Vivo Wound Healing Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maver, T.; Maver, U.; Stana Kleinschek, K.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Dungir, S.G.; Katja, D.G.; Kamu, V.S. Aktivitas Antioksidan Ekstrak Fenolik dari Kulit Buah Manggis (Garcinia mangostana L.). J. Ternak Trop. 2012, 3, 15–21. [Google Scholar] [CrossRef]
- Goh, S.H.; Jantan, I.; Gray, A.I.; Waterman, P.G. Prenylated xanthones from Garcinia opaca. Phytochemistry 1992, 31, 1383–1386. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Mustafa, A.; Fischer, J.W.; Singh, A.; Zhong, W.; Shekhani, M.O.; Meske, L.; Havighurst, T.; Kim, K.M.; Verma, A.K. α-Mangostin: A Dietary Antioxidant Derived from the Pericarp of Garcinia mangostana L. Inhibits Pancreatic Tumor Growth in Xenograft Mouse Model. Antioxid. Redox Signal. 2014, 21, 682–699. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Wiriyachitra, P. Chemical constituents of Garcinia mangostana. J. Nat. Prod. 1987, 50, 474–478. [Google Scholar] [CrossRef]
- Sandeep Kumar, P.S. Various techniques for solubility enhancement: An overview. J. Am. Med. Assoc. 2016, 5, 23–28. [Google Scholar]
- Uekama, K.; Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier Syst. 1987, 3, 1–40. [Google Scholar] [CrossRef]
- Ma, S.; Chen, W.; Yang, X.; Zhang, N.; Wang, S.; Liu, L.; Yang, L. Alpinetin/hydroxypropyl-β-cyclodextrin host–guest system: Preparation, characterization, inclusion mode, solubilization and stability. J. Pharm. Biomed. Anal. 2012, 67–68, 193–200. [Google Scholar] [CrossRef]
- Maria, D.N.; Mishra, S.R.; Wang, L.; Abd-Elgawad, A.-E.H.; Soliman, O.A.-E.; El-Dahan, M.S.; Jablonski, M.M. Water-soluble Complex of Curcumin with Cyclodextrins: Enhanced Physical Properties For Ocular Drug Delivery. Curr. Drug Deliv. 2017, 14, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Arima, H.; Hirayama, F.; Yamamoto, M.; Horikawa, T.; Sumiyoshi, H.; Noda, S.; Uekama, K. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-beta-cyclodextrin. Pharm. Dev. Technol. 2000, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Derakhshandeh, H.; Yue, K.; Swieszkowski, W. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Anderson, J.M.; Langone, J.J. Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J. Control. Release 1999, 57, 107–113. [Google Scholar] [CrossRef]
- Ambrosio, L.; Demitri, C.; Sannino, A. Superabsorbent Cellulose-Based Hydrogels for Biomedical Applications. In Biomedical Hydrogels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 25–50. [Google Scholar]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef]
- Serri, C.; Argirò, M.; Piras, L.; Mita, D.G.; Saija, A.; Mita, L.; Forte, M.; Giarra, S.; Biondi, M.; Crispi, S.; et al. Nano-precipitated curcumin loaded particles: Effect of carrier size and drug complexation with (2-hydroxypropyl)-β-cyclodextrin on their biological performances. Int. J. Pharm. 2017, 520, 21–28. [Google Scholar] [CrossRef]
- Erawati, T.; Rosita, N.; Hendroprasetyo, W.; Juwita, D.R. Pengaruh Jenis Basis Gel Dan Penambahan NaCl (0, 5%-b/b) Terhadap Intensitas Echo Gelombang Ultrasonik Sediaan Gel Untuk Pemeriksaan USG (Acoustic Coupling Agent). Airlangga J. Pharm. 2005, 2, 1–5. [Google Scholar]
- Tong, W.Q. Applications of Complexation in Formulation of Insoluble Compound. In Water Insoluble Drug Formation; Liu, R., Ed.; Interpharm Press: Englewood, NJ, USA, 2000. [Google Scholar]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Dermawan, D.; Wathoni, N.; Muchtaridi, M. Host-guest interactions of α- Mangostin with (α, β, γ)- Cyclodextrins: Semi-empirical quantum mechanical methods of PM6 and PM7. J. Young Pharm. 2019, 11, 31. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-Mangostin Hydrogel Film Based Chitosan-Alginate for Recurrent Aphthous Stomatitis. Appl. Sci. 2019, 9, 5235. [Google Scholar] [CrossRef]
- Hu, S.C.-S.; Lai, Y.-C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- Hotarat, W.; Phunpee, S.; Rungnim, C.; Wolschann, P.; Kungwan, N.; Ruktanonchai, U.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of alpha-mangostin and hydrophilic beta-cyclodextrins revealed by all-atom molecular dynamics simulations. J. Mol. Liq. 2019, 288, 110965. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Chun, J.Y.; You, S.K.; Lee, M.Y.; Choi, M.J.; Min, S.G. Characterization of β-cyclodextrin self-aggregates for eugenol encapsulation. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Zhang, J.T.; Huang, S.W.; Zhuo, R.X. Preparation and Characterization of Novel Temperature Sensitive Poly (N-isopropylacrylamide-co-acryloyl beta-cyclodextrin) Hydrogels with Fast Shrinking Kinetics. Macromol. Chem. Phys. 2004, 205, 107–113. [Google Scholar] [CrossRef]
- Naibaho, O.H.; Yamlean, P.V.Y.; Wiyono, W. Pengaruh Basis Salep Terhadap Formulasi Sediaan Salep Ekstrak Daun Kemangi (Ocimum sanctum L.) Pada Kulit Punggung Kelinci yang Dibuat Infeksi Staphylococcus aureus. J. Ilm. Farm. 2013, 2, 27–34. [Google Scholar]
- Fitriani, L.; Ismed, F.; Bakhtiar, A. Hydrogel Formulation of Usnic Acid and Antibacterial Activity Test Against Propionibacterium acne. Sci. Pharm. 2018, 87, 1. [Google Scholar] [CrossRef]
- Gooch, J.W. Emulsification and Polymerization of Alkyd Resins; Georgia Institude of Technology: Atlanta, GA, USA, 2002. [Google Scholar]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.F.V.; Collett, J.H.; Bentley, M.V.L.B. Influence of cyclodextrin complexation on the in vitro permeation and skin metabolism of dexamethasone. Int. J. Pharm. 2000, 200, 127–132. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L.; Wang, C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Shankaranarayan, D.; Gopalakrishnan, C.; Kameswaran, L. Pharmacological profile of mangostin and its derivatives. Arch. Int. Pharm. Ther. 1979, 239, 257–269. [Google Scholar]
- Kurahashi, T.; Fujii, J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl- g -cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Chen, M.; Diao, G.; Zhang, E. Study of inclusion complex of b -cyclodextrin and nitrobenzene. Chemosphere 2006, 63, 522–529. [Google Scholar] [CrossRef]
- Pal, R.; Islam, M.A.; Hossain, T.; Saha, A. Molecular Modeling on Structure-Function Analysis of Human Progesterone Receptor Modulators. Cientia Pharm. 2011, 79, 461–477. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5281650, Alpha-Mangostin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin (accessed on 18 September 2020).
- Jangampalli, P.; Kumar, N.; Matcha, B. Modeling, molecular docking, probing catalytic binding mode of acetyl-CoA malate synthase G in Brucella melitensis 16M. Biochem. Biophys. Rep. 2016, 8, 192–199. [Google Scholar]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Qasaymeh, R.M.; Rotondo, D.; Oosthuizen, C.B.; Lall, N.; Seidel, V. Predictive Binding A ffi nity of Plant-Derived Natural Products Towards the Protein Kinase G Enzyme of Mycobacterium tuberculosis (MtPknG). Plants 2019, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Hasanah, A.N.; Mohammed, A.F.A.; Pratiwi, E.D.; Mahmudah, R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018, 10, 57–61. [Google Scholar] [CrossRef][Green Version]
- Ditjen, P.O.M. Formularium Kosmetika Indonesia; Departemen Kesehatan RI: Jakarta, Indonesia, 1985. [Google Scholar]
- Xu, D.; Lin, Y.; Bauer, R.; Chen, H.-R.; Yang, R.-Q.; Zou, H.-Q.; Yan, Y.-H. Organoleptic Evaluation of Amomi Fructus and Its Further Background Verified via Morphological Measurement and GC Coupled with E-Nose. Evid. Based Complement. Altern. Med. 2018, 2018, 4689767. [Google Scholar] [CrossRef] [PubMed]
- Kurniawansyah, I.S.; Sopyan, I.; Wathoni, N.; Fillah, D.L.; Praditya, R.U. Application and Characterization of in Situ Gel. Int. J. Appl. Pharm. 2018, 10, 34–37. [Google Scholar] [CrossRef][Green Version]
- Gabriela, M.; Dantas, B.; Alan, S.; Bomfim, G.; Mahara, C.; Damasceno, D.; Rolim, L.A.; Rolim-neto, P.J.; Carvalho, F.O.; Quintans-junior, L.J.; et al. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrcková, M. Microscopic structure of swollen hydrogels by scanning electron and light microscopies: Artifacts and reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef]
- Wathoni, N.; Sriwidodo; Insani, U.C. Characterization and optimization of natural maltodextrin-based niosome. J. Appl. Pharm. Sci. 2013, 3, 68–71. [Google Scholar]
- Strictest, I.; Confidence, C.; One, P.; Strictest, I.; Confidence, C. Table of of contents. In Proceedings of the 30th Annual International Conference of IEEE Engineering in Medicine and Biology Society (EMBC’08), Vancouver, BC, Canada, 20–24 August 2008. [Google Scholar]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and effect of structure on swelling properties of hydrogels based on high methylated pectin and acrylic polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef]
- Djajadisastra, J.; Mun’im, A.; Dessy, N.P. Formulasi Gel Topikal Dari Ekstrak Nerii Folium Dalam Sediaan Anti Jerawat. J. Farm. Indones. 2009, 4, 210–216. [Google Scholar]
- Kono, H.; Onishi, K.; Nakamura, T. Characterization and bisphenol A adsorption capacity of β-cyclodextrin-carboxymethylcellulose-based hydrogels. Carbohydr. Polym. 2013, 98, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.G.; Leite, L.L.R.; de Lima, I.S.; Barreto, H.M.; Nunes, L.C.C.; Ribeiro, A.B.; Osajima, J.A.; da Silva Filho, E.C. Chitosan Hydrogel in combination with Nerolidol for healing wounds. Carbohydr. Polym. 2016, 152, 409–418. [Google Scholar] [CrossRef] [PubMed]




| Parameter | α-M HG | α-M/HP-β-CD PM HG | α-M/HP-β-CD CX HG |
|---|---|---|---|
| Slope (% min-0.5) | 1.25 ± 0.13 | 1.55 ± 0.06 | 2.02 ± 0.01 |
| Correlation Coefficient (r) | 0.96 ± 0.002 | 0.99 ± 0.0002 | 0.99 ± 0.00 |
| Formulation | α-M HG | α-M/HP-β-CD PM HG | α-M/HP-β-CD CX HG |
|---|---|---|---|
| α-M | 25 mg | - | - |
| α-M/HP-β-CD PM | - | 25 mg | - |
| α-M/HP-β-CD CX | - | - | 25 mg |
| Na-CMC | 0.4 g | 0.4 g | 0.4 g |
| Glycerin | 2 g | 2 g | 2 g |
| Propylene glycol | 1 g | 1 g | 1 g |
| Methylparaben | 0.03 g | 0.03 g | 0.03 g |
| Distilled water added | 20 mL | 20 mL | 20 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wathoni, N.; Sari, D.P.; Suharyani, I.; Motoyama, K.; Mohammed, A.F.A.; Cahyanto, A.; Abdassah, M.; Muchtaridi, M. Enhancement of α-Mangostin Wound Healing Ability by Complexation with 2-Hydroxypropyl-β-Cyclodextrin in Hydrogel Formulation. Pharmaceuticals 2020, 13, 290. https://doi.org/10.3390/ph13100290
Wathoni N, Sari DP, Suharyani I, Motoyama K, Mohammed AFA, Cahyanto A, Abdassah M, Muchtaridi M. Enhancement of α-Mangostin Wound Healing Ability by Complexation with 2-Hydroxypropyl-β-Cyclodextrin in Hydrogel Formulation. Pharmaceuticals. 2020; 13(10):290. https://doi.org/10.3390/ph13100290
Chicago/Turabian StyleWathoni, Nasrul, Diah Permata Sari, Ine Suharyani, Keiichi Motoyama, Ahmed Fouad Abdelwahab Mohammed, Arief Cahyanto, Marline Abdassah, and Muchtaridi Muchtaridi. 2020. "Enhancement of α-Mangostin Wound Healing Ability by Complexation with 2-Hydroxypropyl-β-Cyclodextrin in Hydrogel Formulation" Pharmaceuticals 13, no. 10: 290. https://doi.org/10.3390/ph13100290
APA StyleWathoni, N., Sari, D. P., Suharyani, I., Motoyama, K., Mohammed, A. F. A., Cahyanto, A., Abdassah, M., & Muchtaridi, M. (2020). Enhancement of α-Mangostin Wound Healing Ability by Complexation with 2-Hydroxypropyl-β-Cyclodextrin in Hydrogel Formulation. Pharmaceuticals, 13(10), 290. https://doi.org/10.3390/ph13100290









