The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy
Abstract
1. Introduction
2. Clinical Benefits of Vonoprazan
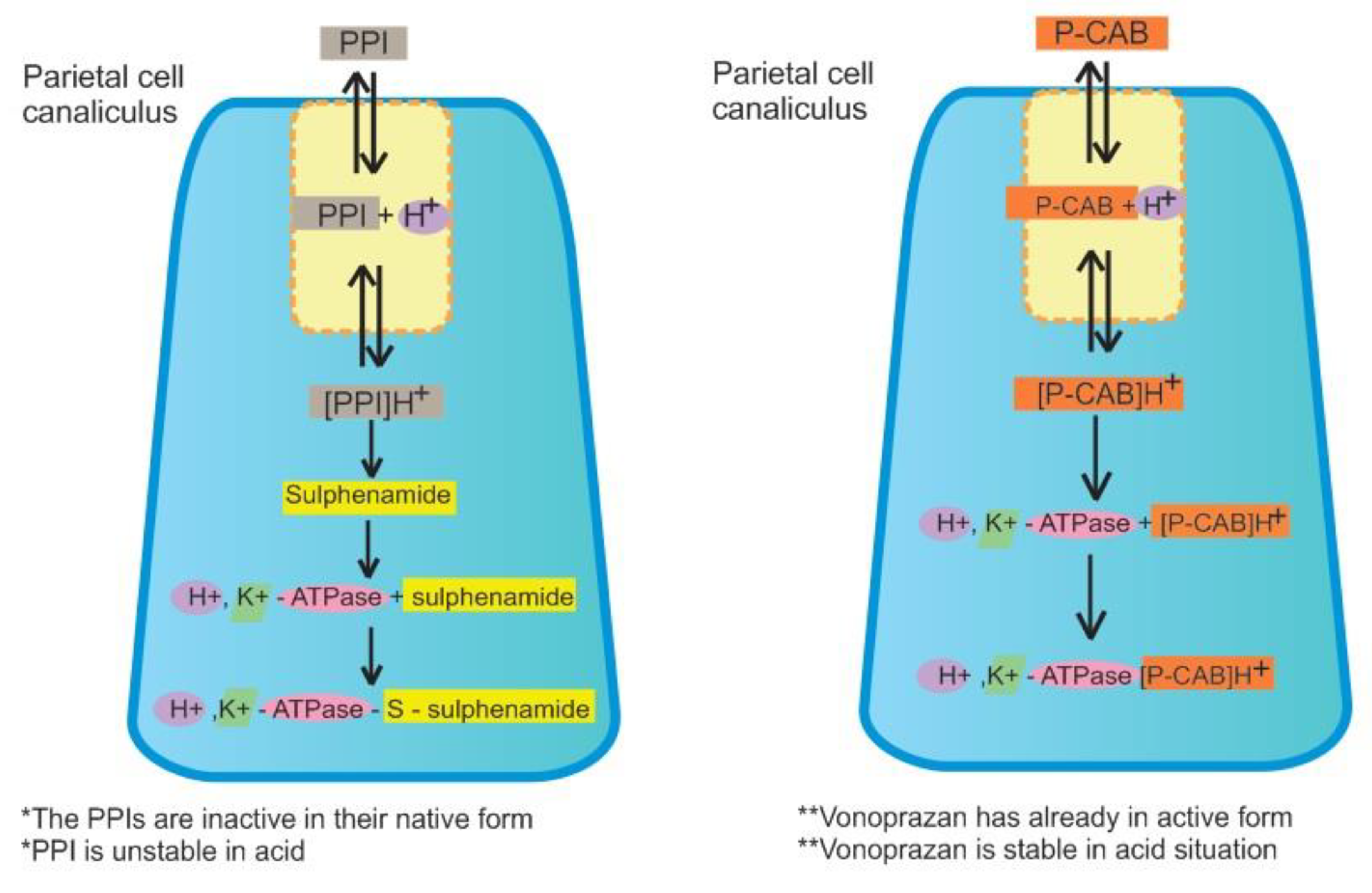
2.1. Pharmacological Aspects
2.2. Vonoprazan and H. pylori Eradication
2.3. Safety and Adverse Events
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, Y.; Wan, J.H.; Li, X.Y.; Zhu, Y.; Graham, D.Y.; Lu, N.H. Systematic review with meta-analysis: The global recurrence rate of Helicobacter pylori. Aliment. Pharmacol. Ther. 2017, 46, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, L.Y.; Lu, H.P.; Liu, J.Z.; Guo, L.S. Recurrence of Helicobacter pylori infection: Incidence and influential factors. Chin. Med. J. (Engl.) 2019, 132, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Syam, A.F.; Miftahussurur, M.; Makmun, D.; Nusi, I.A.; Zain, L.H.; Zulkhairi; Akil, F.; Uswan, W.B.; Simanjuntak, D.; Uchida, T.; et al. Risk factors and prevalence of Helicobacter pylori in five largest islands of Indonesia: A preliminary study. PLoS ONE 2015, 10, e0140186. [Google Scholar] [CrossRef]
- Abadi, A.T.B.; Ierardi, E. Vonoprazan and Helicobacter pylori treatment: A lesson from Japan or a limited geographic phenomenon? Front. Pharmacol. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Lyu, Q.J.; Pu, Q.H.; Zhong, X.F.; Zhang, J. Efficacy and safety of vonoprazan-based versus proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: A meta-analysis of randomized clinical trials. BioMed Res. Int. 2019, 2019, 9781212–9781218. [Google Scholar] [CrossRef]
- Floch, P.; Mégraud, F.; Lehours, P. Helicobacter pylori strains and gastric MALT lymphoma. Toxins 2017, 9, 132. [Google Scholar] [CrossRef]
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018, 13, 51–57. [Google Scholar] [CrossRef]
- Seta, T.; Takahashi, Y.; Noguchi, Y.; Shikata, S.; Sakai, T.; Sakai, K.; Yamashita, Y.; Nakayama, T. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: A systematic review and meta-analysis comparing risk ratio with risk difference. PLoS ONE 2017, 12, e0183321. [Google Scholar] [CrossRef]
- Ford, A.; Forman, D.; Hunt, R.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Suzuki, H.; Mori, H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J. Gastroenterol. 2018, 53, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; La Fortezza, R.F.; Principi, M.; Barone, M.; Leo, A. Di Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J. Gastroenterol. 2019, 25, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Putra, B.P.; Miftahussurur, M. Vonoprazan-based therapy has lower failure rate in eradicating Helicobacter pylori compared to proton pum inhibitors-based therapy: A meta-analysis of randomized controlled trials. New Armen. Med. J. 2019, 13, 22–30. [Google Scholar]
- Graham, D.Y.; Dore, M.P. Update on the use of vonoprazan: A competitive acid blocker. Gastroenterology 2018, 154, 462–466. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Inatomi, N.; Matsukawa, J.; Sakurai, Y.; Otake, K. Potassium-competitive acid blockers: Advanced therapeutic option for acid-related diseases. Pharmacol. Ther. 2016, 168, 12–22. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Ofosu, A.; Gaduputi, V. Potassium-competitive acid blockers - are they the next generation of proton pump inhibitors? World J. Gastrointest. Pharmacol. Ther. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Kato, M.; Ota, H.; Okuda, M.; Kikuchi, S.; Satoh, K.; Shimoyama, T.; Suzuki, H.; Handa, O.; Furuta, T.; Mabe, K.; et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter 2019, 24, e12597. [Google Scholar] [CrossRef]
- Mori, H.; Suzuki, H. Role of acid suppression in acid-related diseases: Proton pump inhibitor and potassium-competitive acid blocker. J. Neurogastroenterol. Motil. 2019, 25, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Son, B.K.; Kim, G.H.; Jung, H.K.; Jung, H.Y.; Chung, I.K.; Sung, I.K.; Kim, J.I.; Kim, J.H.; Lee, J.S.; et al. Randomised phase 3 trial: Tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment. Pharmacol. Ther. 2019, 49, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Take, Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J. Pharmacol. Exp. Ther. 2018, 364, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, K.; Kinoshita, Y.; Habu, Y.; Oshima, T.; Manabe, N.; Fujiwara, Y.; Nagahara, A.; Kawamura, O.; Iwakiri, R.; Ozawa, S.; et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J. Gastroenterol. 2016, 51, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–238. [Google Scholar] [CrossRef]
- Syam, A.F.; Simadibrata, M.; Makmun, D.; Abdullah, M.; Fauzi, A.; Renaldi, K.; Maulahela, H.; Utari, A.P. National consensus on management of dyspepsia and Helicobacter pylori infection. Acta Med. Indones. 2017, 49, 279–287. [Google Scholar]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69.e4. [Google Scholar] [CrossRef]
- Chang, J.Y.; Shim, K.N.; Tae, C.H.; Lee, K.E.; Lee, J.; Lee, K.H.; Moon, C.M.; Kim, S.E.; Jung, H.K.; Jung, S.A. Triple therapy versus sequential therapy for the first-line Helicobacter pylori eradication. BMC Gastroenterol. 2017, 17, 16. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Yamaoka, Y. Appropriate first-line regimens to combat Helicobacter pylori antibiotic resistance: An Asian perspective. Molecules 2015, 20, 6068–6092. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Shrestha, P.K.; Subsomwong, P.; Sharma, R.P.; Yamaoka, Y. Emerging Helicobacter pylori levofloxacin resistance and novel genetic mutation in Nepal. BMC Microbiol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Lamichhane, B.; Tay, C.Y.; Pai, G.C.; Lingadakai, R.; Balaraju, G.; Shetty, S.; Ballal, M.; Chua, E.G. High primary resistance to metronidazole and levofloxacin, and a moderate resistance to clarithromycin in Helicobacter pylori isolated from Karnataka patients. Gut Pathog. 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Waskito, L.A.; Syam, A.F.; Nusi, I.A.; Siregar, G.; Richardo, M.; Bakry, A.F.; Rezkitha, Y.A.A.; Wibawa, I.D.N.; Yamaoka, Y. Alternative eradication regimens for helicobacter pylori infection in indonesian regions with high metronidazole and levofloxacin resistance. Infect. Drug Resist. 2019, 12, 345–358. [Google Scholar] [CrossRef]
- Echizen, H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacokinet. 2016, 55, 409–418. [Google Scholar] [CrossRef]
- Jenkins, H.; Sakurai, Y.; Nishimura, A.; Okamoto, H.; Hibberd, M.; Jenkins, R.; Yoneyama, T.; Ashida, K.; Ogama, Y.; Warrington, S. Randomised clinical trial: Safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment. Pharmacol. Ther. 2015, 41, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Nishimura, A.; Kennedy, G.; Hibberd, M.; Jenkins, R.; Okamoto, H.; Yoneyama, T.; Jenkins, H.; Ashida, K.; Irie, S.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising Tak-438 (Vonoprazan) doses in healthy male Japanese/Non-Japanese Subjects. Clin. Transl. Gastroenterol. 2015, 6, e94. [Google Scholar] [CrossRef]
- Shin, J.M.; Inatomi, N.; Munson, K.; Strugatsky, D.; Tokhtaeva, E.; Vagin, O.; Sachs, G. Characterization of a Novel Potassium-Competitive Acid Blocker of the Gastric H,K-ATPase, 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine Monofumarate (TAK-438). J. Pharmacol. Exp. Ther. 2011, 339, 412–420. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Sun, Y.; Zhang, M.; Guo, C.; Mirza, I.A.; Li, Y.Q. Vonoprazan: A novel and potent alternative in the treatment of acid-related diseases. Dig. Dis. Sci. 2018, 63, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Wang, S.; Zhou, Q.; Dai, D.; Shi, J.; Xu, X.; Luo, Q. Cytochrome P450-based drug-drug interactions of vonoprazan in vitro and in vivo. Front. Pharmacol. 2020, 11, 53. [Google Scholar] [CrossRef]
- Jenkins, H.; Jenkins, R.; Patat, A. Effect of multiple oral doses of the potent CYP3A4 inhibitor clarithromycin on the pharmacokinetics of a single oral dose of vonoprazan: A phase I, open-label, sequential design study. Clin. Drug Investig. 2017, 37, 311–316. [Google Scholar] [CrossRef]
- Kagami, T.; Sahara, S.; Ichikawa, H.; Uotani, T.; Yamade, M.; Sugimoto, M.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; Sugimoto, K.; et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment. Pharmacol. Ther. 2016, 43, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Ishimura, N.; Ishihara, S. Advantages and disadvantages of long-term proton pump inhibitor use. J. Neurogastroenterol. Motil. 2018, 24, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Arai, E.; Taki, M.; Kondo, T.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Randomised clinical trial: Vonoprazan versus lansoprazole for the initial relief of heartburn in patients with erosive oesophagitis. Aliment. Pharmacol. Ther. 2019, 49, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Miwa, H. Potent potassium-competitive acid blockers: A new era for the treatment of acid-related diseases. J. Neurogastroenterol. Motil. 2018, 24, 334–344. [Google Scholar] [CrossRef]
- Akazawa, Y.; Fukuda, D.; Fukuda, Y. Vonoprazan-based therapy for Helicobacter pylori eradication: Experience and clinical evidence. Therap. Adv. Gastroenterol. 2016, 9, 845–852. [Google Scholar] [CrossRef]
- Sugano, K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: Safety and clinical evidence to date. Therap. Adv. Gastroenterol. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Yao, X.; Smolka, A.J. Gastric parietal cell physiology and Helicobacter pylori–induced disease. Gastroenterology 2019, 156, 2158–2173. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Vonoprazan: First global approval. Drugs 2015, 75, 439–443. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Tan, X.; Dai, Y.; Xie, C.; Li, X.; Lu, Q.; Kou, F.; Jiang, H.; Li, J. Direct comparison of the efficacy and safety of vonoprazan versus proton-pump inhibitors for gastroesophageal reflux disease: A systematic review and meta-analysis. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Ashida, K.; Sakurai, Y.; Hori, T.; Kudou, K.; Nishimura, a.; Hiramatsu, N.; Umegaki, E.; Iwakiri, K. Randomised clinical trial: Vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment. Pharmacol. Ther. 2016, 43, 240–251. [Google Scholar] [CrossRef]
- Mizokami, Y.; Oda, K.; Funao, N.; Nishimura, A.; Soen, S.; Kawai, T.; Ashida, K.; Sugano, K. Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: Randomised, lansoprazole-controlled non-inferiority and single-blind extension study. Gut 2018, 67, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Uedo, N.; Watari, J.; Mori, Y.; Sakurai, Y.; Takanami, Y.; Nishimura, a.; Tatsumi, T.; Sakaki, N. Randomised clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers–results from two phase 3, non-inferiority randomised controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Oda, K.; Funao, N.; Nishimura, A.; Matsumoto, Y.; Mizokami, Y.; Ashida, K.; Sugano, K. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: Randomised phase 3 study. Gut 2018, 67, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Poonsombudlert, K.; Ungprasert, P. Vonoprazan versus proton-pump inhibitors for gastric endoscopic submucosal dissection-induced ulcers: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1416–1421. [Google Scholar] [CrossRef]
- Sugimoto, M.; Yamaoka, Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front. Pharmacol. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Waskito, L.A.; Miftahussurur, M.; Lusida, M.I.; Syam, A.F.; Suzuki, R.; Subsomwong, P.; Uchida, T.; Hamdan, M.; Nasronudin; Yamaoka, Y. Distribution and clinical associations of integrating conjugative elements and cag pathogenicity islands of Helicobacter pylori in Indonesia. Sci. Rep. 2018, 8, 6073. [Google Scholar] [CrossRef]
- Doohan, D.; Miftahussurur, M.; Matsuo, Y.; Kido, Y.; Akada, J.; Matsuhisa, T.; Yee, T.T.; Htet, K.; Aftab, H.; Vilaichone, R.K.; et al. Characterization of a novel Helicobacter pylori East Asian-type CagA ELISA for detecting patients infected with various cagA genotypes. Med. Microbiol. Immunol. 2020, 209, 29–40. [Google Scholar] [CrossRef]
- Subsomwong, P.; Miftahussurur, M.; Uchida, T.; Vilaichone, R.K.; Ratanachu-Ek, T.; Mahachai, V.; Yamaoka, Y. Prevalence, risk factors, and virulence genes of Helicobacter pylori among dyspeptic patients in two different gastric cancer risk regions of Thailand. PLoS ONE 2017, 12, e0187113. [Google Scholar] [CrossRef]
- Li, M.; Oshima, T.; Horikawa, T.; Tozawa, K.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Murakami, K.; Sakurai, Y.; Shiino, M.; Funao, N.; Nishimura, A.; Asaka, M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 2016, 65, 1439–1446. [Google Scholar] [CrossRef]
- Maruyama, M.; Tanaka, N.; Kubota, D.; Miyajima, M.; Kimura, T.; Tokutake, K.; Imai, R.; Fujisawa, T.; Mori, H.; Matsuda, Y.; et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: A randomized controlled trial. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sue, S.; Ogushi, M.; Arima, I.; Kuwashima, H.; Nakao, S.; Naito, M.; Komatsu, K.; Kaneko, H.; Tamura, T.; Sasaki, T.; et al. Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial. Helicobacter 2018, 23, e12456. [Google Scholar] [CrossRef]
- Ozaki, H.; Harada, S.; Takeuchi, T.; Kawaguchi, S.; Takahashi, Y.; Kojima, Y.; Ota, K.; Hongo, Y.; Ashida, K.; Sakaguchi, M.; et al. Vonoprazan, a novel potassium-competitive acid blocker, should be used for the Helicobacter pylori eradication therapy as first choice: A large sample study of vonoprazan in real world compared with our randomized control trial using second-generation pro. Digestion 2018, 97, 212–218. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Iwatsuka, K.; Moriyama, M. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am. J. Gastroenterol. 2016, 111, 949–956. [Google Scholar] [CrossRef]
- Shinozaki, S.; Nomoto, H.; Kondo, Y.; Sakamoto, H.; Hayashi, Y.; Yamamoto, H.; Lefor, A.K.; Osawa, H. Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J. Med. Sci. 2016, 32, 255–260. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Mochizuki, S.; Matsuo, K.; Isomura, Y.; Seto, M.; Suzuki, N.; et al. Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: A multicenter retrospective study in clinical practice. J. Dig. Dis. 2016, 17, 670–675. [Google Scholar] [CrossRef]
- Noda, H.; Noguchi, S.; Yoshimine, T.; Goji, S.; Adachi, K.; Tamura, Y.; Izawa, S.; Ebi, M.; Yamamoto, S.; Ogasawara, N.; et al. A novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against helicobacter pylori. J. Gastrointest. Liver Dis. 2016, 25, 283–288. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shiotani, A.; Katsumata, R.; Fujita, M.; Nakato, R.; Murao, T.; Ishii, M.; Kamada, T.; Haruma, K.; Graham, D.Y. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: The effect of clarithromycin resistance. Dig. Dis. Sci. 2016, 61, 3215–3220. [Google Scholar] [CrossRef]
- Yamada, S.; Kawakami, T.; Nakatsugawa, Y.; Suzuki, T.; Fujii, H.; Tomatsuri, N.; Nakamura, H.; Sato, H.; Okuyama, Y.; Kimura, H.; et al. Usefulness of vonoprazan, a potassium ion-competitive acid blocker, for primary eradication of Helicobacter pylori. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 550. [Google Scholar] [CrossRef]
- Tsujimae, M.; Yamashita, H.; Hashimura, H.; Kano, C.; Shimoyama, K.; Kanamori, A.; Matsumoto, K.; Koizumi, A.; Momose, K.; Eguchi, T.; et al. A comparative study of a new class of gastric acid suppressant agent named vonoparazan versus esomeprazole for the eradication of Helicobacter pylori. Digestion 2017, 94, 240–246. [Google Scholar] [CrossRef]
- Kajihara, Y.; Shimoyama, T.; Mizuki, I. Analysis of the cost-effectiveness of using vonoprazan–amoxicillin–clarithromycin triple therapy for first-line Helicobacter pylori eradication. Scand. J. Gastroenterol. 2017, 52, 238–241. [Google Scholar] [CrossRef]
- Sakurai, K.; Suda, H.; Ido, Y.; Takeichi, T.; Okuda, A.; Hasuda, K.; Hattori, M. Comparative study: Vonoprazan and proton pump inhibitors in Helicobacter pylori eradication therapy. World J. Gastroenterol. 2017, 23, 668–675. [Google Scholar] [CrossRef]
- Sue, S.; Kuwashima, H.; Iwata, Y.; Oka, H.; Arima, I.; Fukuchi, T.; Sanga, K.; Inokuchi, Y.; Ishii, Y.; Kanno, M.; et al. The superiority of vonoprazan-based first-line triple therapy with clarithromycin: A prospective multi-center cohort study on Helicobacter pylori eradication. Intern. Med. 2017, 56, 1277–1285. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Hibi, T. Quinolone-based therapy for Helicobacter pylori eradication. J. Clin. Biochem. Nutr. 2009, 44, 119–124. [Google Scholar] [CrossRef]
- Tanabe, H.; Yoshino, K.; Ando, K.; Nomura, Y.; Ohta, K.; Satoh, K.; Ichiishi, E.; Ishizuka, A.; Otake, T.; Kohgo, Y.; et al. Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Shinozaki, S.; Shinozaki, S.; Kobayashi, Y.; Osawa, H.; Sakamoto, H.; Hayashi, Y.; Yamamoto, H.; Lefor, A.K. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: Systematic review and meta-analysis. Digestion 2020, 3223, 1–7. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H.; Fujimoto, A.; Kinoshita, H.; Yoshida, S.; Isomura, Y.; Toyoshima, A.; Kanai, T.; Yahagi, N.; Toyoshima, O. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J. Clin. Biochem. Nutr. 2017, 60, 208–210. [Google Scholar] [CrossRef]
- Sue, S.; Shibata, W.; Sasaki, T.; Kaneko, H.; Irie, K.; Kondo, M.; Maeda, S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J. Gastroenterol. Hepatol. 2019, 34, 686–692. [Google Scholar] [CrossRef]
- Saito, Y.; Konno, K.; Sato, M.; Nakano, M.; Kato, Y.; Saito, H.; Serizawa, H. Vonoprazan-based third-line therapy has a higher eradication rate against sitafloxacin-resistant. Cancers 2019, 11, 116. [Google Scholar] [CrossRef]
- Kiyotoki, S.; Nishikawa, J.; Sakaida, I. Efficacy of vonoprazan for helicobacter pylori eradication. Intern. Med. 2020, 59, 153–161. [Google Scholar] [CrossRef]
- Okamura, T.; Suga, T.; Nagaya, T.; Arakura, N.; Matsumoto, T.; Nakayama, Y.; Tanaka, E. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: A multi-generational comparison. Helicobacter 2014, 19, 214–220. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Aftab, H.; Shrestha, P.K.; Sharma, R.P.; Subsomwong, P.; Waskito, L.A.; Doohan, D.; Fauzia, K.A.; Yamaoka, Y. Effective therapeutic regimens in two South Asian countries with high resistance to major Helicobacter pylori antibiotics. Antimicrob. Resist. Infect. Control. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Chaudhary, P.H.; Tawar, M.G. Pharmacognostic and phytopharmacological overview on Bombax ceiba. Syst. Rev. Pharm. 2019, 10, 20–25. [Google Scholar] [CrossRef]
- Ratnasari, N.; Rezkitha, Y.A.A.; Adnyana, I.K.; Alfaray, R.I.; Fauzia, K.A.; Doohan, D.; Panjaitan, A.; Priskila, Y.; Yulinah, E.; Khomsan, A.; et al. Anti-helicobacter pylori effects of propolis ethanol extract on clarithromycin and metronidazole resistant strains. Syst. Rev. Pharm. 2020, 11, 429–434. [Google Scholar] [CrossRef]
- Scott, D.R.; Munson, K.B.; Marcus, E.A.; Lambrecht, N.W.G.; Sachs, G. The binding selectivity of vonoprazan (TAK-438) to the gastric H+,K+-ATPase. Aliment. Pharmacol. Ther. 2015, 42, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Lundell, L.; Vieth, M.; Gibson, F.; Nagy, P.; Kahrilas, P.J. Systematic review: The effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment. Pharmacol. Ther. 2015, 42, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Kang, A.J.; Merchant, J.L. Pathophysiology of gastric NETs: Role of gastrin and menin. Curr. Gastroenterol. Rep. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Martinsen, T.C.; Fossmark, R.; Waldum, H.L. The phylogeny and biological function of gastric juice—Microbiological consequences of removing gastric acid. Int. J. Mol. Sci. 2019, 20, 6031. [Google Scholar] [CrossRef]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 125–133. [Google Scholar] [CrossRef]
- Kristanto, A.; Adiwinata, R.; Rasidi, J.; Phang, B.B.; Adiwinata, S.; Richard, T.; Uwan, W.B.; Syam, A.F. Long-term risks of proton pump inhibitor administration: A literature review. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2017, 18, 169–176. [Google Scholar] [CrossRef][Green Version]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther. Adv. Drug Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Habib, A.; Najmi, A.K. Proton pump inhibitors use and risk of chronic kidney disease: Evidence-based meta-analysis of observational studies. Clin. Epidemiol. Glob. Health 2019, 7, 46–52. [Google Scholar] [CrossRef]
| American College of Gastroenterology [25] Indonesian Society of Gastroenterology [26] | Japanese Society for Helicobacter Research [20] | The Toronto Consensus [27] | The Maastricht V/Florence Consensus Report [17] | |
|---|---|---|---|---|
| First Line | If Clarithromycin-resistant strains < 20% PPI * Amoxicillin Clarithromycin If Clarithromycin-resistant strains > 20% PPI * Bismuth subsalicylate Metronidazole Tetracycline | PPI * or Vonoprazan Amoxicillin Clarithromycin | If Clarithromycin-resistant strains < 15% PPI * Amoxicillin/Metronidazole Clarithromycin If Clarithromycin-resistant strains > 15% PPI * Amoxicillin Metronidazole Clarithromycin or PPI * Bismuth Metronidazole Tetracycline | If Clarithromycin-resistant strains < 15% PPI * Amoxicillin Clarithromycin If Clarithromycin-resistant strains > 15% PPI * Amoxicillin Metronidazole |
| Second Line | PPI * Bismuth subsalicylate Metronidazole or PPI * Amoxicillin Levofloxacin | PPI * Amoxicillin Metronidazole | PPI * Amoxicillin Levofloxacin or PPI * Bismuth Metronidazole Tetracycline | PPI * Amoxicillin Levofloxacin or PPI * Bismuth Metronidazole Tetracycline |
| Third Line | PPI * Amoxicillin Levofloxacin Rifabutin | PPI * Amoxicillin/Metronidazole Sitafloxacin | PPI * Amoxicillin Rifabutin | Regimens based on the bacterial culture susceptibility test |
| Parameter | PPI | Vonoprazan | |
|---|---|---|---|
| First Generation PPI | Second Generation PPI | ||
| Acid activation | Yes | No | |
| Active drug | No | Yes | |
| Acid Stability | No | Yes | |
| Main P450 metabolizer | CYP2C19 | CYP3A4 | |
| Meal’s influence | Yes | No | |
| Mechanism of Action | Covalent bond to gastric proton pump | Potassium ion competitive reversible inhibitor to gastric proton pump | |
| Days required for reaching maximal acid suppression | 3–5 | 1 | |
| pH > 4 holding time (%) | OMZ 30.4 LPZ 39.1 | ESO 43.1 RPZ 42.8 | 10 mg 38.4–43.1 20 mg 62.7–63.3 |
| Time Needed to Reach Maximum Plasma Concentration (h) | OMZ 1–4 LPZ 1.2–2.1 | ESO 1–3.5 RPZ 1.14 | 10 mg 1.75 20 mg 1.50 |
| Half-life (h) | OMZ 0.5–1.2 LPZ 0.9–2.1 | ESO 1.3–1.6 RPZ 0.6–1.4 | 10 mg 6.95 ± 1.03 20 mg 6.85 ± 0.80 |
| Cmax (μmol/L) | OMZ 0.23–23.2 LPZ 1.62–3.25 | ESO 2.1–2.4 RPZ 1.14 | 10 mg 9.7 ± 2.1 μg/L 20 mg 25.0 ± 5.6 μg/L |
| AUC (μmol.h/L) | OMZ 0.58–3.47 LPZ 4.60–13.5 | ESO 4.2 RPZ 2.22 | 10 mg 60.1 ± 9.0 μg.h/L 20 mg 160.3 ± 38.6 μg.h/L |
| Study | VPZ-Based Regimen | PPI-Based Regimen | ||
|---|---|---|---|---|
| Regimen | Eradication Rate | Regimen | Eradication Rate | |
| RCT | ||||
| Murakami et al., 2016 [60] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 90.9% | LPZ: 30 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 75.1% |
| Maruyama et al., 2017 [61] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 95.8% | LPZ: 30 mg bid or RPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 69.6% |
| Sue et al., 2017 [62] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 87.3% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 76.5% |
| Ozaki et al., 2018 [63] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 90.9% | RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 72.8% |
| Non-RCT | ||||
| Suzuki et al., 2016 [64] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 89.0% | LPZ: 30 mg bid or RPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 74.2% |
| Shinozaki et al., 2016 [65] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 82.9% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 73.9% |
| Shichijo et al., 2016 [66] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 87.2% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 72.4% |
| Noda et al., 2016 [67] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 400 mg bid | 89.7% | OMZ: 20 mg bid, LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 73.9% |
| Matsumoto et al., 2016 [68] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 89.6% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 71.9% |
| Yamada et al., 2016 [69] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 85.7% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 73.2% |
| Tsujimae et al., 2016 [70] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 84.6% | ESO: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 79.1% |
| Kajihara et al., 2016 [71] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 400 mg bid | 94.6% | RPZ: 10 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 86.7% |
| Sakurai et al., 2017 [72] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 87.9% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 66.9% |
| Sue et al., 2017 [73] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 84.9% | OMZ: 20 mg bid, LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 78.8% |
| Nishizawa et al., 2017 [74] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 62.3% | LPZ: 30 mg bid or RPZ: 10 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 47.1% |
| Tanabe et al., 2018 [75] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 91.5% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 79.4% |
| Study | VPZ-Based Regimen | PPI-Based Regimen | ||
|---|---|---|---|---|
| Regimen | Eradication Rate | Regimen | Eradication Rate | |
| RCT | ||||
| Murakami et al., 2016 [60] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 82.0% | LPZ: 30 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 40.0% |
| Non-RCT | ||||
| Noda et al., 2016 [67] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 400 mg bid | 87.5% | OMZ: 20 mg bid, LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 53.8% |
| Matsumoto et al., 2016 [68] | VPZ: 20 mg bid AMX: 750 mg bid CLR: 200 mg bid | 76.1% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid CLR: 200 or 400 mg bid | 40.2% |
| Study | VPZ-Based Regimen | PPI-Based Regimen | ||
|---|---|---|---|---|
| Regimen | Eradication Rate | Regimen | Eradication Rate | |
| Yamada et al., 2016 [69] | VPZ: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 89.6% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 89.9% |
| Tsujimae et al., 2016 [70] | VPZ: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 89.1% | ESO: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 83.3% |
| Sakurai et al., 2017 [72] | VPZ: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 96.1% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 89.7% |
| Sue et al., 2017 [73] | VPZ: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 80.5% | LPZ: 30 mg bid, RPZ: 10 mg bid or ESO: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 81.5% |
| Nishizawa et al., 2017 [77] | VPZ: 20 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 71.8% | LPZ: 30 mg bid or RPZ: 10 mg bid AMX: 750 mg bid MNZ: 250 mg bid | 73.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miftahussurur, M.; Pratama Putra, B.; Yamaoka, Y. The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals 2020, 13, 276. https://doi.org/10.3390/ph13100276
Miftahussurur M, Pratama Putra B, Yamaoka Y. The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals. 2020; 13(10):276. https://doi.org/10.3390/ph13100276
Chicago/Turabian StyleMiftahussurur, Muhammad, Boby Pratama Putra, and Yoshio Yamaoka. 2020. "The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy" Pharmaceuticals 13, no. 10: 276. https://doi.org/10.3390/ph13100276
APA StyleMiftahussurur, M., Pratama Putra, B., & Yamaoka, Y. (2020). The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals, 13(10), 276. https://doi.org/10.3390/ph13100276







