Metal-Based Complexes as Pharmaceuticals for Molecular Imaging of the Liver
Abstract
1. Introduction
2. Metal Complexes in Liver Specific MRI
2.1. MRI Principle and Relevant Metals
2.2. Gd-Based Liver Specific CA Gd-EOB-DTPA and Gd-BOPTA
2.3. Other Gd-Based Liver Imaging Agents
2.4. Side Effects of Gd-Based CA and Gd Deposition
2.5. Liver-Specific CA Based on Mn(II) and Other Metals
3. Metal Complexes in Scintigraphic Liver Imaging
3.1. Scintigraphic Imaging and Relevant Metal Nuclides
3.2. 99mTc-Complexes for Hepatobiliary Scintigraphy
3.3. 99mTc Serum Albumin Scintigraphy and Asiaglycoprotein Receptor Imaging
3.4. Colloid Scintigraphy
4. Metal Complexes in Liver Specific Pet Diagnostics
4.1. PET Imaging and Relevant Metal Nuclides
4.2. Advances in the Development of Liver Specific PET Tracers
5. Conclusions
5.1. Liver-Targeting Metal Complexes—Influence of Ligand and Metal
- -
- Use of glycoprotein conjugates, e.g., galactosyl albumin (NGA, GSA) or galactosylated copolymers, to achieve active uptake into the hepatocytes via targeting of AGPR;
- -
- Generation of small lipophilic, preferably anionic complexes like Gd-EOB-DTPA, 99mTc-IDAs, 99mTc-PMT, or 68Ga-BP-IDA [208], or use of derivatives of natural liver targeting substrates like bile acids [223] and bromosulfophthalein [208], to achieve active uptake into the hepatocytes via transporters like OATP;
- -
- Active uptake into the Kupffer cells of the RES via phagocytosis, e.g., using nanoparticles like iron hydroxides or crystalline coated silica, colloids (for example 99mTc-sulfur colloid, 99mTc albumin colloid) or liposomes;
- -
- Indirect-targeting mechanisms, e.g., Mn(II) uptake into the hepatocytes following in vivo demetallation of Mn-DPDP.
5.2. Medical Application is Determined by Biodistribution and Imaging Technique
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AGP | asiaglycoprotein |
| AGPR | asiaglycoprotein receptor |
| t-butyl-HBED | N,N′-Bis(5-t-butyl-2-hydroxy3-methylbenzyl)ethylenediamine-N,N′-diacetic acid |
| BOPTA | benzyloxymethyl-DTPA |
| BRIDA | 3-bromo-2,4,6-trimethyl-IDA (mebrofenin) |
| BP-IDA | tetra-bromo-ortho-cresolphthalein IDA |
| CA | contrast agent |
| Cy2DOT | hexadecahydro-dibenzo[b,h][1,4,7,10]tetraazacyclododecine5,8,13,16-tetraacetic acid |
| DAZA | 1,4-diazepane-6-amine |
| 3,4-DiP-LICAM | N,N′-bis(iso-propyl)-N,N′,N″-tris(2,3-dihydroxybenzoyl)-l,5,10-triazadecane |
| DISIDA | 2,6-diisopropyl-IDA |
| 2,5-BPA-DO3A | 10-[2-[(2,5-bis(isopropyl)phenyl)amino]-2-oxoethyl]-DO3A |
| DO3A | 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid |
| DOTA | 1,4,7-10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DTPA | diethylenetriamine pentaacetic acid |
| DPDP | dipyridoxyl-ethylendiamindiacetate-diphosphate |
| EDTA | ethylenediamine tetraacetic acid |
| EHIDA | 2,6-diethyl-IDA |
| EMA | European Medicines Agency |
| EOB | p-ethoxybenzyl |
| GSA | galactosyl serum albumin |
| HCC | hepatocellular carcinoma |
| HIDA | 2,6-dimethyl-IDA |
| IOTIDA | 3-iodo-2,4,6-trimethyl-IDA |
| ID | injected dose |
| IDA | phenylcarbamoylmethyl iminidoacetic acid |
| i.v. | intravenous |
| MRI | magnetic resonance imaging |
| MRP | multidrug resistance protein |
| NAFDL | non-alcoholic fatty liver disease |
| NGA | galactosyl-neoglycoalbumin |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetic acid |
| NSF | nephrogenic systemic fibrosis |
| NTCP | Na+/taurocholate co-transporting polypeptide |
| OATP | organic anion transporter |
| PET | positron emission tomography |
| p.i. | post injection |
| PMT | N-pyridoxyl-5-methyltryptophane |
| PIPIDA | p-isopropyl-IDA |
| PyC3A | N-picolyl-N,N′,N′-trans-1,2-cyclohexylenediaminetriacetate |
| PyC3A-3-OBn | N-[(3-benzyloxy)picolyl]-N,N′,N′-trans-1,2-cyclohexylenediaminetriacetate |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RES | reticuloendothelial system |
| SOD | superoxide dismutase |
| SPECT | single photon emission tomography |
| SPIO | superparamagnetic iron oxide |
| TEtOHB-DAZA | N,1,4-Tri(4-ethoxy-2-hydroxybenzyl)-DAZA |
| USPIO | Ultra-small superparamagnetic iron oxide |
References
- Geisel, D.; Hamm, B.; Denecke, T.; Ludemann, L. Imaging-based liver function tests—Past, present and future. Fortschr. Röntgenstr. 2015, 187, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Feine, U.; Zum Winkel, K. Nuklearmedizin-Szintigraphische Diagnostik, 2nd ed.; Georg Thieme Verlag: Stuttgart, Germany, 1995. [Google Scholar]
- Ghosh, S.; Das, T.; Sarma, H.D.; Banerjee, S. Preparation and preliminary bioevaluation of 68Ga-oxine in lipiodol as a potential liver imaging agent. J. Radioanal. Nucl. Chem. 2016, 311, 263–268. [Google Scholar] [CrossRef]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.-C.; Roudot-Thoraval, F. The burden of liver disease in Europe: A review of available epidemiological data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Alfke, H.; Nocken, F.; Heverhagen, J.T.; Klose, K.J. Molecular radiology. II: Molecular imaging. Fortschr. Röntgenstr. 2001, 173, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Meikle, S.R.; Beekman, F.J.; Rose, S.E. Complementary molecular imaging technologies: High resolution SPECT, PET and MRI. Drug Discov. Today Technol. 2006, 3, 187–194. [Google Scholar] [CrossRef]
- Rodriguez, A.O. Principles of magnetic resonance imaging. Rev. Mex. Fis. 2004, 50, 272–286. [Google Scholar]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Fischbach, F.; Fischbach, K. MRT der Leber—Diagnostik, Differenzialdiagnose, Therapieansätze; Georg Thieme Verlag: Stuttgart, Germany, 2017. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Donato, H.; Candelaria, I.; Caseiro-Alves, F.; Franca, M. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef]
- Bui, T.; Stevenson, J.; Hoekman, J.; Zhang, S.; Maravilla, K.; Ho, R.J.Y. Novel Gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Toth, E. MRI Contrast Agents. In Ligand Design in Medicinal Inorganic Chemistry, 1st ed.; Storr, T., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 321–354. [Google Scholar]
- Ba-Ssalamah, A.; Happel, B.; Kettenbach, J.; Dirisamer, A.; Wrba, F.; Längle, F.; Schima, W. MRT der Leber. Der Radiologe 2004, 44, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S. Primer on molecular imaging technology. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Dill, T. Contraindications to magnetic resonance imaging: Non-invasive imaging. Heart 2008, 94, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Van Beers, B.E.; Pastor, C.M.; Hussain, H.K. Primovist, Eovist—What to expect. J. Hepatol. 2012, 57, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Clément, O.; Siauve, N.; Lewin, M.; de Kerviler, E.; Cuénod, C.-A.; Frija, G. Contrast agents in magnetic resonance imaging of the liver: Present and future. Biomed. Pharmacother. 1998, 52, 51–58. [Google Scholar] [CrossRef]
- El-Gazzar, M.F.; Kohla, M.A.S.; El-Sakhawy, M.M.; Husseiny, M.M.; Yousef, R.R.H.; El-Shorbagy, S.H. Use of gadobenate dimeglumine dynamic MRI for detection of early hepatocellular carcinoma in atypical hepatic focal lesions. Hepatoma Res. 2017, 3, 123–128. [Google Scholar] [CrossRef][Green Version]
- Thian, Y.L.; Riddell, A.M.; Koh, D.-M. Liver-specific agents for contrast-enhanced MRI: Role in oncological imaging. Cancer Imaging 2013, 13, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Ba-Ssalamah, A.; Wibmer, A.; Fragner, R.; Hodge, J.C.; Herold, C.J.; Bastati, N.; Trauner, M.; Bashir, M.R.; Van Beers, B.E. Hepatic gadoxetic acid uptake as a measure of diffuse liver disease: Where are we? J. Magn. Reason. Imaging 2017, 45, 646–659. [Google Scholar] [CrossRef]
- Leonhardt, M.; Keiser, M.; Oswald, S.; Kühn, J.; Jia, J.; Grube, M.; Kroemer, H.; Siegmund, W.; Weitschies, W. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-Eob-dtpa: Role of human organic anion transporters. Drug Metab. Dispos. 2010, 38, 1024–1028. [Google Scholar] [CrossRef]
- Frydrychowicz, A.; Lubner, M.G.; Brown, J.J.; Merkle, E.M.; Nagle, S.K.; Rofsky, N.M.; Reeder, S.B. Hepatobiliary MR imaging with gadolinium-based contrast agents. J. Magn. Reason. Imaging 2012, 35, 492–511. [Google Scholar] [CrossRef]
- Schima, W.; Petersein, J.; Hahn, P.F.; Harisinghani, M.; Halpern, E.; Saini, S. Contrast-enhanced MR imaging of the liver: Comparison between Gd-bopta and mangafodipir. J. Magn. Reason. Imaging 1997, 7, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.E.; Kim, S.Y.; Lee, S.S.; Kim, K.W.; Won, H.J.; Shin, Y.M.; Kim, P.N.; Lee, M.-G. Assessment of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig. Dis. 2012, 30, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, L.; Bondioni, M.P.; Faccioli, N.; Gambarini, S.; Tinti, R.; Schneider, G.; Kirchin, M. Solid focal liver lesions: Dynamic and late enhancement patterns with the dual phase contrast agent gadobenate dimeglumine. J. Gastrointest. Cancer 2010, 41, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Akpinar, E.; Balli, O.; Tirnaksiz, B.; Akata, D.; Akhan, O.; Karcaaltincaba, M. Clinical applications of gadobenate dimeglumine-enhanced magnetic resonance cholangiography: An expanded pictorial review. Jpn. J. Radiol. 2011, 29, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.R.; Heimbach, J.K.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef]
- Jeong, W.K.; Kim, Y.K.; Song, K.D.; Choi, D.; Lim, H.K. The MR imaging diagnosis of liver diseases using gadoxetic acid: Emphasis on hepatobiliary phase. Clin. Mol. Hepatol. 2013, 19, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.K.; Ma, N.; Vreugdenburg, T.D.; Cameron, A.L.; Maddern, G.; Maddern, G. Gadoxetic acid-enhanced MRI for the characterization of hepatocellular carcinoma: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2017, 45, 281–290. [Google Scholar] [CrossRef]
- Gotra, A.; Vu, K.-N.; Kauffmann, C.; Tang, A.; Gotra, A.; Gallix, B.; Sivakumaran, L.; Sivakumaran, L.; Kadoury, S.; Tang, A.; et al. Liver segmentation: Indications, techniques and future directions. Insights Imaging 2017, 8, 377–392. [Google Scholar] [CrossRef]
- Palmucci, S.; Roccasalva, F.; Piccoli, M.; Fuccio, S.G.; Foti, P.V.; Milone, P.; Ragozzino, A.; Ettorre, G.C. Contrast-enhanced magnetic resonance cholangiography: Practical tips and clinical indications for biliary disease management. Gastroenterol. Res. Pract. 2017. [Google Scholar] [CrossRef]
- Rassam, F.; Cieslak, K.P.; van Gulik, T.M.; Zhang, T.; van Vliet, L.J.; Vos, F.M.; Lavini, C.; Stoker, J.; Bennink, R.J.; Runge, J.H.; et al. Comparison between dynamic gadoxetate-enhanced MRI and (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for quantitative assessment of liver function. Eur. Radiol. 2019, 29, 5063–5072. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, K.P.; Runge, J.H.; Heger, M.; Stoker, J.; Bennink, R.J.; van Gulik, T.M. New perspectives in the assessment of future remnant liver. Dig. Surg. 2014, 31, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, L.; Penny, J.; Nicholls, G.; Woodhouse, N.; Ble, F.-X.; Hubbard, C.P.L.; Naish, J.H. Quantitative assessment of liver function using gadoxetate-enhanced magnetic resonance imaging: Monitoring transporter-mediated processes in healthy volunteers. Investig. Radiol. 2017, 52, 111–119. [Google Scholar] [CrossRef] [PubMed]
- You, M.-W.; Kim, H.J.; Lim, H.-S.; Kim, S.Y.; Byun, J.H.; Kim, K.W.; Hwang, D.W.; Lee, Y.-J. Assessment of liver function using pharmacokinetic parameters of Gd-Eob-dtpa: Experimental study in rat hepatectomy model. Contrast Media Mol. Imaging 2018, 6321316/6321311. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Akata, D.; Karcaaltincaba, M. Liver function assessment by magnetic resonance imaging. Semin. Ultrasound CT MR 2016, 37, 549–560. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Shimada, M.; Hanaoka, J.; Kanamoto, M.; Ikemoto, T.; Morine, Y.; Imura, S.; Harada, M. Possible utility of MRI using Gd-EOB-DTPA for estimating liver functional reserve. J. Gastroenterol. 2012, 47, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Amparo, E.E.; Johnson, R.R., Jr. Gadolinium-labeled pharmaceuticals as potential MRI contrast agents for liver and biliary tract. J. Label. Compd. Radiopharm. 1987, 24, 1131–1141. [Google Scholar] [CrossRef]
- Karwowski, B.; Witczak, M.; Mikiciuk-Olasik, E.; Studniarek, M. Gadolinium Gd(III) complexes with derivatives of nitriloacetic acid: Synthesis and biological properties. Acta Pol. Pharm. 2008, 65, 535–541. [Google Scholar]
- Markowicz-Piasecka, M.; Sikora, J.; Szymanski, P.; Kozak, O.; Studniarek, M.; Mikiciuk-Olasik, E. Pamam dendrimers as potential carriers of gadolinium complexes of iminodiacetic acid derivatives for magnetic resonance imaging. J. Nanomater. 2015, 394827/394821. [Google Scholar] [CrossRef]
- Baek, A.R.; Kim, H.-K.; Park, S.; Lee, G.H.; Kang, H.J.; Jung, J.-C.; Park, J.-S.; Ryeom, H.-K.; Kim, T.-J.; Chang, Y. Gadolinium complex of 1,4,7,10-tetraazacyclododecane-1,4,7-trisacetic acid (DO3A)-ethoxybenzyl (EOB) conjugate as a new macrocyclic hepatobiliary mri contrast agent. J. Med. Chem. 2017, 60, 4861–4868. [Google Scholar] [CrossRef]
- Harrison, A.; Walker, C.A.; Pereira, K.A.; Parker, D.; Royle, L.; Pulukkody, K.; Norman, T.J. Hepato-biliary and renal excretion in mice of charged and neutral gadolinium complexes of cyclic tetra-aza-phosphinic and carboxylic acids. Magn. Reson. Imaging 1993, 11, 761–770. [Google Scholar] [CrossRef]
- Runge, V.M.; Wells, J.W.; Williams, N.M. Comparison of gadolinium Cy2DOTA, a new hepatobiliary agent, and gadolinium HP-DO3A, an extracellular agent, in healthy liver and metastatic disease. Investig. Radiol. 1995, 30, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M.; Wells, J.W.; Williams, N.M. Evaluation of gadolinium 2,5-BPA-DO3A, a new macrocyclic hepatobiliary chelate, in normal liver and metastatic disease on high field magnetic resonance imaging. Investig. Radiol. 1996, 31, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, E.R.; Neubeck, R.; Song, B.; Wagler, T.; Ranganathan, R.S.; Sukumaran, K.; Wedeking, P.W.; Nunn, A.; Runge, V.M.; Tweedle, M.F. Synthesis, characterization, and imaging performance of a new class of macrocyclic hepatobiliary MR contrast agents. Investig. Radiol. 2000, 35, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Anelli, P.L.; Lattuada, L.; Lorusso, V.; Lux, G.; Morisetti, A.; Morosini, P.; Serleti, M.; Uggeri, F. Conjugates of gadolinium complexes to bile acids as hepatocyte-directed contrast agents for magnetic resonance imaging. J. Med. Chem. 2004, 47, 3629–3641. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Zanzoni, S.; Ragona, L.; Gianolio, E.; Aime, S.; Assfalg, M.; Molinari, H. Solution structure of the supramolecular adduct between a liver cytosolic bile acid binding protein and a bile acid-based gadolinium(III)-chelate, a potential hepatospecific magnetic resonance imaging contrast agent. J. Med. Chem. 2008, 51, 6782–6792. [Google Scholar] [CrossRef] [PubMed]
- Muhler, A.; Platzek, J.; Raduchel, B.; Frenzel, T.; Weinmann, H.J. Characterization of a gadolinium-labeled cholesterol derivative as an organ-specific contrast agent for adrenal MR imaging. J. Magn. Reson. Imaging 1995, 5, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.R.; Buonocore, M.H.; Wisner, E.R.; Katzberg, R.W.; Stadalnik, R.C. A molecular receptor-binding contrast agent for magnetic resonance imaging of the liver. Acad. Radiol. 1995, 2, 497–506. [Google Scholar] [CrossRef]
- Xiao, Y.; Xue, R.; You, T.; Li, X.; Pei, F.; Wang, X.; Lei, H. Gadolinium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid conjugate of arabinogalactan as a potential liver-targeting magnetic resonance imaging contrast agent. Carbohydr. Res. 2014, 395, 9–14. [Google Scholar] [CrossRef]
- Unger, E.; Cardenas, D.; Zerella, A.; Fajardo, L.L.; Tilcock, C. Biodistribution and clearance of liposomal gadolinium-DTPA. Investig. Radiol. 1990, 25, 638–644. [Google Scholar] [CrossRef]
- Schwendener, R.A.; Wuethrich, R.; Duewell, S.; Wehrli, E.; Von Schulthess, G.K. A pharmacokinetic and MRI study of unilamellar gadolinium-, manganese-, and iron-dtpa-stearate liposomes as organ-specific contrast agents. Investig. Radiol. 1990, 25, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Watcharin, W.; Zeuzem, S.; Piiper, A.; Schmithals, C.; Pleli, T.; Koberle, V.; Korkusuz, H.; Huebner, F.; Vogl, T.J.; Korf, H.W.; et al. Biodegradable human serum albumin nanoparticles as contrast agents for the detection of hepatocellular carcinoma by magnetic resonance imaging. Eur. J. Pharm. Biopharm. 2014, 87, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Watcharin, W.; Schmithals, C.; Pleli, T.; Koeberle, V.; Korkusuz, H.; Huebner, F.; Waidmann, O.; Zeuzem, S.; Korf, H.-W.; Terfort, A.; et al. Detection of hepatocellular carcinoma in transgenic mice by Gd-DTPA- and rhodamine 123-conjugated human serum albumin nanoparticles in T1 magnetic resonance imaging. J. Control. Release 2015, 199, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Ma, L.; Jin, J.; Shen, T.; Wei, R.; Wang, X.; Ai, H.; Chen, Z.; Gao, J. Albumin-based nanoparticles loaded with hydrophobic gadolinium chelates as T1-T2 dual-mode contrast agents for accurate liver tumor imaging. Nanoscale 2017, 9, 4516–4523. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Yang, H.; Grossniklaus, H.E.; Qiao, J.; Pu, F.; Jiang, J.; Hubbard, K.; Salarian, M.; Yang, J.J.; Hekmatyar, K.; et al. Protein MRI contrast agent with unprecedented metal selectivity and sensitivity for liver cancer imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, H.; Li, Y.; Chen, B.; Liu, S.; Shi, D. Bioinspired synthesis of gadolinium-based hybrid nanoparticles as MRI blood pool contrast agents with high relaxivity. J. Mater. Chem. 2012, 22, 14494–14501. [Google Scholar] [CrossRef]
- Primovist® Full Prescribing Information; Bayer Vital GmbH: Reading, UK, 2017.
- MultiHance® Full Prescribing Information; Bracco Diagnostics Inc.: Monroe Township, NJ, USA, 2018.
- Information on Primovist® Brochure; Bayer Vital GmbH.: Leverkusen, Germany, 2014.
- Endrikat, J.; Breuer, J.; Endrikat, J.; Kim, S.Y.; Sakaguchi, T.; Dohanish, S. Safety of gadoxetate disodium: Results from six clinical phase IV studies in 8194 patients. Acta Radiol. 2016, 57, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Kang, H.-R.; Kim, M.-H.; Lee, W.; Min, K.-U.; Han, M.-H.; Cho, S.-H. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012, 264, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Cascella, M.; Fusco, R.; Dell’Aprovitola, N.; Catalano, O.; Filice, S.; Schiavone, V.; Izzo, F.; Cuomo, A.; Petrillo, A. Immediate adverse reactions to gadolinium-based MR contrast media: A retrospective analysis on 10,608 examinations. BioMed Res. Int. 2016, 3918292. [Google Scholar] [CrossRef] [PubMed]
- Neeley, C.; Moritz, M.; Brown, J.J.; Zhou, Y. Acute side effects of three commonly used gadolinium contrast agents in the paediatric population. Br. J. Radiol. 2016, 89, 20160027. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Schurholz, H.; Kirchin, M.A.; Bucker, A.; Fries, P. Safety and adverse effects during 24 hours after contrast-enhanced MRI with gadobenate dimeglumine (multihance) in children. Pediatr. Radiol. 2013, 43, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.J.; Todd, D.J.; Kay, J. Gadolinium-induced fibrosis. Annu. Rev. Med. 2016, 67, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.S.; Moore, K.G.; Gillis, S.N.; Ford, K.F.; Gray, T.; Steinwinder, A.H.; Graham, A. GbCAs and risk for nephrogenic systemic fibrosis: A literature review. Radiol. Technol. 2017, 88, 583–589. [Google Scholar] [PubMed]
- Penfield, J.G.; Reilly, R.F., Jr. What nephrologists need to know about gadolinium. Nat. Clin. Pract. Nephrol. 2007, 3, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rodriguez, J.; Lai, S.; Ehst, B.D.; Fine, D.M.; Bluemke, D.A. Nephrogenic systemic fibrosis: Incidence, associations, and effect of risk factor assessment—report of 33 cases. Radiology 2009, 250, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Semelka, R.C.; Ramalho, M.; Nunes, R.H.; Al Obaidy, M.; Castillo, M. Gadolinium-based contrast agent accumulation and toxicity: An update. Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef]
- Olchowy, C.; Cebulski, K.; Lasecki, M.; Chaber, R.; Olchowy, A.; Kalwak, K.; Zaleska-Dorobisz, U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—A systematic review. PLoS ONE 2017, 12, e0171704. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.i.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef]
- Roberts, D.R.; Lindhorst, S.M.; Welsh, C.T.; Maravilla, K.R.; Herring, M.N.; Braun, K.A.; Thiers, B.H.; Davis, W.C. High levels of gadolinium deposition in the skin of a patient with normal renal function. Investig. Radiol. 2016, 51, 280–289. [Google Scholar] [CrossRef]
- White, G.W.; Gibby, W.A.; Tweedle, M.F. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (Prohance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig. Radiol. 2006, 41, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Chehabeddine, L.; Al, S.T.; Baalbaki, M.; Saleh, E.; Khoury, S.J.; Hannoun, S.; Khoury, S.J.; Hannoun, S. Cumulative administrations of gadolinium-based contrast agents: Risks of accumulation and toxicity of linear vs macrocyclic agents. Crit. Rev. Toxicol. 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, T.; Apte, C.; Jost, G.; Schoeckel, L.; Lohrke, J.; Pietsch, H. Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: Comparative study in rats. Investig. Radiol. 2017, 52, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Prybylski, J.P.; Semelka, R.C.; Jay, M. The stability of gadolinium-based contrast agents in human serum: A reanalysis of literature data and association with clinical outcomes. Magn. Reson. Imaging 2017, 38, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, D.A.; Vojinovic, S.; Benedeto-Stojanov, D.; Ljubisavljevic, S.; Stojanov, D.A.; Aracki-Trenkic, A.; Vojinovic, S.; Ljubisavljevic, S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1w magnetic resonance images in patients with relapsing-remitting multiple sclerosis: Correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur. Radiol. 2016, 26, 807–815. [Google Scholar] [PubMed]
- Gianolio, E.; Bardini, P.; Arena, F.; Stefania, R.; Di, G.E.; Iani, R.; Aime, S. Gadolinium retention in the rat brain: Assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology 2017, 285, 839–849. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA’s Final Opinion Confirms Restrictions on Use of Linear Gadolinium Agents in Body Scans; EMA/457616/2017; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Marti-Bonmati, L.; Marti-Bonmati, E. Retention of gadolinium compounds used in magnetic resonance imaging: A critical review and the recommendations of regulatory agencies. Radiologia 2017, 59, 469–477. [Google Scholar] [CrossRef]
- Rocklage, S.M.; Cacheris, W.P.; Quay, S.C.; Ekkehardt Hahn, F.; Raymond, K.N. Manganese(II) N,N′-Dipyridoxylethylenediamine-N,N′-diacetate 5,5′-Bis(phosphate). Synthesis and Characterization of a Paramagnetic Chelate for Magnetic Resonance Imaging Enhancement. Inorg. Chem. 1989, 28, 477–485. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Koren, S.A.; Abisambra, J.F.; Cloyd, R.A.; Cloyd, R.A.; Koren, S.A.; Abisambra, J.F.; Koren, S.A.; Abisambra, J.F.; Abisambra, J.F. Manganese-enhanced magnetic resonance imaging: Overview and central nervous system applications with a focus on neurodegeneration. Front. Aging Neurosci. 2018, 10, 403. [Google Scholar] [CrossRef]
- Vogl, T.J.; Hamm, B.; Schnell, B.; Eibl-Eibesfeldt, B.; Steiner, S.; Lissner, J. The clinical value of Mn-DPDP: A new paramagnetic hepatobiliary contrast medium for magnetic resonance tomography of the liver. Fortschr. Röntgenstr. 1991, 155, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.; Chezmar, J.; Rubin, D.L.; Weinreb, J.; Freeny, P.; Schmiedl, U.P.; Brown, J.J.; Borrello, J.A.; Lee, J.K.; Semelka, R.C.; et al. Efficacy and safety of mangafodipir trisodium (MnDPDP) injection for hepatic mri in adults: Results of the U.S. Multicenter phase III clinical trials. Efficacy of early imaging. J. Magn. Reson. Imaging 2000, 12, 689–701. [Google Scholar] [CrossRef]
- Reimer, P.; Schneider, G.; Schima, W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: Properties, clinical development and applications. Eur. Radiol. 2004, 14, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Rummeny, E.J.; Torres, C.G.; Kurdziel, J.C.; Nilsen, G.; Op de Beeck, B.; Lundby, B. MnDPDP for MR imaging of the liver. Results of an independent image evaluation of the european phase III studies. Acta Radiol. 1997, 38, 638–642. [Google Scholar] [PubMed]
- Kettritz, U.; Schlund, J.F.; Wilbur, K.; Eisenberg, L.B.; Semelka, R.C. Comparison of gadolinium chelates with manganese-DPDP for liver lesion detection and characterization: Preliminary results. Magn. Reson. Imaging 1996, 14, 1185–1190. [Google Scholar] [CrossRef]
- Rummeny, E.; Ehrenheim, C.; Gehl, H.B.; Hamm, B.; Laniado, M.; Lodemann, K.P.; Schmiedel, E.; Steudel, A.; Vogl, T.G. Manganese-DPDP as a hepatobiliary contrast agent in the magnetic resonance imaging of liver tumors. Results of clinical phase II trials in germany including 141 patients. Investig. Radiol. 1991, 26. [Google Scholar] [CrossRef] [PubMed]
- Sahani, D.V.; O’Malley, M.E.; Bhat, S.; Hahn, P.F.; Saini, S. Contrast-enhanced MRI of the liver with mangafodipir trisodium: Imaging technique and results. J. Comput. Assist. Tomogr. 2002, 26, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ahlstrom, H.; Ekholm, S.; Fagertun, H.; Hellstrom, M.; Hemmingsson, A.; Holtas, S.; Isberg, B.; Jonnson, E.; Lonnemark-Magnusson, M.; et al. Diagnostic efficacy of MnDPDP in MR imaging of the liver. A phase III multicentre study. Acta Radiol. 1997, 38, 643–649. [Google Scholar] [PubMed]
- Toft, K.G.; Hustvedt, S.O.; Grant, D.; Martinsen, I.; Gordon, P.B.; Friisk, G.A.; Korsmo, A.J.; Skotland, T. Metabolism and pharmacokinetics of Mndpdp in man. Acta Radiol. 1997, 38, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Chabanova, E.; Logager, V.B.; Moller, J.M.; Thomsen, H.S. Manganese based MR contrast agents: Formulation and clinical applications. Open Drug Saf. J. 2011, 2, 29–38. [Google Scholar] [CrossRef][Green Version]
- Misselwitz, B.; Muehler, A.; Weinmann, H.-J. A toxicologic risk for using manganese complexes? A literature survey of existing data through several medical specialties. Investig. Radiol. 1995, 30, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; Bacic, G.; Swartz, H.M. Evidence for the dissociation of the hepatobiliary MRI contrast agent Mn-DPDP. Magn. Reson. Med. 1996, 35, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.O.; Stark, D.D.; Leese, P.T.; Pfefferbaum, A.; Rocklage, S.M.; Quay, S.C. Hepatobiliary MR imaging: First human experience with MnDPDP. Radiology 1991, 178, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- Aicher, K.P.; Laniado, M.; Kopp, A.F.; Gronewaller, E.; Duda, S.H.; Claussen, C.D. Mn-DPDP-enhanced MR imaging of malignant liver lesions: Efficacy and safety in 20 patients. J. Magn. Reson. Imaging 1993, 3, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; Duhart, H.M.; Newport, G.D.; Lipe, G.W.; Slikker, W., Jr. Manganese-induced reactive oxygen species: Comparison between Mn+2 and Mn+3. Neurodegeneration 1995, 4, 329–334. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Biophys. Acta 1999, 1411, 385–400. [Google Scholar] [CrossRef]
- Liu, G.-F.; Filipovic, M.; Heinemann, F.W.; Ivanovic-Burmazovic, I. Seven-coordinate iron and manganese complexes with acyclic and rigid pentadentate chelates and their superoxide dismutase activity. Inorg. Chem. 2007, 46, 8825–8835. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W. Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 2005, 5, 345–354. [Google Scholar] [CrossRef]
- Platzek, J.; Mareski, P.; Niedballa, U.; Raduechel, B.; Weinmann, H.-J.; Misselwitz, B. Perfluoroalkyl-Containing Tetraazacyclododecane Metal Complexes Comprising Sugar Residues, Method for Their Preparation and Use as Imaging Agents. Patent Number WO2002014309A1, 21 February 2002. [Google Scholar]
- Larsen, L.E.; Grant, D. General toxicology of MnDPDP. Acta Radiol. 1997, 38, 770–779. [Google Scholar] [CrossRef]
- Jynge, P.; Brurok, H.; Asplund, A.; Towart, R.; Refsum, H.; Karlsson, J.O. Cardiovascular safety of MnDPDP and MnCl2. Acta Radiol 1997, 38, 740–749. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Pan, D.; Caruthers, S.D.; Senpan, A.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Revisiting an old friend: Manganese-based MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 162–173. [Google Scholar] [CrossRef]
- Gale, E.M.; Atanasova, I.P.; Blasi, F.; Ay, I.; Caravan, P. A manganese alternative to gadolinium for mri contrast. J. Am. Chem. Soc. 2015, 137, 15548–15557. [Google Scholar] [CrossRef]
- Gale, E.M.; Wey, H.-Y.; Ramsay, I.; Yen, Y.-F.; Sosnovik, D.E.; Caravan, P. A manganese-based alternative to gadolinium: Contrast-enhanced mr angiography, excretion, pharmacokinetics, and metabolism. Radiology 2018, 286, 865–872. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ramsay, I.A.; Erstad, D.J.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Manganese-based contrast agents for magnetic resonance imaging of liver tumors: Structure-activity relationships and lead candidate evaluation. J. Med. Chem. 2018, 61, 8811–8824. [Google Scholar] [CrossRef]
- Botta, M.; Carniato, F.; Esteban-Gomez, D.; Platas-Iglesias, C.; Tei, L. Mn(ii) compounds as an alternative to gd-based mri probes. Future Med. Chem. 2019, 11, 1461–1483. [Google Scholar] [CrossRef]
- Islam, M.K.; Kim, S.; Kim, H.-K.; Park, S.; Lee, G.-H.; Kang, H.J.; Jung, J.-C.; Kim, T.-J.; Chang, Y.; Park, J.-S. Manganese complex of ethylenediaminetetraacetic acid (edta)-benzothiazole aniline (bta) conjugate as a potential liver-targeting MRI contrast agent. J. Med. Chem. 2017, 60, 2993–3001. [Google Scholar] [CrossRef]
- Zhu, J.; Gale, E.M.; Atanasova, I.; Rietz, T.A.; Caravan, P. Hexameric Mn(II) dendrimer as MRI contrast agent. Chemistry 2014, 20, 14507–14513. [Google Scholar] [CrossRef]
- Leander, P.; Golman, K.; Klaveness, J.; Holtz, E.; Olsson, M.; Leunbach, I. MRI contrast media for the liver. Efficacy in conditions of acute biliary obstruction. Investig. Radiol. 1990, 25, 1130–1134. [Google Scholar] [CrossRef]
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer cell metabolism and function. J. Enzymol. Metab. 2015, 1. [Google Scholar]
- Lu, J.; Ma, S.; Sun, J.; Xia, C.; Liu, C.; Wang, Z.; Zhao, X.; Gao, F.; Gong, Q.; Song, B.; et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 2009, 30, 2919–2928. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Choi, D.; Jang, M.-S.; Lee, J. Biocompatible and biodegradable Fe3+-melanoidin chelate as a potentially safe contrast agent for liver MRI. Bioconjug. Chem. 2018, 29, 2426–2435. [Google Scholar] [CrossRef]
- Rahmim, A. PET vs. SPECT: In the context of ongoing developments. Iran. J. Nucl. Med. 2006, 14, 1–20. [Google Scholar]
- Kharissova, O.V.; Mendez-Rojas, M.A.; Kharisov, B.I.; Ortiz Mendez, U.; Elizondo Martinez, P. Metal complexes containing natural and artificial radioactive elements and their applications. Molecules 2014, 19, 10755–10802. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Bartholoma, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Loberg, M.D.; Cooper, M.; Harvey, E.; Callery, P.; Faith, W. Development of new radiopharmaceuticals based on n-substitution of iminodiacetic acid. J. Nucl. Med. 1976, 17, 633–638. [Google Scholar]
- Büll, U.; Schicha, H.; Biersack, H.-J.; Knapp, W.H.; Reiners, C.; Schober, O. Nuklearmedizin, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2001. [Google Scholar]
- Bennink, R.J.; Dinant, S.; Erdogan, D.; Heijnen, B.H.; Straatsburg, I.H.; van Vliet, A.K.; van Gulik, T.M. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J. Nucl. Med. 2004, 45, 965–971. [Google Scholar]
- Krishnamurthy, G.T.; Krishnamurthy, S. Cholescintigraphic measurement of liver function: How is it different from other methods? Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1103–1106. [Google Scholar] [CrossRef]
- Rassam, F.; Olthof, P.B.; van Gulik, T.M.; Richardson, H.; Bennink, R.J. Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl. Med. Commun. 2019, 40, 297–307. [Google Scholar] [CrossRef]
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and biochemical basis of clinical liver function tests: A review. Ann. Surg. 2013, 257, 27–36. [Google Scholar] [CrossRef]
- De Graaf, W.; Hausler, S.; Heger, M.; van Ginhoven, T.M.; van Cappellen, G.; Bennink, R.J.; Kullak-Ublick, G.A.; Hesselmann, R.; van Gulik, T.M.; Stieger, B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J. Hepatol. 2011, 54, 738–745. [Google Scholar] [CrossRef]
- Chervu, L.R.; Nunn, A.D.; Loberg, M.D. Radiopharmaceuticals for hepatobiliary imaging. Semin. Nucl. Med. 1982, 12, 5–17. [Google Scholar] [CrossRef]
- Rassam, F.; Olthof, P.B.; van Gulik, T.M.; Bennink, R.J. Current modalities for the assessment of future remnant liver function. Visc. Med. 2017, 33, 442–448. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Singh, S.; Hazarika, D. Liver functional volumetry by Tc-99m mebrofenin hepatobiliary scintigraphy before major liver resection: A game changer. Indian J. Nucl. Med. 2018, 33, 277–283. [Google Scholar] [CrossRef]
- Krishnamurthy, G.T.; Turner, F.E. Pharmacokinetics and clinical application of technetium 99m-labeled hepatobiliary agents. Semin. Nucl. Med. 1990, 20, 130–149. [Google Scholar] [CrossRef]
- Nunn, A.D.; Loberg, M.D.; Conley, R.A. A structure-distribution-relationship approach leading to the development of Tc-99m mebrofenin: An improved cholescintigraphic agent. J. Nucl. Med. 1983, 24, 423–430. [Google Scholar]
- de Graaf, W.; Bennink, R.J.; Vetelainen, R.; van Gulik, T.M. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J. Nucl. Med. 2010, 51, 742–752. [Google Scholar] [CrossRef]
- EHIDA® Full Prescribing Information; ROTOP GmbH: Dresden, Germany, 2014.
- Bridatec® Full Prescribing Information; GE Healthcare Buchler GmbH & Co. KG: Braunschweig, Germany, 2013.
- Krishnamurthy, S.; Krishnamurthy, G.T. Technetium-99m-iminodiacetic acid organic anions: Review of biokinetics and clinical application in hepatology. Hepatology 1989, 9, 139–153. [Google Scholar] [CrossRef]
- Ghibellini, G.; Leslie, E.M.; Pollack, G.M.; Brouwer, K.L.R. Use of Tc-99m mebrofenin as a clinical probe to assess altered hepatobiliary transport: Integration of in vitro, pharmacokinetic modeling, and simulation studies. Pharm. Res. 2008, 25, 1851–1860. [Google Scholar] [CrossRef]
- Choletec® Instruction Leaflet; Bracco Diagnostics Inc.: Monroe Township, NJ, USA, 2014.
- Fraser, I.A.; Shaffer, P.; Love, J.; Staubus, A.E.; Hinkle, G.; Olsen, J.; Carey, L.C.; Fabri, P.J.; Ellison, E.C. Pharmacokinetic studies of disida disposition. I. Animal studies. Eur. J. Nucl. Med. 1988, 14, 431–435. [Google Scholar] [CrossRef]
- Fraser, I.A.; Shaffer, P.; Staubus, A.E.; Tuttle, S.; Carey, L.C.; Ellison, E.C. DISIDA kinetics measure liver function in dogs. Nucl. Med. Commun. 1989, 10, 435–447. [Google Scholar] [CrossRef]
- Zmbova, B.; Konstantinovska-Djokic, D.; Kostic, K.; Obradovic, V. Synthesis and quality control of 2,6-diisopropyl IDA and its labelling with technetium-99m. Isotopenpraxis 1987, 23, 278–281. [Google Scholar]
- Gambhir, S.S.; Hawkins, R.A.; Huang, S.C.; Hall, T.R.; Busuttil, R.W.; Phelps, M.E. Tracer kinetic modeling approaches for the quantification of hepatic function with technetium-99m DISIDA and scintigraphy. J. Nucl. Med. 1989, 30, 1507–1518. [Google Scholar]
- Park, S.H.; Gwon, H.J.; Park, J.S.; Park, K.B. Synthesis and radiochemical labeling of N-(2,6-diisopropylacetanilido)-iminodiacetic acid and its analogues under microwave irradiation: A hepatobiliary imaging agent. QSAR Comb. Sci. 2004, 23, 868–874. [Google Scholar] [CrossRef]
- Hong, Y.-D.; Jang, B.-S.; Choi, S.-M.; Park, W.-W.; Park, K.-B.; Choi, S.-J. Preparation and in-vivo evaluation of 99mTc-IOTIDA for cholescintigraphy. Appl. Radiat. Isot. 2004, 61, 1273–1278. [Google Scholar] [CrossRef]
- Chauhan, U.P.S.; Mishra, P.; Chander, J. Technetium-99m-diethylmonoiodo-IDA: A radiopharmaceutical for hepatobiliary scintigraphy. Appl. Radiat. Isot. 1993, 44, 843–848. [Google Scholar] [CrossRef]
- Horiuchi, K.; Saji, H.; Arano, Y.; Yokoyama, A. Ligandin binding phthalein complexone complex of technetium for hepatic function studies. Eur. J. Nucl. Med. 1990, 16, 137–142. [Google Scholar] [CrossRef]
- Kato-Azuma, M. Technetium-99m(tin)-N-pyridoxylaminates: A new series of hepatobiliary imaging agents. J. Nucl. Med. 1982, 23, 517–524. [Google Scholar]
- Kato-Azuma, M. Lipophilic derivatives of 99mTc(Sn)pyridoxylidenephenylalanine: A structure distribution relationship (sdr) study on technetium-99m complexes. Int. J. Appl. Radiat. Isot. 1982, 33, 937–944. [Google Scholar] [CrossRef]
- Oyamada, H.; Yamazaki, S.; Makuuchi, M.; Hasegawa, H. Clinical significance of technetium-99m-N-pyridoxyl-5-methyltryptophan (technetium-99m-PMT) in the diagnosis of intrahepatic masses. Radioisotopes 1989, 38, 244–251. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kubo, S.; Tanaka, H.; Takemura, S.; Yamazaki, K.; Hirohashi, K.; Shiomi, S. Bile leakage after living donor liver transplantation demonstrated with hepatobiliary scan using 99mTcPMT. Ann. Nucl. Med. 2003, 17, 507–509. [Google Scholar] [CrossRef]
- Kobayashi, M. Transport mechanisms of hepatic uptake and bile excretion in clinical hepatobiliary scintigraphy with 99mTc-N-pyridoxyl-5-methyltryptophan. Nucl. Med. Biol. 2014, 338. [Google Scholar] [CrossRef]
- Chen, M.; Will, Y. Methods in Pharmacology and Toxicology: Drug-Induced Liver Toxicity; Springer: New York, NY, USA, 2018. [Google Scholar]
- Ono, Y.; Yamamoto, Y.; Itoh, S.; Arai, H.; Aga, F.; Nishiyama, Y. SPECT/CT imaging in 99mTc-PMT hepatobiliary scintigraphy to detect bone metastases from hepatocellular carcinoma. Clin. Nucl. Med. 2012, 37, 1011–1012. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, S.-J.; Hong, Y.-D. Characterization of 99mTc(CO)3-iminodiacetic acid (IDA) and its comparison with 99mTc-(IDA)2 using compounds for hepatobiliary scintigraphy. J. Radioanal. Nucl. Chem. 2012, 292, 203–209. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, H.; Xu, X.; Zhang, C.; Shen, Y.-M. Synthesis and characterization of organometallic rhenium(I) and technetium(I) bile acid complexes. J. Organomet. Chem. 2009, 694, 3247–3253. [Google Scholar] [CrossRef]
- Betebenner, D.A.; Carney, P.L.; Zimmer, A.M.; Kazikiewicz, J.M.; Brucher, E.; Sherry, A.D.; Johnson, D.K. Hepatobiliary delivery of polyaminopolycarboxylate chelates: Synthesis and characterization of a cholic acid conjugate of edta and biodistribution and imaging studies with its indium-111 chelate. Bioconjug. Chem. 1991, 2, 117–123. [Google Scholar] [CrossRef]
- Iimuro, Y. ICG clearance test and 99mTc-GSA SPECT/CT fusion images. Visc. Med. 2017, 33, 449–454. [Google Scholar] [CrossRef]
- Kokudo, N.; Vera, D.R.; Makuuchi, M. Clinical application of TcGSA. Nucl. Med. Biol. 2003, 845–849. [Google Scholar] [CrossRef]
- Stadalnik, R.C.; Vera, D.R. The evolution of (99m)Tc-NGA as a clinically useful receptor-binding radiopharmaceutical. Nucl. Med. Biol. 2001, 28, 499–503. [Google Scholar] [CrossRef]
- Kotani, K.; Kawabe, J.; Higashiyama, S.; Yoshida, A.; Kawamura, E.; Tamori, A.; Shiomi, S.; Kawada, N. Heterogenous liver uptake of Tc-99m-GSA as quantified through SPECT/CT helps to evaluate the degree of liver fibrosis. Medicine 2018, 97, e11765. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Z.; Zhang, P.; Song, M.; Zhao, Z.; Wu, X.; Zhang, X. Kit formulated asialoglycoprotein receptor targeting tracer based on copolymer for liver SPECT imaging. Nucl. Med. Biol. 2014, 41, 587–593. [Google Scholar] [CrossRef]
- Yang, W.; Mou, T.; Shao, G.; Wang, F.; Zhang, X.; Liu, B. Copolymer-based hepatocyte asialoglycoprotein receptor targeting agent for spect. J. Nucl. Med. 2011, 52, 978–985. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Z.; Zhang, P.; Li, Y.; Su, X.; You, L.; Gao, M.; Liu, C.; Wu, H.; Zhang, X. Simplified quantification method for in vivo SPECT/CT imaging of asialoglycoprotein receptor with 99mTc-p(VLA-co-VNI) to assess and stage hepatic fibrosis in mice. Sci. Rep. 2016, 6, 25377. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, D.; Liu, C.; Chen, G.; Zhuang, R.; Song, M.; Wu, H.; Zhang, X. A novel copolymer-based functional SPECT/MR imaging agent for asialoglycoprotein receptor targeting. Mol. Imaging 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Kim, E.-M.; Jeong, H.-J.; Park, I.-K.; Cho, C.-S.; Kim, C.-G.; Bom, H.-S. Hepatocyte-targeted nuclear imaging using 99mTc-galactosylated chitosan: Conjugation, targeting, and biodistribution. J. Nucl. Med. 2004, 46, 141–145. [Google Scholar]
- Vera, D.R.; Hall, D.J.; Hoh, C.K.; Gallant, P.; McIntosh, L.M.; Mattrey, R.F. Cy5.5-DTPA-galactosyl-dextran: A fluorescent probe for in vivo measurement of receptor biochemistry. Nucl. Med. Biol. 2005, 32, 687–693. [Google Scholar] [CrossRef]
- Arano, Y.; Mukai, T.; Akizawa, H.; Uezono, T.; Motonari, H.; Wakisaka, K.; Kairiyama, C.; Yokoyama, A. Radiolabeled metabolites of proteins play a critical role in radioactivity elimination from the liver. Nucl. Med. Biol. 1995, 22, 555–564. [Google Scholar] [CrossRef]
- Lee, R.T.; Wang, M.-H.; Lin, W.-J.; Lee, Y.C. New and more efficient multivalent glyco-ligands for asialoglycoprotein receptor of mammalian hepatocytes. Bioorg. Med. Chem. 2011, 19, 2494–2500. [Google Scholar] [CrossRef]
- Wang, M.-H.; Chien, C.-Y.; Wang, P.-Y.; Yu, H.-M.; Lee, H.-S.; Lin, W.-J. The specificity and accuracy of 111In-hexavalent lactoside in estimating liver reserve and its threshold value for mortality in mice. J. Hepatol. 2015, 63, 370–377. [Google Scholar] [CrossRef]
- Wang, M.-H.; Chien, C.-Y.; Yu, H.-M.; Wang, P.-Y.; Lin, W.-J. Use of 111In-hexavalent lactoside for liver reserve estimation in rodents with thioacetamide-induced hepatic fibrosis. Mol. Pharm. 2018, 15, 4417–4425. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Neves, M.; Geraldes, C.F.G.C.; de Lima, J.J.P. 153Sm3+ and 111In3+ DTPA derivatives with high hepatic specificity: In vivo and in vitro studies. J. Inorg. Biochem. 2002, 91, 312–319. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Bligh, S.W.A.; Chowdhury, A.H.M.S.; Geraldes, C.F.G.C.; De Lima, J.J.P. Characterization of 111In3+ complexes of dtpa amide derivatives: Biodistribution and clearance studied by gamma imaging. Nucl. Med. Biol. 2000, 27, 605–610. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakano, S.; Ishiguro, S.; Imaoka, S.; Sasaki, Y.; Tanaka, S.; Kasugai, H.; Kojima, J.; Ishigami, S. Comparison of delayed hepatobiliary imaging using 99mTc-Sn-N-pyridoxyl-5-methyltryptophan and 67Ga-citrate imaging for diagnosis of hepatocellular carcinoma. Eur. J. Nucl. Med. 1988, 14, 414–418. [Google Scholar] [CrossRef]
- Noujaim, A.A.; Lentle, B.C.; Hill, J.R.; Terner, U.K.; Wong, H. On the role of transferrin in the uptake of gallium by tumor cells. Int. J. Nucl. Med. Biol. 1979, 6, 193–199. [Google Scholar] [CrossRef]
- Murahashi, H. Study on the pathway of gallium-67 uptake into cultured tumor cells. Kanagawa Shigaku 1988, 23, 1–14. [Google Scholar]
- Ohkubo, Y.; Shibuya, A.; Kohno, H.; Kubodera, A. Involvement of transferrin in the uptake of 67ga in inflammatory and normal tissues. Int. J. Radiat. Appl. Instrum. B 1989, 16, 337–341. [Google Scholar] [CrossRef]
- Levin, J.; Kew, M.C. Gallium-67-citrate scanning in primary cancer of the liver: Diagnostic value in the presence of cirrhosis and relation to alpha-fetoprotein. J. Nucl. Med. 1975, 16, 949–951. [Google Scholar]
- Matesan, M.M.; Bowen, S.R.; Chapman, T.R.; Miyaoka, R.S.; Velez, J.W.; Wanner, M.F.; Nyflot, M.J.; Apisarnthanarax, S.; Vesselle, H.J. Assessment of functional liver reserve: Old and new in 99mTc-sulfur colloid scintigraphy. Nucl. Med. Commun. 2017, 38, 577–586. [Google Scholar] [CrossRef]
- Geslien, G.E.; Pinsky, S.M.; Poth, R.K.; Johnson, M.C. The sensitivity and specificity of 99mTc-sulfur colloid liver imaging in diffuse hepatocellular disease. Radiology 1976, 118, 115–119. [Google Scholar] [CrossRef]
- Bekerman, C.; Gottschalk, A. Diagnostic significance of the relative uptake of liver compared with spleen in technetium-99m-sulfur colloid scintiphotography. J. Nucl. Med. 1971, 12, 237–240. [Google Scholar]
- Huet, P.M.; Chartrand, R.; Marleau, D. Extrahepatic uptake of 99mTc-phytate: Its mechanism and significance in chronic liver disease. Gastroenterology 1980, 78, 76–80. [Google Scholar] [CrossRef]
- Noronha, O.P.; Sewatkar, A.B. Comparison of three re agents-99mTc-phytate, 99mTc-sulfur colloid and 99mTc-Sb2S3 colloid in the rodent species. Int. J. Radiat. Appl. Instrum. B 1986, 13, 67–73. [Google Scholar] [CrossRef]
- Arzoumanian, A.; Rosenthall, L.; Seto, H. Clinical comparison of 99mTc-labeled preformed phytate colloid and sulfur colloid: Concise communication. J. Nucl. Med. 1977, 18, 118–120. [Google Scholar]
- Fernandes, R.S.; Mota, L.G.; Kalbasi, A.; Moghbel, M.; Werner, T.J.; Alavi, A.; Rubello, D.; Cardoso, V.N.; de Barros, A.L.B. 99mTc-phytate as a diagnostic probe for assessing inflammatory reaction in malignant tumors. Nucl. Med. Commun. 2015, 36, 1042–1048. [Google Scholar] [CrossRef]
- Haubner, R.; Vera, D.R.; Farshchi-Heydari, S.; Helbok, A.; Rangger, C.; Putzer, D.; Virgolini, I.J. Development of (68)Ga-labelled DTPA galactosyl human serum albumin for liver function imaging. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1245–1255. [Google Scholar] [CrossRef]
- Phytacis®—Full Prescribing Information; CIS Bio International: Gif-sur-Yvette, France, 2017.
- Anger, H.O. Scintillation camera. Rev. Sci. Instrum. 1958, 29, 27–33. [Google Scholar] [CrossRef]
- Price, T.W.; Greenman, J.; Stasiuk, G.J. Current advances in ligand design for inorganic positron emission tomography tracers 68Ga, 64Cu, 89Zr and 44Sc. Dalton Trans. 2016, 45, 15702–15724. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Velikyan, I. Continued rapid growth in 68Ga applications: Update 2013 to june 2014. J. Label. Compd. Radiopharm. 2015, 58, 99–121. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An overview of PET radiochemistry, part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef]
- Roesch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef]
- Velikyan, I. 68Ga-based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Ram, R.; Vatsa, R.; Bhusari, P.; Shukla, J.; Mittal, B.R.; Dash, A. Detailed evaluation of different 68Ge/68Ga generators: An attempt toward achieving efficient 68Ga radiopharmacy. J. Label. Compd. Radiopharm. 2016, 59, 87–94. [Google Scholar] [CrossRef]
- Fani, M.; Andre, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 67–77. [Google Scholar] [CrossRef]
- Buck, A.K.; Stollfuss, J.C.; Stahl, A.; Beer, A.J.; Meisetschlager, G.; Schwaiger, M. Nuclear medical diagnostics for liver tumors. Internist 2007, 48, 26–29. [Google Scholar]
- Detry, O.; Govaerts, L.; Deroover, A.; Vandermeulen, M.; Meurisse, N.; Malenga, S.; Bletard, N.; Mbendi, C.; Lamproye, A.; Honore, P.; et al. Prognostic value of 18F-FDG PET/CT in liver transplantation for hepatocarcinoma. World J. Gastroenterol. 2015, 21, 3049–3054. [Google Scholar] [CrossRef]
- Cheung, T.T.; Ho, C.L.; Lo, C.M.; Chen, S.; Chan, S.C.; Chok, K.S.H.; Fung, J.Y.; Chan, A.C.Y.; Sharr, W.; Yau, T.; et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of milan criteria: Surgeon’s perspective. J. Nucl. Med. 2013, 54, 192–200. [Google Scholar] [CrossRef]
- Aparici, C.M.; Behr, S.C.; Seo, Y.; Kelley, R.K.; Corvera, C.; Gao, K.T.; Aggarwal, R.; Evans, M.J. Imaging hepatocellular carcinoma with 68Ga-citrate PET: First clinical experience. Mol. Imaging 2017, 16, 1–4. [Google Scholar] [CrossRef]
- McInnes, L.E.; Rudd, S.E.; Donnelly, P.S. Copper, gallium and zirconium positron emission tomography imaging agents: The importance of metal ion speciation. Coord. Chem. Rev. 2017, 352, 499–516. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Matys, R.; Hauser, H.; Clorius, J.H.; Maier-Borst, W. A Ga-68-labeled tetrabromophthalein (Ga-68 BP-IDA) for positron imaging of hepatobiliary function: Concise communication. J. Nucl. Med. 1983, 24, 593–602. [Google Scholar]
- Pfeifer-Leeg, M.; Szabo, G.; Baranyai, Z.; Niksch, T.; Weigand, W.; Freesmeyer, M. Synthesis and characterization of GaIII, YIII, and LuIII complexes with etifenin and analogues. Z. Anorg. Allg. Chem. 2016, 642, 486–491. [Google Scholar] [CrossRef]
- Mathias, C.J.; Sun, Y.; Welch, M.J.; Green, M.A.; Thomas, J.A.; Wade, K.R.; Martell, A.E. Targeting radiopharmaceuticals: Comparative biodistribution studies of gallium and indium complexes of multidentate ligands. Nucl. Med. Biol. 1988, 15, 69–81. [Google Scholar] [CrossRef]
- Moerlein, S.M.; Welch, M.J. Tricatecholamide analogs of enterobactin as gallium- and indium-binding radiopharmaceuticals. J. Nucl. Med. 1981, 22, 710–719. [Google Scholar]
- Schuhmacher, J.; Maier-Borst, W.; Wellman, H.N. Liver and kidney imaging with gallium-68-labeled dihydroxyanthraquinones. J. Nucl. Med. 1980, 21, 983–987. [Google Scholar]
- Kumar, B.; Miller, T.R.; Siegel, B.A.; Mathias, C.J.; Markham, J.; Ehrhardt, G.J.; Welch, M.J. Positron tomographic imaging of the liver: 68Ga iron hydroxide colloid. Am. J. Roentgenol. 1981, 136, 685–690. [Google Scholar] [CrossRef]
- Vera, D.R. Gallium-labeled deferoxamine-galactosyl-neoglycoalbumin: A radiopharmaceutical for regional measurement of hepatic receptor biochemistry. J. Nucl. Med. 1992, 33, 1160–1166. [Google Scholar]
- Haubner, R.; Schmid, A.M.; Maurer, A.; Rangger, C.; Roig, L.G.; Pichler, B.J.; Virgolini, I.J. [68Ga]NOTA-galactosyl human serum albumin: A tracer for liver function imaging with improved stability. Mol. Imaging Biol. 2017, 19, 723–730. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, J.M.; Yoo, B.C.; Hong, M.K.; Kim, Y.J.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K. Ga-68-labeled neolactosylated human serum albumin (LSA) for PET imaging of hepatic asialoglycoprotein receptor. Nucl. Med. Biol. 2014, 42, 53–58. [Google Scholar] [CrossRef]
- Yu, H.-M.; Chan, C.-H.; Chen, J.-H.; Chien, C.-Y.; Wang, P.-Y.; Juan, W.-C.; Yang, C.-H.; Hsia, H.-T.; Wang, M.-H.; Lin, W.-J. Development of single vial kits for preparation of 68Ga-labelled hexavalent lactoside for PET imaging of asialoglycoprotein receptor. J. Label. Compd. Radiopharm. 2018, 61, 885–894. [Google Scholar] [CrossRef]
- Greiser, J.; Niksch, T.; Freesmeyer, M.; Weigand, W. Investigations on the Ga(III) complex of EOB-DTPA and its 68Ga radiolabeled analogue. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Greiser, J.; Kuehnel, C.; Goerls, H.; Weigand, W.; Freesmeyer, M. N,1,4-tri(4-alkoxy-2-hydroxybenzyl)-DAZA: Efficient one-pot synthesis and labelling with 68Ga for PET liver imaging in ovo. Dalton Trans. 2018, 47, 9000–9007. [Google Scholar] [CrossRef]
- Waldron, B.P.; Parker, D.; Burchardt, C.; Yufit, D.S.; Zimny, M.; Roesch, F. Structure and stability of hexadentate complexes of ligands based on aazta for efficient PET labelling with gallium-68. Chem. Commun. 2013, 49, 579–581. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.P.; Roesch, F.; Parker, D. Approaching ’kit-type’ labelling with 68Ga: The DATA chelators. Chem. Med. Chem. 2015, 10, 1019–1026. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, D.; Hu, P.; Li, X.; Shi, H.; Cheng, D.; Zhang, L. Synthesis and evaluation of 18F-labeled bile acid compound: A potential PET imaging agent for FXR-related diseases. Nucl. Med. Biol. 2014, 41, 495–500. [Google Scholar] [CrossRef]
- Testa, A.; Dall’Angelo, S.; Mingarelli, M.; Augello, A.; Schweiger, L.; Welch, A.; Elmore, C.S.; Sharma, P.; Zanda, M. Design, synthesis, in vitro characterization and preliminary imaging studies on fluorinated bile acid derivatives as PET tracers to study hepatic transporters. Bioorg. Med. Chem. 2017, 25, 963–976. [Google Scholar] [CrossRef][Green Version]
- Testa, A.; Zanda, M.; Elmore, C.S.; Sharma, P. PET tracers to study clinically relevant hepatic transporters. Mol. Pharm. 2015, 12, 2203–2216. [Google Scholar] [CrossRef]
- Langer, O. Use of PET imaging to evaluate transporter-mediated drug-drug interactions. J. Clin. Pharmacol. 2016, 56, S143–CS156. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.-S.; Chen, Y.; Kang, C.S.; Sun, X.; Wu, N. Novel 64Cu-radiolabeled bile acid conjugates for targeted PET imaging. Bioorg. Med. Chem. Lett. 2015, 25, 1082–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wachsmann, J.; Peng, F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 221–231. [Google Scholar] [CrossRef]
- Huang, S.K.; Lee, K.D.; Hong, K.; Friend, D.S.; Papahadjopoulos, D. Microscopic localization of sterically-stabilized liposomes in colon carcinoma-bearing mice. Cancer Res. 1992, 52, 5135–5143. [Google Scholar] [PubMed]
- Zanzi, I.; Srivastava, S.C.; Meinken, G.E.; Robeson, W.; Mausner, L.F.; Fairchild, R.G.; Margouleff, D.A. New cholescintigraphic agent: Ruthenium-97-DISIDA. Nucl. Med. Biol. 1989, 16, 397–403. [Google Scholar] [CrossRef]
- Ryan, J.; Cooper, M.; Loberg, M.; Harvey, E.; Sikorski, S. Technetium-99m-labeled n-(2,6-dimethylphenylcarbamoylmethyl) iminodiacetic acid (Tc-99m HIDA): A new radiopharmaceutical for hepatobiliary imaging studies. J. Nucl. Med. 1977, 18, 997–1004. [Google Scholar]
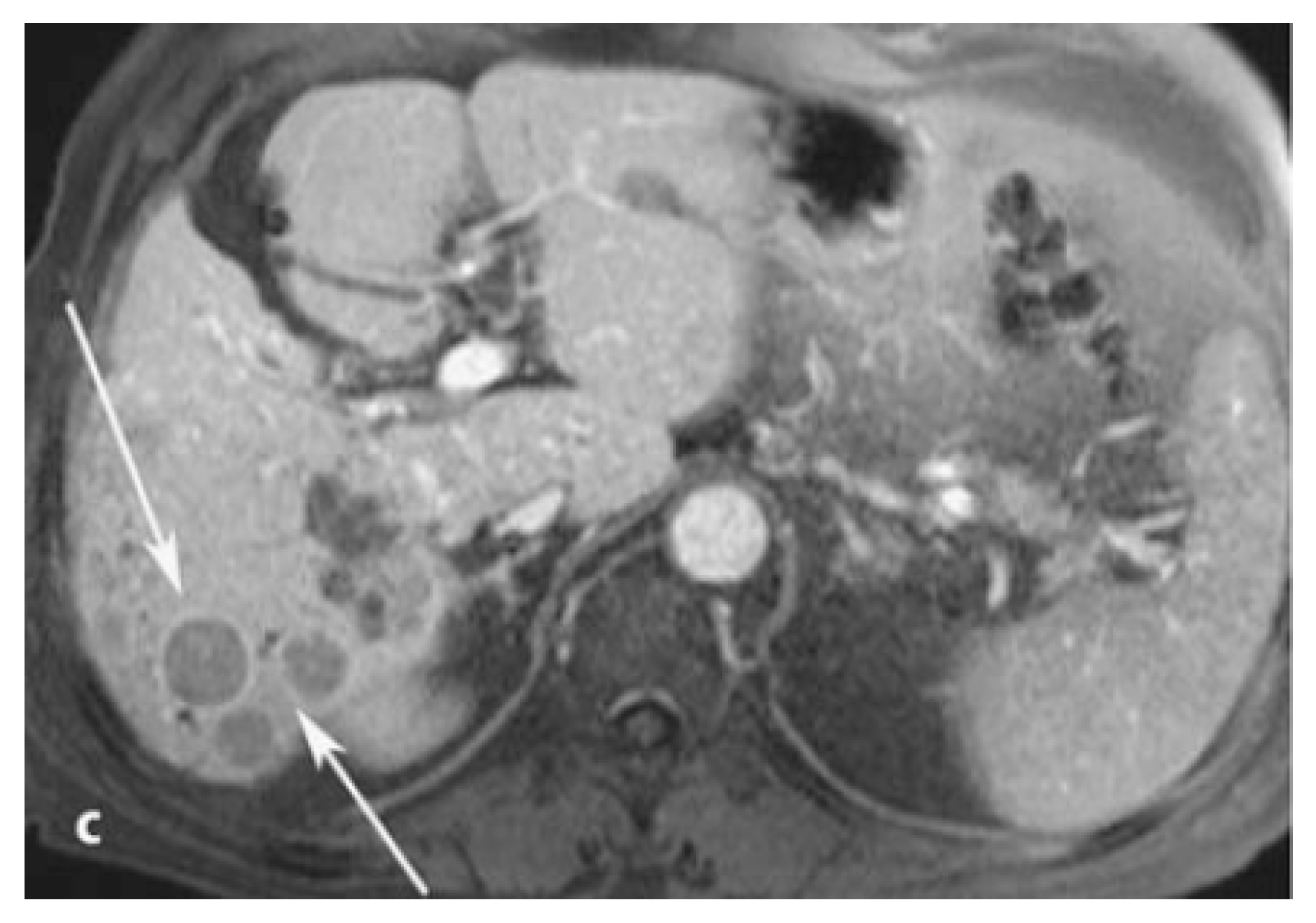
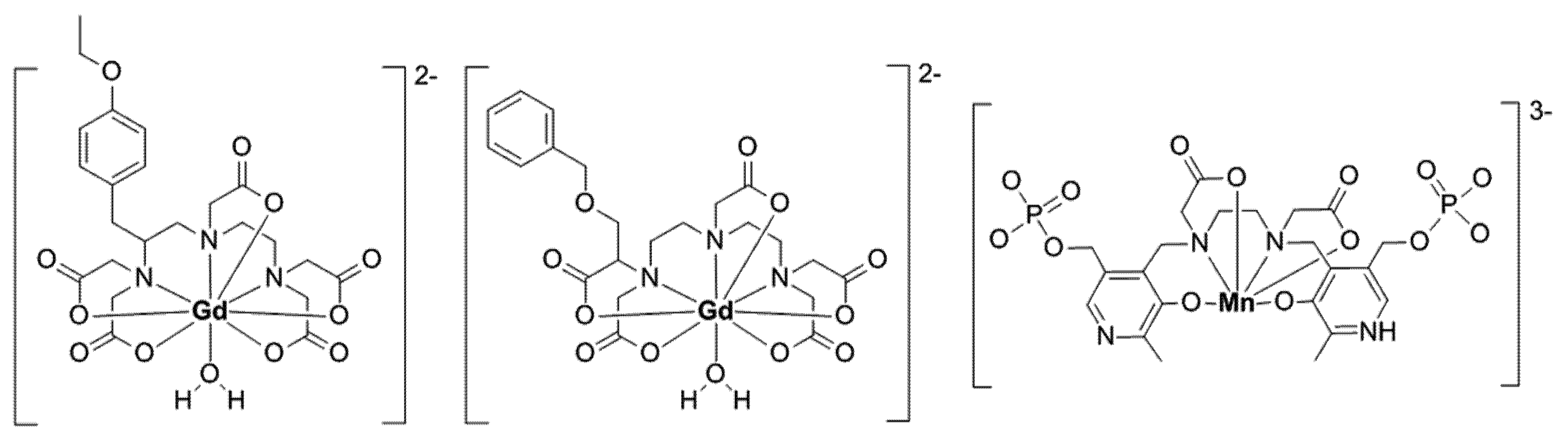
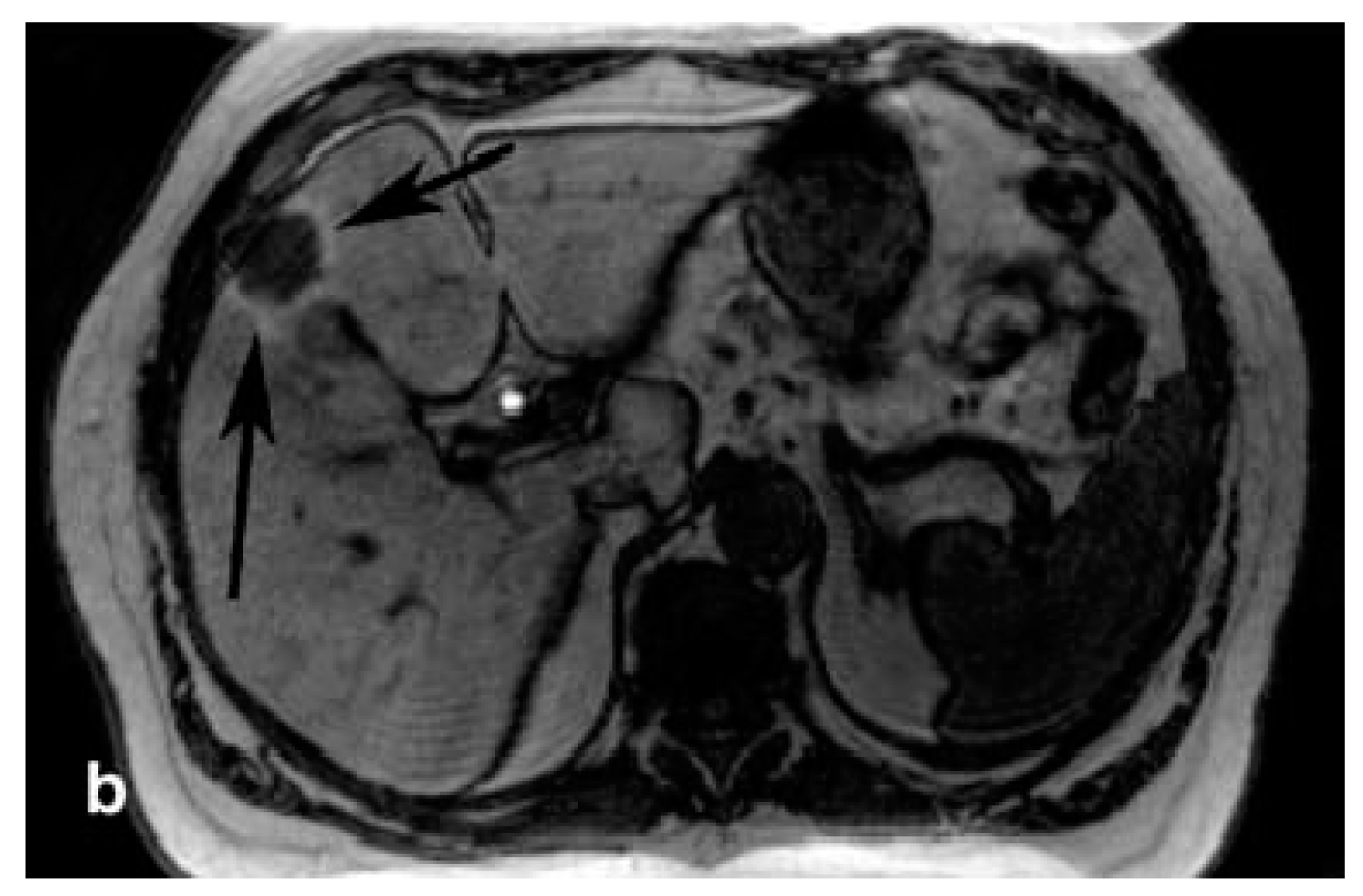


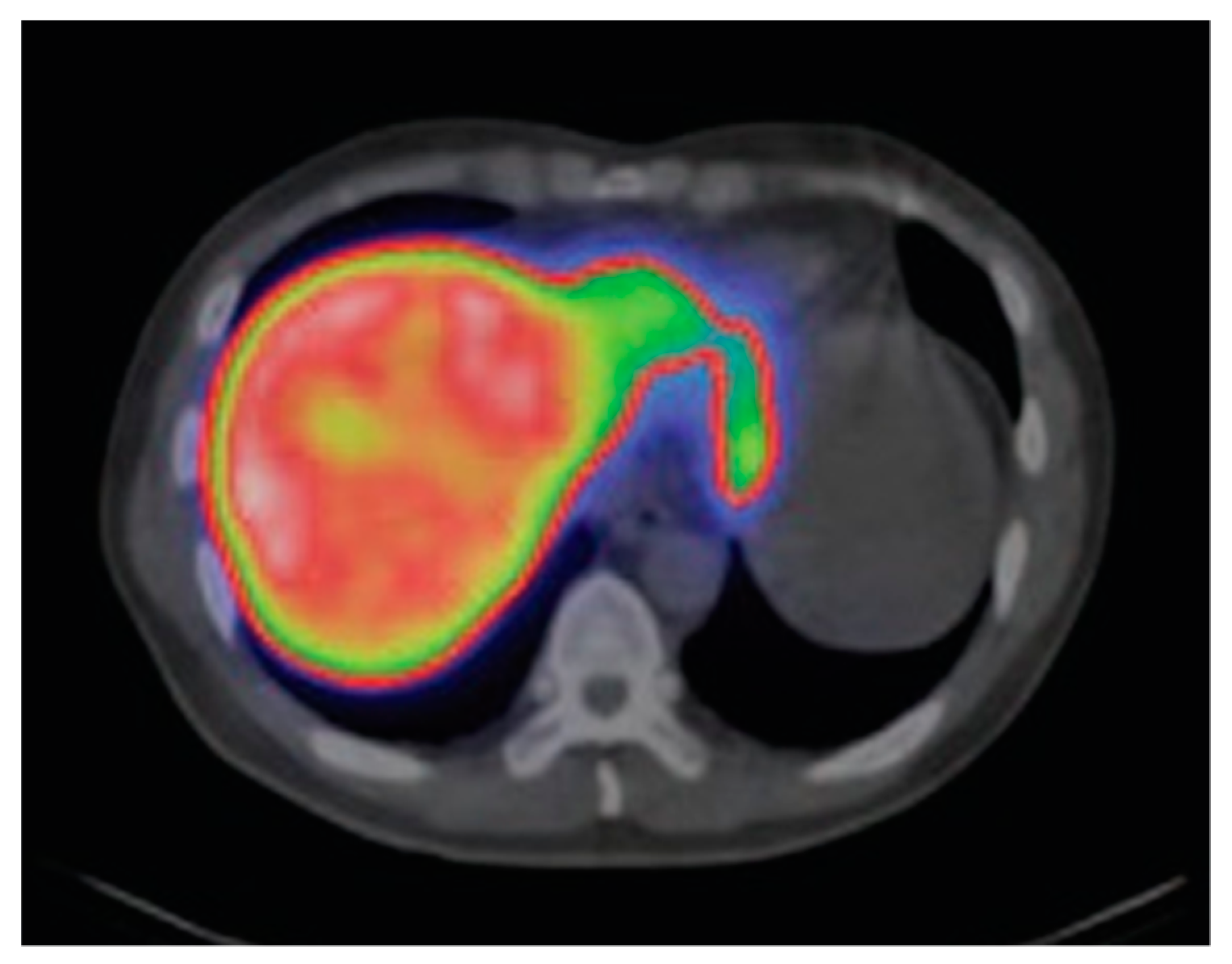
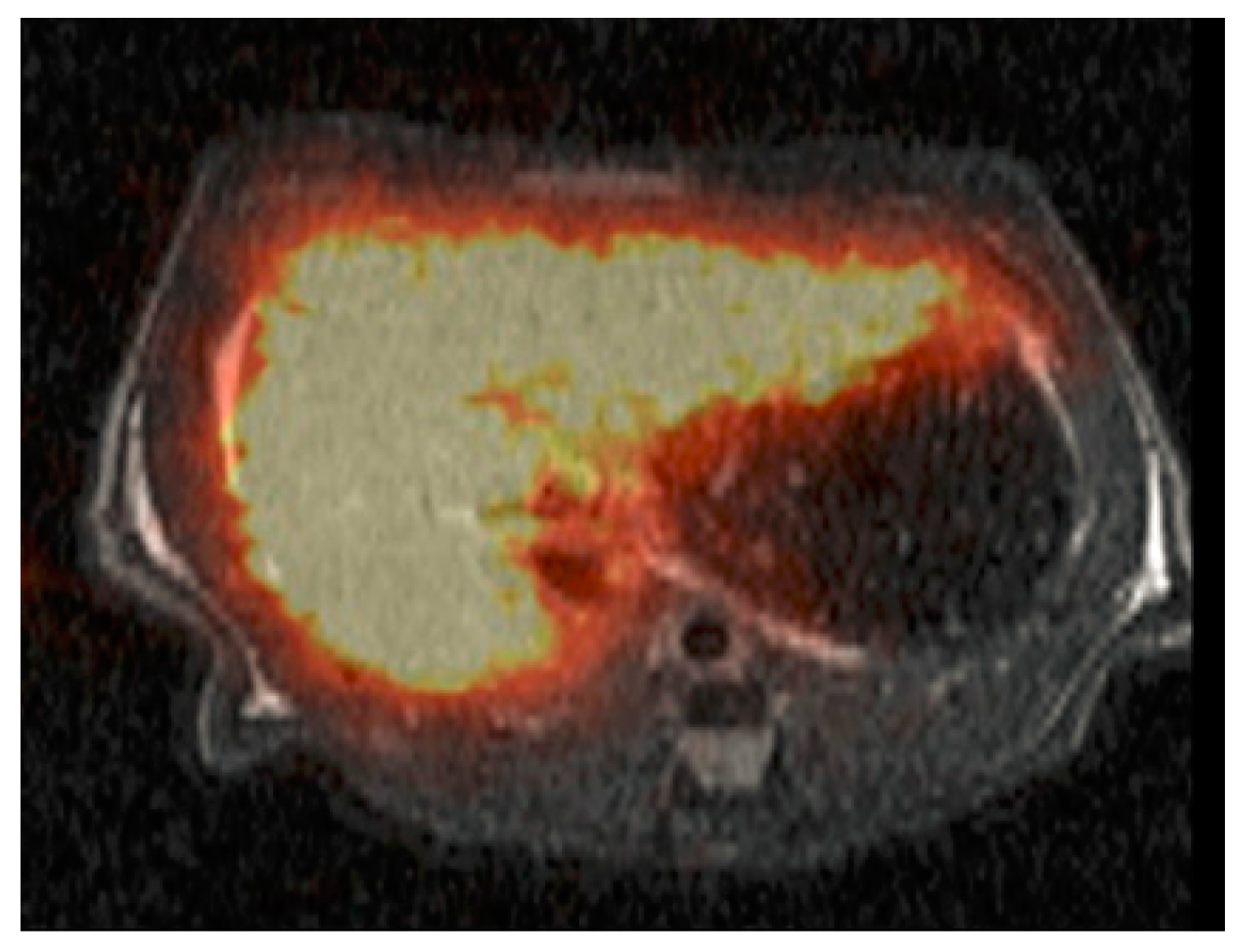
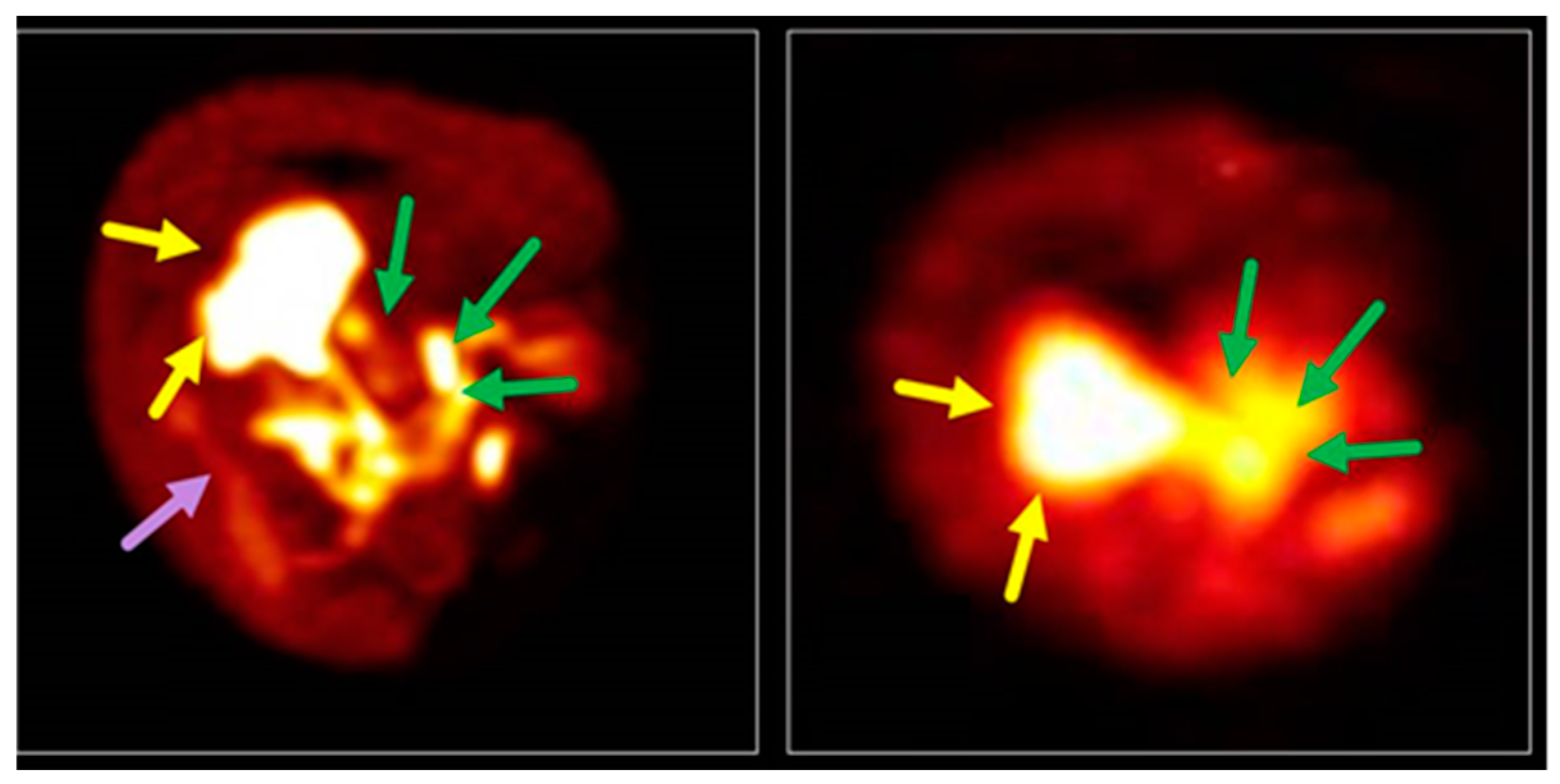
| Complex (Commercial Source: Tradename) | Biodistribution 1/Target Excretion | Use and Limitations | Applied Dose | |
|---|---|---|---|---|
| MRI | Gd-DTPA (Bayer: Magnevist®) | extracellular, non-specific, renal excretion | lesion characterization, only early phase visualization | 0.1–0.2 mmol/kg [23] |
| Gd-EOB-DTPA (Bayer: Primovist®, Eovist®) | uptake by hepatocytes (50%) with biliary excretion | lesion characterization in early and late phase, cholangiography, Gd-EOB-DTPA can be used for liver volumetry, quantification and liver function test | 0.025–0.05 mmol/kg [60] | |
| Gd-BOPTA (Bracco Diagnostic: MultiHance®) | uptake by hepatocytes (5%) with biliary excretion, mostly renal excretion | 0.05–0.1 mmol/kg [23] | ||
| Mn-DPDP (GE Healthcare: Teslascan®) | dissociation in vivo, uptake of M(II) by hepatocytes (>60%), biliary excretion | lesion characterization, cholangiography, no bolus injection | 5 µmol/kg [90] | |
| SPECT | 99mTc-mebrofenin (Bracco Diagnostic: Choletec®, or GE Healthcare: Bridatec®) | uptake by hepatocytes (>98%) with biliary excretion | hepatobiliary scintigraphy, liver volumetry, liver function test, diagnosis of chronic liver diseases, no lesion differentiation | 0.06 mmol 2 per kit [142] |
| 99mTc-etifenin (ROTOP: EHIDA®) | uptake by hepatocytes (82%) with biliary excretion | 0.06 mmol 2 per kit [141] | ||
| 99mTc-PMT (Japan Medi-Physics Co., Chiba [158]) | uptake by hepatocytes (>90%) with biliary excretion | 6.0 mmol 2 per kit [154] | ||
| 99mTc-GSA (Nihon Medi-Physics, Tokyo [192]) | exclusive uptake by AGPR on hepatocytes, no excretion | liver volumetry, regional hepatic function, diagnosis of chronic liver diseases, no visualization of biliary structures | 0.04 µmol 2 per kit 3 | |
| 99mTc-Phytate (Curium: Phytacis®) | uptake in Kupffer cells (75% liver), no excretion, phagocytosis | 0.03 mmol 2 per kit [193] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiser, J.; Weigand, W.; Freesmeyer, M. Metal-Based Complexes as Pharmaceuticals for Molecular Imaging of the Liver. Pharmaceuticals 2019, 12, 137. https://doi.org/10.3390/ph12030137
Greiser J, Weigand W, Freesmeyer M. Metal-Based Complexes as Pharmaceuticals for Molecular Imaging of the Liver. Pharmaceuticals. 2019; 12(3):137. https://doi.org/10.3390/ph12030137
Chicago/Turabian StyleGreiser, Julia, Wolfgang Weigand, and Martin Freesmeyer. 2019. "Metal-Based Complexes as Pharmaceuticals for Molecular Imaging of the Liver" Pharmaceuticals 12, no. 3: 137. https://doi.org/10.3390/ph12030137
APA StyleGreiser, J., Weigand, W., & Freesmeyer, M. (2019). Metal-Based Complexes as Pharmaceuticals for Molecular Imaging of the Liver. Pharmaceuticals, 12(3), 137. https://doi.org/10.3390/ph12030137






