The Impact of Childhood Maltreatment on Intravenous Ketamine Outcomes for Adult Patients with Treatment-Resistant Depression
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Single Infusion
2.2.1. Treatment Effect
2.2.2. Effect of Childhood Maltreatment
QIDS-SR
Response and Remission Rates
2.2.3. Influence of Demographic and Treatment Variables on Maltreatment Effects
2.3. Repeated Infusions
2.3.1. Treatment Effect
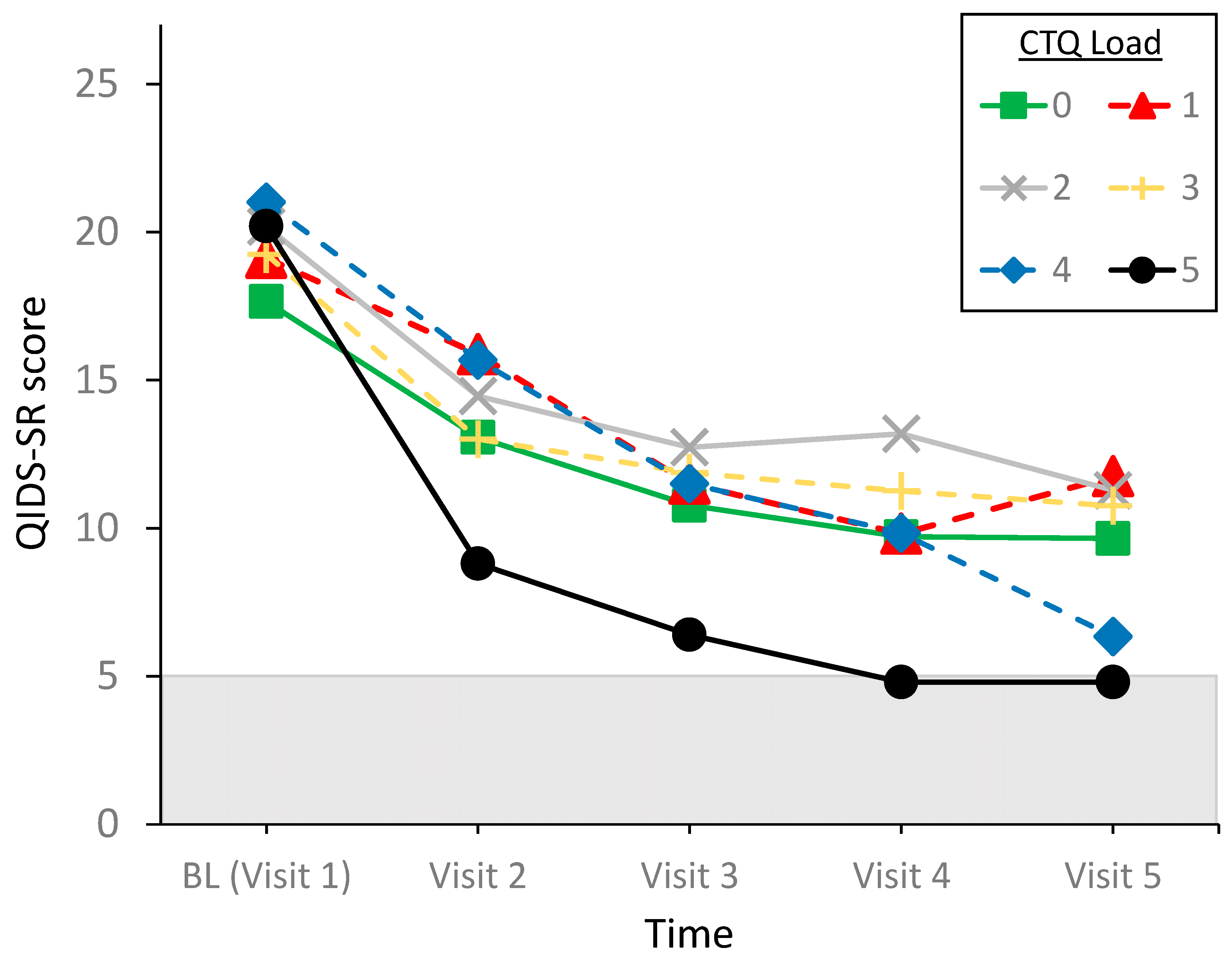
2.3.2. Effect of Childhood Maltreatment
QIDS-SR
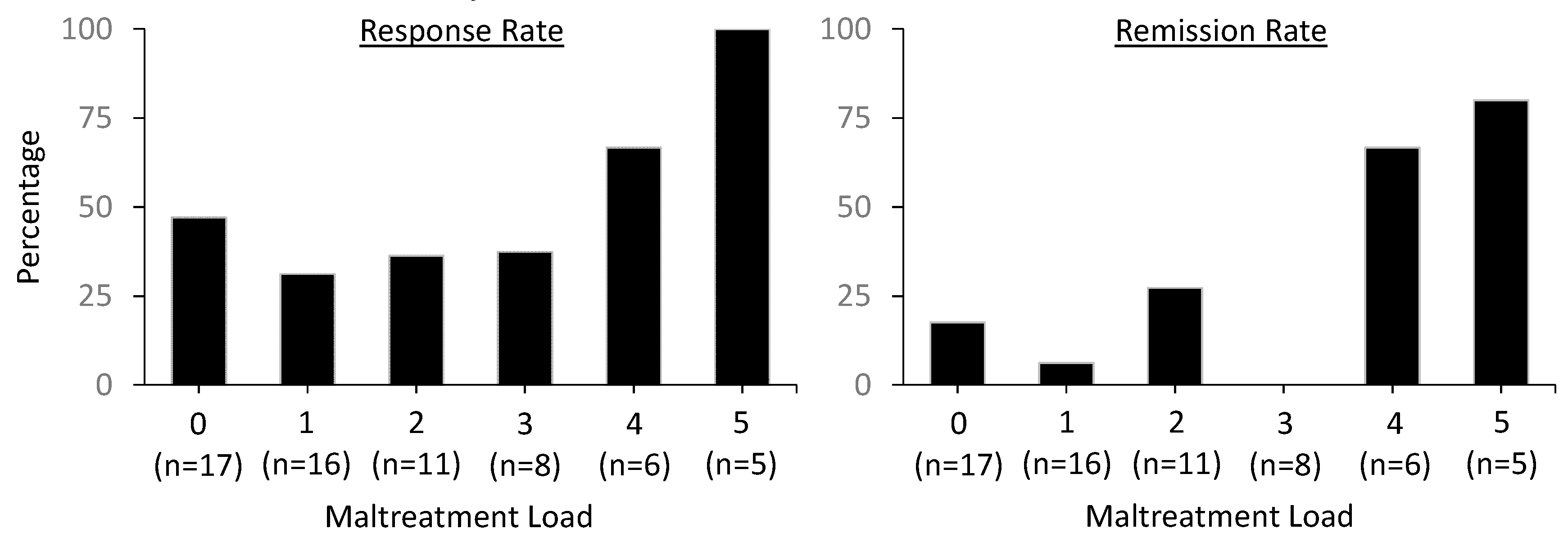
Response and Remission Rates
2.3.3. Influence of Demographic and Treatment Variables on Maltreatment Effects
2.3.4. Intent-to-Treat Analysis
3. Discussion
4. Materials and Methods
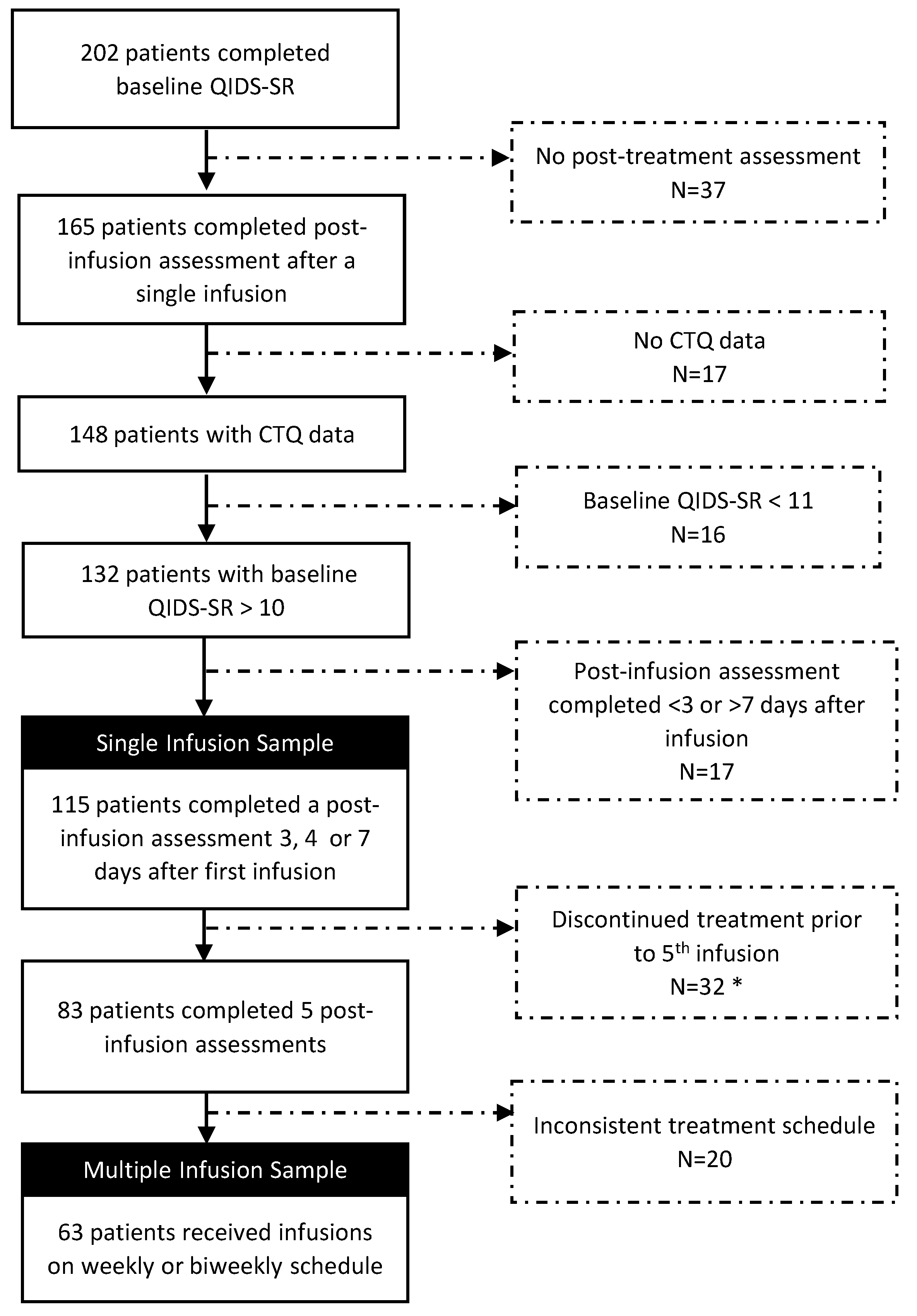
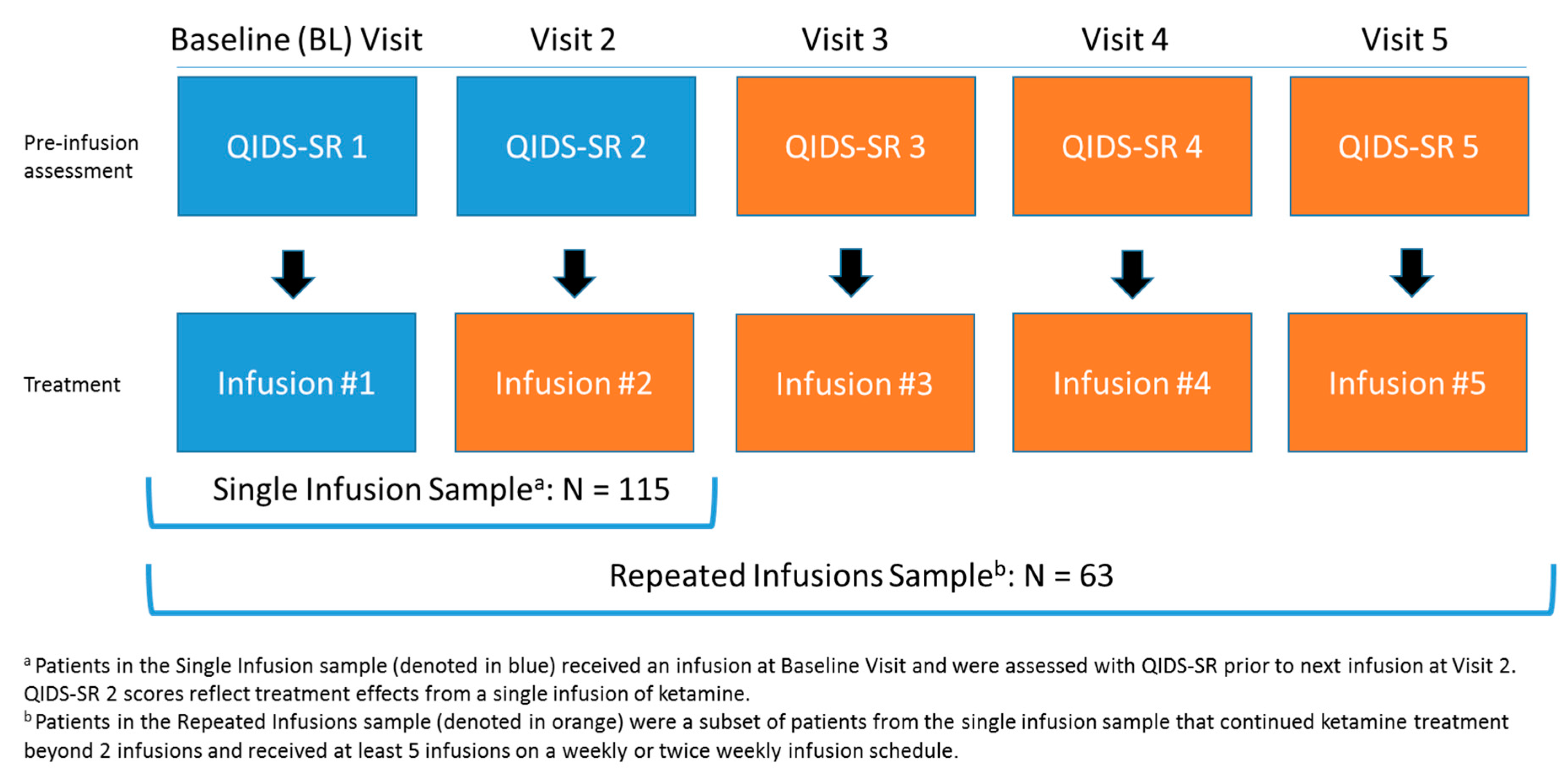
4.1. Study Samples
4.2. Administration of IV Ketamine
4.3. Data Set
4.4. Materials
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Single Infusion (N = 115) | Repeated Infusions (N = 63) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 43.78 | 14.45 | 19–76 | 43.25 | 13.67 | 19–70 |
| Weight (kg) | 78.18 | 18.98 | 47–127 | 76.87 | 18.58 | 47–127 |
| BMI | 26.69 | 5.56 | 16.3–43.1 | 26.55 | 5.41 | 16.3–43.1 |
| Dose (mg) 1 | 47.07 | 26.79 | 15–240 | 57.59 | 38.22 | 20–238 |
| Dose/kg (mg) 1 | 0.62 | 0.38 | 0.32–3.02 | 0.76 | 0.48 | 0.37–3.00 |
| QIDS-SR | ||||||
| Baseline | 18.63 | 3.70 | 11–26 | 19.19 | 3.71 | 11–26 |
| Total medications | 2.2 | 1.8 | 0–7 | 2.3 | 1.6 | 0–7 |
| Infusion costs ($) | 444.73 | 50.82 | 375–565 | 459.51 | 56.25 | 375–565 |
| N | % | N | % | |||
| Time of post-infusion assessment (TPIA) | Infusion Schedule | |||||
| Day 3 | 74 | 64.3 | Twice weekly | 41 | 65.1 | |
| Day 7 | 41 | 35.7 | Once weekly | 22 | 34.9 | |
| Gender (m/f) | 52/63 | 45.2/54.8 | 26/37 | 41.3/58.7 | ||
| Diagnosis | ||||||
| MDD | 88 | 76.5 | 49 | 77.8 | ||
| BD | 26 | 22.6 | 14 | 22.2 | ||
| AD | 54 | 47.0 | 29 | 46.0 | ||
| PTSD | 13 | 11.3 | 7 | 11.1 | ||
| Pain | 7 | 6.1 | 5 | 7.9 | ||
| Medication | ||||||
| Benzodiazepine * | 44 | 38.3 | 23 | 36.5 | ||
| SSRI | 38 | 33.0 | 22 | 34.9 | ||
| Anticonvulsant | 37 | 32.2 | 22 | 34.9 | ||
| SNRI | 27 | 23.5 | 14 | 22.2 | ||
| Antipsychotic | 26 | 22.6 | 15 | 23.8 | ||
| AAD | 25 | 21.7 | 13 | 20.6 | ||
| Psychostimulant | 20 | 17.4 | 11 | 17.5 | ||
| Hypnotic | 12 | 10.4 | 8 | 12.7 | ||
| Opioid | 9 | 7.8 | 8 | 12.7 | ||
| Lithium | 9 | 7.8 | 4 | 6.3 | ||
| TCA | 6 | 5.2 | 4 | 6.3 | ||
| Anxiolytic | 5 | 4.4 | 2 | 3.2 | ||
Appendix B
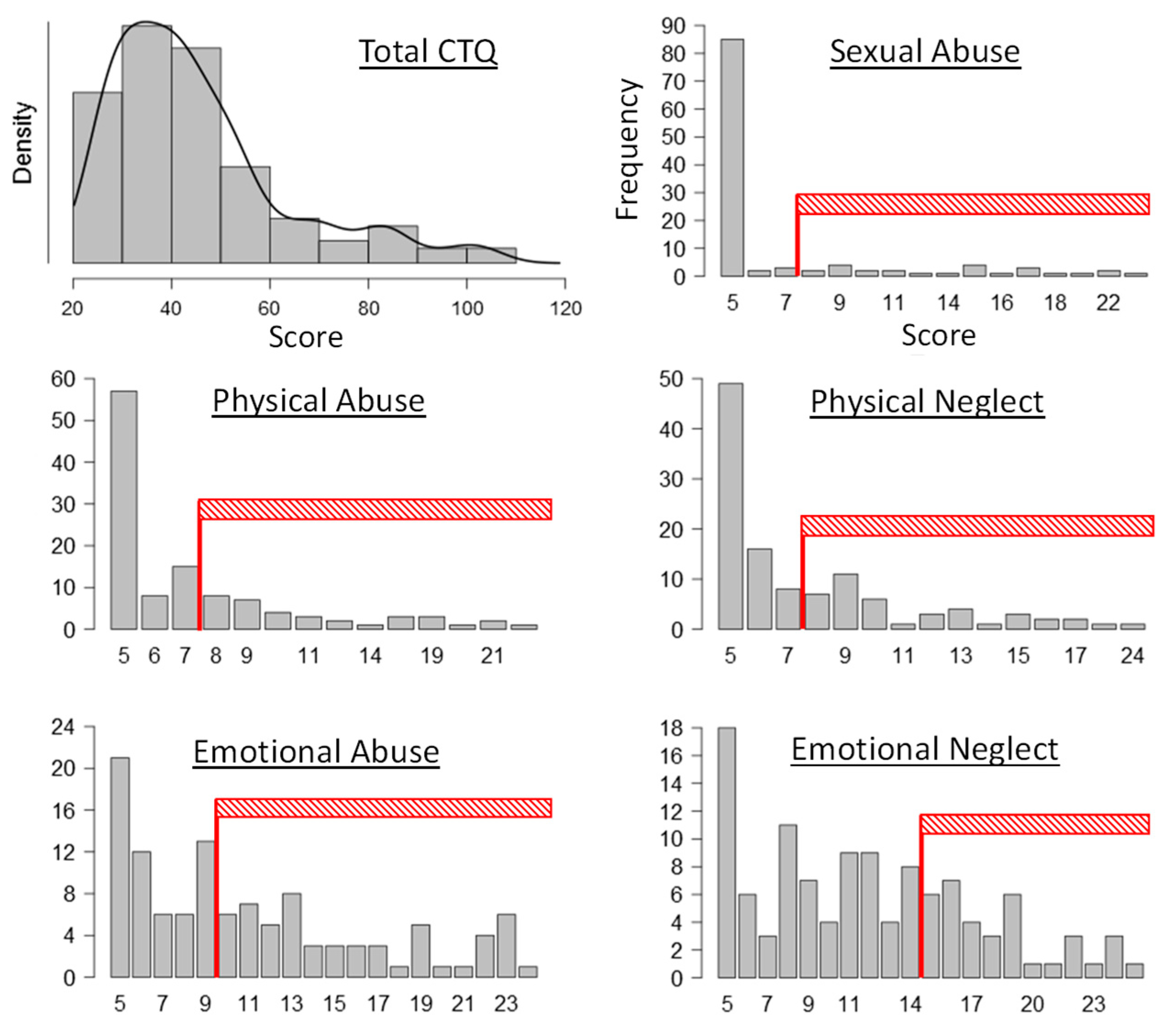
Appendix C
| CTQ Maltreatment Load | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | F/X2 | p | |
| n | 34 | 26 | 23 | 14 | 8 | 10 | - | - |
| Gender (m/f) | 18/16 | 13/13 | 11/12 | 6/8 | 4/4 | 0/10 | 9.48 | 0.09 |
| Age | 41.56 (15.75) | 40.77 (14.97) | 43.74 (13.20) | 51.64 (14.25) | 47.63 (13.93) | 45.20 (9.22) | 1.37 | 0.24 |
| Weight (kg) | 81.41 (17.95) | 81.15 (23.85) | 74.96 (18.94) | 76.21 (15.87) | 77.21 (13.59) | 70.41 (11.52) | 0.84 | 0.52 |
| Dose (mg) 1 | 51.47 (37.51) | 47.02 (15.17) | 47.39 (30.18) | 45.36 (22.14) | 39.38 (7.76) | 40 (11.30) | 4.33 | 0.50 |
| Dose (mg/kg) 1 | 0.64 (0.47) | 0.59 (0.13) | 0.69 (0.58) | 0.59 (0.21) | 0.51 (0.07) | 0.56 (0.09) | 3.50 | 0.62 |
| TPIA (3/7) | 24/10 | 19/7 | 15/8 | 9/5 | 4/4 | 3/7 | 7.31 | 0.20 |
| CTQ | ||||||||
| SA 1 | 5.03 (0.17) | 5.46 (1.17) | 6.30 (2.75) | 8.5 (4.80) | 8 (5.58) | 17.2 (4.80) | 51.24 | <0.001 |
| PA 1 | 5.38 (0.74) | 6.42 (2.52) | 7.22 (2.43) | 7.57 (3.74) | 11.63 (5.73) | 15.6 (6.15) | 44.04 | <0.001 |
| PN 1 | 5.21 (0.48) | 6.35 (1.67) | 7.44 (2.95) | 9.07 (2.73) | 10.88 (3.31) | 15.20 (4.32) | 63.51 | <0.001 |
| EA 1 | 6.38 (1.58) | 9.77 (4.81) | 11.30 (3.54) | 16.57 (5.85) | 16.25 (4.23) | 18.3 (4.62) | 64.23 | <0.001 |
| EN | 7.77 (2.78) | 9.42 (3.60) | 13 (3.94) | 15.79 (4.56) | 18.5 (3.38) | 19.7 (3.34) | 30.80 | <0.001 |
| Total | 29.77 (3.92) | 37.42 (5.61) | 45.26 (3.98) | 57.50 (9.15) | 65.25 (13.83) | 86 (13.65) | 120.09 | <0.001 |
| Diagnosis (y/n) | ||||||||
| MDD | 25/9 | 21/5 | 17/6 | 11/3 | 7/1 | 7/3 | 1.32 | 0.93 |
| BD | 9/25 | 5/21 | 5/18 | 3/11 | 1/7 | 3/7 | 1.26 | 0.94 |
| AD | 14/20 | 12/14 | 11/12 | 7/7 | 5/3 | 5/5 | 1.34 | 0.93 |
| PTSD | 2/32 | 3/23 | 2/21 | 3/11 | 0/8 | 7/3 | 7.09 | 0.21 |
| Pain | 2/32 | 1/25 | 3/20 | 0/14 | 0/8 | 1/9 | 3.87 | 0.57 |
| Medications (y/n) | ||||||||
| Benzodiazepine | 16/18 | 9/17 | 7/16 | 7/7 | 1/7 | 4/6 | 4.93 | 0.42 |
| SSRI | 10/24 | 10/16 | 9/14 | 7/7 | 1/7 | 1/9 | 6.68 | 0.25 |
| Anticonvulsant | 14/20 | 6/20 | 11/12 | 3/11 | 1/7 | 2/8 | 7.67 | 0.18 |
| SNRI | 14/20 | 5/21 | 2/21 | 3/11 | 1/7 | 2/8 | 9.62 | 0.09 |
| Antipsychotic | 8/26 | 8/18 | 5/18 | 2/12 | 0/8 | 3/7 | 4.22 | 0.52 |
| AAD | 7/27 | 7/19 | 6/17 | 2/12 | 1/7 | 2/8 | 1.57 | 0.91 |
| Psychostimulant | 10/24 | 3/23 | 4/19 | 0/14 | 1/7 | 2/8 | 7.17 | 0.21 |
| Hypnotic | 1/33 | 3/23 | 3/20 | 3/11 | 1/7 | 1/9 | 4.09 | 0.54 |
| Opioid | 2/32 | 2/24 | 1/22 | 1/13 | 2/6 | 1/9 | 3.91 | 0.56 |
| Lithium | 2/32 | 3/23 | 3/20 | 0/14 | 0/8 | 1/9 | 3.48 | 0.63 |
| TCA | 3/31 | 3/23 | 0/23 | 0/14 | 0/8 | 0/10 | 6.02 | 0.30 |
| Anxiolytic | 3/31 | 1/25 | 1/22 | 0/14 | 0/8 | 0/10 | 3.11 | 0.68 |
| Total medications | 2.65 (1.69) | 2.31 (1.49) | 2.26 (2.09) | 2 (1.71) | 1.13 (1.64) | 1.9 (1.97) | 1.14 | 0.34 |
Appendix D
| F(1,108) | p | µ2p | |
|---|---|---|---|
| Main Effects | |||
| Time | 7.74 | 0.006 | 0.067 |
| Gender | 4.10 | 0.045 | 0.037 |
| Age | 4.27 | 0.041 | 0.038 |
| TPIA | 2.94 | 0.09 | 0.027 |
| Dose | 0.36 | 0.55 | 0.003 |
| Load | 0.12 | 0.73 | 0.001 |
| Interaction effects | |||
| Time × load | 7.86 | 0.006 | 0.068 |
| Time × TPIA | 3.03 | 0.085 | 0.027 |
| Time × gender | 0.12 | 0.73 | 0.001 |
| Time × age | 0.04 | 0.85 | <0.001 |
| Gender × TPIA | 0.16 | 0.69 | 0.002 |
| Time × gender × TPIA | 0.35 | 0.56 | 0.003 |
Appendix E
Influence of Diagnosis and Psychopharmacological Treatment on Maltreatment Effects
| Single Infusion | Repeated Infusions | |||||
|---|---|---|---|---|---|---|
| N | F | p | N | F | p | |
| Diagnosis | ||||||
| MDD | 88 | 11.74 | <0.001 | 49 | 4.77 | 0.001 |
| BD | 26 | 11.73 | <0.001 | 14 | 2.00 | 0.095 |
| AD | 54 | 12.03 | <0.001 | 29 | 4.60 | <0.001 |
| PTSD | 13 | 12.96 | <0.001 | 7 | 4.64 | 0.001 |
| Pain | 7 | 11.83 | <0.001 | 5 | 4.63 | 0.001 |
| Medication | ||||||
| SSRI | 38 | 12.15 | <0.001 | 22 | 3.75 | 0.006 |
| SNRI | 27 | 10.30 | 0.002 | 14 | 3.69 | 0.006 |
| Antipsychotic | 26 | 12.84 | <0.001 | 15 | 1.51 | 0.199 |
| anticonvulsant | 37 | 12.42 | <0.001 | 22 | 3.57 | 0.008 |
| Psychostimulant | 20 | 12.69 | <0.001 | 11 | 2.94 | 0.021 |
| Benzodiazepine | 44 | 12.20 | <0.001 | 23 | 3.96 | 0.004 |
| Hypnotic | 12 | 10.96 | 0.001 | 8 | 2.32 | 0.057 |
| AAD | 25 | 11.84 | <0.001 | 13 | 1.89 | 0.114 |
| Number of concurrent medications | 3.62 | 0.005 | 1.68 | 0.039 | ||
Appendix F
Appendix G
| CTQ Maltreatment Load | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | F/X2 | p | |
| n | 17 | 16 | 11 | 8 | 6 | 5 | - | - |
| Gender (m/f) | 8/9 | 8/8 | 4/7 | 3/5 | 3/3 | 0/5 | 4.60 | 0.47 |
| Age | 39.94 (16.75) | 37.63 (11.26) | 44.46 (10.45) | 53.13 (11.09) | 49.50 (11.19) | 46.60 (14.52) | 2.07 | 0.08 |
| Weight (kg) | 80.02 (16.95) | 82.61 (22.68) | 69.85 (20.49) | 71.78 (13.24) | 72.12 (15.72) | 69.67 (10.56) | 1.07 | 0.39 |
| Dose (mg) 1 | 62.83 (43.63) | 53.27 (23.61) | 54.15 (52.30) | 40.61 (14.90) | 46.41 (16.34) | 45.82 (19.89) | 5.14 | 0.40 |
| Dose (mg/kg) 1 | 0.79 (0.53) | 0.65 (0.22) | 0.78 (0.69) | 0.58 (0.27) | 0.66 (0.26) | 0.64 (0.20) | 2.31 | 0.81 |
| Schedule (3/7) | 11/6 | 10/6 | 9/2 | 5/3 | 4/2 | 2/3 | 2.82 | 0.73 |
| CTQ | ||||||||
| SA 1 | 5.06 (0.24) | 5.63 (1.41) | 6.27 (2.61) | 8.88 (4.94) | 8.17 (6.40) | 18.6 (4.62) | 25.86 | <0.001 |
| PA 1 | 5.24 (0.56) | 6.50 (3.03) | 5.91 (1.81) | 6.38 (2.07) | 10.5 (5.47) | 18 (6.33) | 25.58 | <0.001 |
| PN 1 | 5.24 (0.56) | 6.32 (1.62) | 8.82 (3.57) | 9.5 (2) | 11.67 (3.39) | 14.6 (3.85) | 40.28 | <0.001 |
| EA 1 | 6 (1.5) | 9.88 (4.76) | 11.91 (3.75) | 16.13 (6.33) | 16.33 (4.63) | 18.4 (3.91) | 35.13 | <0.001 |
| EN | 6.82 (2.22) | 9.81 (4.02) | 13.82 (5.23) | 15.75 (5.85) | 18.83 (3.82) | 18.6 (3.51) | 14.06 | <0.001 |
| Total | 28.35 (3.26) | 38.13 (5.81) | 46.73 (4.45) | 56.63 (10.91) | 65. 5 (15.45) | 88.2 (7.53) | 66.99 | <0.001 |
| Diagnosis (y/n) | ||||||||
| MDD | 13/4 | 12/4 | 9/2 | 6/2 | 5/1 | 4/1 | 0.35 | 0.99 |
| BD | 43/13 | 4/12 | 2/9 | 2/6 | 1/5 | ¼ | 0.35 | 0.99 |
| AD | 7/10 | 7/9 | 6/5 | 2/6 | 5/1 | 2/3 | 5.37 | 0.37 |
| PTSD | 2/15 | 3/13 | 1/10 | 1/7 | 0/6 | 0/5 | 2.39 | 0.79 |
| Pain | 1/16 | 1/15 | 2/9 | 0/8 | 0/6 | 1/4 | 3.94 | 0.56 |
| Medications (y/n) | ||||||||
| Benzodiazepine | 7/10 | 6/10 | 3/8 | 4/4 | 1/5 | 2/3 | 2.25 | 0.81 |
| SSRI | 5/12 | 7/9 | 5/6 | 4/4 | 1/5 | 0/5 | 5.68 | 0.34 |
| Anticonvulsant | 7/10 | 5/11 | 6/5 | 2/6 | 1/5 | 1/4 | 3.97 | 0.55 |
| SNRI | 7/10 | 3/13 | 0/11 | 2/6 | 1/5 | 1/4 | 6.95 | 0.23 |
| Antipsychotic | 4/13 | 7/9 | 2/9 | 1/7 | 0/6 | 1/4 | 6.18 | 0.29 |
| AAD | 3/14 | 4/12 | 3/8 | 2/6 | 1/5 | 0/5 | 2.03 | 0.85 |
| Psychostimulant | 6/11 | 1/15 | 2/9 | 0/8 | 1/5 | 1/4 | 6.87 | 0.23 |
| Hypnotic | 0/17 | 2/14 | 2/9 | 3/5 | 1/5 | 0/5 | 8.02 | 0.16 |
| Opioid | 2/15 | 2/14 | 1/10 | 1/7 | 2/4 | 0/5 | 3.18 | 0.67 |
| Lithium | 1/16 | 1/15 | 1/10 | 0/8 | 0/6 | 1/4 | 2.66 | 0.75 |
| TCA | 1/16 | 3/13 | 0/11 | 0/8 | 0/6 | 0/5 | 6.18 | 0.29 |
| Anxiolytic | 1/16 | 1/15 | 0/11 | 0/8 | 0/6 | 0/5 | 1.88 | 0.87 |
| Total medications | 2.59 (1.54) | 2.63 (1.46) | 2.27 (1.95) | 2.38 (1.51) | 1.50 (1.76) | 1.40 (2.07) | 0.80 | 0.55 |
Appendix H
References
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68 (Suppl. 8), 17–25. [Google Scholar]
- Johnston, K.M.; Powell, L.C.; Anderson, I.M.; Szabo, S.; Cline, S. The burden of treatment-resistant depression: A systematic review of the economic and quality of life literature. J. Affect. Disord. 2019, 242, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Reutfors, J.; Andersson, T.M.-L.; Brenner, P.; Brandt, L.; DiBernardo, A.; Li, G.; Hägg, D.; Wingård, L.; Bodén, R. Mortality in treatment-resistant unipolar depression: A register-based cohort study in Sweden. J. Affect. Disord. 2018, 238, 674–679. [Google Scholar] [CrossRef]
- Krystal, J.H.; Abdallah, C.G.; Sanacora, G.; Charney, D.S.; Duman, R.S. Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 2019, 101, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 2010, 67, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.B.; Fedgchin, M.; Daly, E.J.; De Boer, P.; Cooper, K.; Lim, P.; Pinter, C.; Murrough, J.W.; Sanacora, G.; Shelton, R.C.; et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am. J. Psychiatry 2016, 173, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; De Feyter, H.M.; Averill, L.A.; Jiang, L.; Averill, C.L.; Chowdhury, G.M.I.; Purohit, P.; de Graaf, R.A.; Esterlis, I.; Juchem, C.; et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 2018, 43, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, N.; Yang, C.; Li, X.-M.; Zhou, Z.-Q.; Yang, J.-J. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry 2014, 29, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.N.; Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Foulkes, A.; Iqbal, S.; Mahoney, J.J.; De La Garza, R.; Charney, D.S.; et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol. 2014, 17, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Abelaira, H.M.; Réus, G.Z.; Neotti, M.V.; Quevedo, J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014, 101, 10–14. [Google Scholar] [CrossRef] [PubMed]
- McGirr, A.; Berlim, M.T.; Bond, D.J.; Fleck, M.P.; Yatham, L.N.; Lam, R.W. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol. Med. 2015, 45, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Romeo, B.; Choucha, W.; Fossati, P.; Rotge, J.-Y. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015, 230, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Chawla, J.M.; Hagi, K.; Zarate, C.A.; Kane, J.M.; Bauer, M.; Correll, C.U. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: A meta-analysis of efficacy, safety and time trajectories. Psychol. Med. 2016, 46, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Uher, R.; Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012, 169, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Klumparendt, A.; Doebler, P.; Ehring, T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br. J. Psychiatry 2017, 210, 96–104. [Google Scholar] [CrossRef]
- Wildeman, C.; Emanuel, N.; Leventhal, J.M.; Putnam-Hornstein, E.; Waldfogel, J.; Lee, H. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014, 168, 706–713. [Google Scholar] [CrossRef]
- Feder, A.; Parides, M.K.; Murrough, J.W.; Perez, A.M.; Morgan, J.E.; Saxena, S.; Kirkwood, K.; Aan Het Rot, M.; Lapidus, K.A.B.; Wan, L.-B.; et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry 2014, 71, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Albott, C.S.; Lim, K.O.; Forbes, M.K.; Erbes, C.; Tye, S.J.; Grabowski, J.G.; Thuras, P.; Batres-Y-Carr, T.M.; Wels, J.; Shiroma, P.R. Efficacy, safety, and durability of repeated ketamine infusions for comorbid posttraumatic stress disorder and treatment-resistant depression. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Rush, A.J.; Ibrahim, H.M.; Carmody, T.J.; Biggs, M.M.; Suppes, T.; Crismon, M.L.; Shores-Wilson, K.; Toprac, M.G.; Dennehy, E.B.; et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol. Med. 2004, 34, 73–82. [Google Scholar] [PubMed]
- Walker, E.A.; Unutzer, J.; Rutter, C.; Gelfand, A.; Saunders, K.; VonKorff, M.; Koss, M.P.; Katon, W. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch. Gen. Psychiatry 1999, 56, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Williams, D.R. Bayesian alternatives for common null-hypothesis significance tests in psychiatry: A non-technical guide using JASP. BMC Psychiatry 2018, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Papakostas, G.I.; Hoeppner, B.; Mazzone, E.; Judge, H.; Cusin, C.; Mathew, S.; Sanacora, G.; Iosifescu, D.; DeBattista, C.; et al. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J. Psychiatr. Res. 2019, 110, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Lijffijt, M.; O’Brien, B.; Salas, R.; Mathew, S.J.; Swann, A.C. Interactions of immediate and long-term action regulation in the course and complications of bipolar disorder. Philos. Trans. R. Soc. B Biol. Sci. 2018, 374, 20180132. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Koenen, K.C.; Bromet, E.J.; Karam, E.G.; Liu, H.; Petukhova, M.; Ruscio, A.M.; Sampson, N.A.; Stein, D.J.; Aguilar-Gaxiola, S.; et al. Childhood adversities and post-traumatic stress disorder: Evidence for stress sensitisation in the World Mental Health Surveys. Br. J. Psychiatry 2017, 211, 280–288. [Google Scholar] [CrossRef]
- Swann, A.C. Beyond dugs: Addictions in the context of recurrent/progressive psychiatric illness. In Neurobiology of Addiction; Oxford University Press: New York, NY, USA, 2016; ISBN 978-0-19-936789-4. [Google Scholar]
- Garcia, L.S.B.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Stertz, L.; Kapczinski, F.; Gavioli, E.C.; Quevedo, J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 450–455. [Google Scholar] [CrossRef]
- Sripada, S.; Gaytan, O.; Swann, A.; Dafny, N. The role of MK-801 in sensitization to stimulants. Brain Res. Brain Res. Rev. 2001, 35, 97–114. [Google Scholar] [CrossRef]
- Chen, J.C.; Liang, K.W.; Huang, Y.K.; Liang, C.S.; Chiang, Y.C. Significance of glutamate and dopamine neurons in the ventral pallidum in the expression of behavioral sensitization to amphetamine. Life Sci. 2001, 68, 973–983. [Google Scholar] [CrossRef]
- Kalivas, P.W. Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug Alcohol. Depend. 1995, 37, 95–100. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Alesdatter, J.E. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1993, 267, 486–495. [Google Scholar] [PubMed]
- Wolf, M.E. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998, 54, 679–720. [Google Scholar] [CrossRef]
- Wolf, M.E.; White, F.J.; Hu, X.T. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J. Neurosci. 1994, 14, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.J.; Covington, H.E.; Gale, M.C.; Datta, R.; Miczek, K.A. Behavioral sensitization due to social defeat stress in mice: Antagonism at mGluR5 and NMDA receptors. Psychopharmacology 2005, 179, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Battista, M.A.; Hierholzer, R.; Khouzam, H.R.; Barlow, A.; O’Toole, S. Pilot trial of memantine in the treatment of posttraumatic stress disorder. Psychiatry 2007, 70, 167–174. [Google Scholar] [CrossRef]
- Baldwin, J.R.; Reuben, A.; Newbury, J.B.; Danese, A. Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry 2019, 76, 584–593. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Fink, L. Childhood Trauma Questionnaire: A Retrospective Self-Report; Manual; Harcourt & Company: San Antonio, TX, USA, 1998. [Google Scholar]
- Bernstein, D.P.; Fink, L.; Handelsman, L.; Foote, J.; Lovejoy, M.; Wenzel, K.; Sapareto, E.; Ruggiero, J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 1994, 151, 1132–1136. [Google Scholar]
- Mathew, S.J.; Wilkinson, S.T.; Altinay, M.; Asghar-Ali, A.; Chang, L.C.; Collins, K.A.; Dale, R.M.; Hu, B.; Krishnan, K.; Kellner, C.H.; et al. ELEctroconvulsive therapy (ECT) vs. Ketamine in patients with Treatment-resistant Depression: The ELEKT-D study protocol. Contemp. Clin. Trials 2019, 77, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; Monden, R.; van Ravenzwaaij, D.; Wagenmakers, E.-J. Bayesian reanalysis of null results reported in medicine: Strong yet variable evidence for the absence of treatment effects. PLoS ONE 2018, 13, e0195474. [Google Scholar] [CrossRef] [PubMed]
- Marsman, M.; Wagenmakers, E.-J. Bayesian benefits with JASP. Eur. J. Dev. Psychol. 2017, 14, 545–555. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Love, J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Selker, R.; Gronau, Q.F.; Dropmann, D.; Boutin, B.; et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychon. Bull. Rev. 2018, 25, 58–76. [Google Scholar] [CrossRef] [PubMed]
| CTQ Scores | Clinically Significant | Maltreatment Load | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | % | N | % | ||
| Total | 45.33 | 18.11 | - | - | 0 | 34 | 29.6 |
| SA 1 | 7.07 | 4.40 | 24 | 20.9 | 1 | 26 | 22.6 |
| PA 1 | 7.57 | 4.22 | 34 | 29.6 | 2 | 23 | 20.0 |
| PN 1 | 7.64 | 3.68 | 44 | 38.3 | 3 | 14 | 12.2 |
| EA | 11.10 | 5.66 | 56 | 48.7 | 4 | 8 | 7.0 |
| EN | 11.95 | 5.39 | 38 | 33.0 | 5 | 10 | 8.7 |
| CTQ Scores | Clinically Significant | Maltreatment Load | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | % | N | % | ||
| Total | 45.92 | 18.63 | - | - | 0 | 17 | 27.0 |
| SA 1 | 7.27 | 4.69 | 15 | 23.8 | 1 | 16 | 25.4 |
| PA 1 | 7.33 | 4.51 | 14 | 22.2 | 2 | 11 | 17.5 |
| PN 1 | 8.03 | 3.63 | 29 | 46.0 | 3 | 8 | 12.7 |
| EA | 11.27 | 5.74 | 32 | 50.8 | 4 | 6 | 9.5 |
| EN | 12.02 | 5.87 | 21 | 33.3 | 5 | 5 | 7.9 |
| Infusion | Visit | QIDS-SR | t | p | d | BF10 | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Comparison | Change | ||||||
| Baseline (BL) | 1 | 19.19 | 3.71 | - | - | - | - | - | - |
| Infusion 1 (I-1) | 2 | 13.92 | 4.94 | BL vs. I-1 | 5.27 | 9.12 | <0.001 | 1.15 | 1.73 × 10+10 |
| Infusion 2 (I-2) | 3 | 11.16 | 5.32 | I-1 vs. I-2 | 2.76 | 6.50 | <0.001 | 0.82 | 7.9 × 10+5 |
| Infusion 3 (I-3) | 4 | 10.16 | 5.81 | I-2 vs. I-3 | 1.00 | 2.67 | 0.097 | 0.34 | 3.537 |
| Infusion 4 (I-4) | 5 | 9.91 | 5.52 | I-3 vs. I-4 | 0.25 | 0.53 | 0.999 | 0.07 | 0.158 |
| Response Rate | Remission Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| X2 | df | p | BF10 | X2 | df | p | BF10 | |
| Load | 8.95 | 5 | 0.111 | 1.19 | 20.43 | 5 | 0.001 | 41.83 |
| Any | 0.01 | 1 | 0.921 | 0.34 | 0.49 | 1 | 0.485 | 0.35 |
| SA | 3.37 | 1 | 0.066 | 1.18 | 0.98 | 1 | 0.321 | 0.51 |
| PA | 2.41 | 1 | 0.120 | 1.15 | 6.81 | 1 | 0.009 | 6.73 |
| PN | 3.43 | 1 | 0.064 | 1.63 | 9.14 | 1 | 0.002 | 23.97 |
| EA | 0.41 | 1 | 0.521 | 0.37 | 1.99 | 1 | 0.159 | 0.68 |
| EN | 0.51 | 1 | 0.475 | 0.41 | 6.30 | 1 | 0.012 | 5.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, B.; Lijffijt, M.; Wells, A.; Swann, A.C.; Mathew, S.J. The Impact of Childhood Maltreatment on Intravenous Ketamine Outcomes for Adult Patients with Treatment-Resistant Depression. Pharmaceuticals 2019, 12, 133. https://doi.org/10.3390/ph12030133
O’Brien B, Lijffijt M, Wells A, Swann AC, Mathew SJ. The Impact of Childhood Maltreatment on Intravenous Ketamine Outcomes for Adult Patients with Treatment-Resistant Depression. Pharmaceuticals. 2019; 12(3):133. https://doi.org/10.3390/ph12030133
Chicago/Turabian StyleO’Brien, Brittany, Marijn Lijffijt, Allison Wells, Alan C. Swann, and Sanjay J. Mathew. 2019. "The Impact of Childhood Maltreatment on Intravenous Ketamine Outcomes for Adult Patients with Treatment-Resistant Depression" Pharmaceuticals 12, no. 3: 133. https://doi.org/10.3390/ph12030133
APA StyleO’Brien, B., Lijffijt, M., Wells, A., Swann, A. C., & Mathew, S. J. (2019). The Impact of Childhood Maltreatment on Intravenous Ketamine Outcomes for Adult Patients with Treatment-Resistant Depression. Pharmaceuticals, 12(3), 133. https://doi.org/10.3390/ph12030133





