Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Electrophilic Radioiodination of β-CIT and SPE Purification
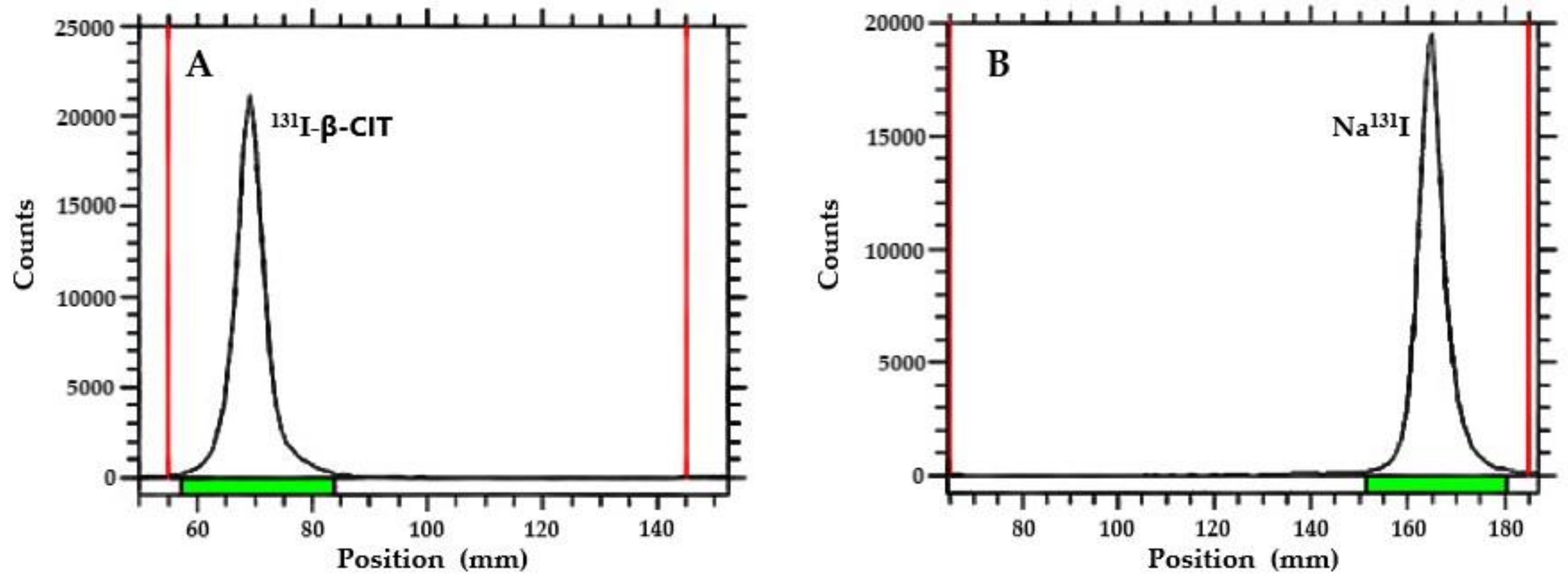
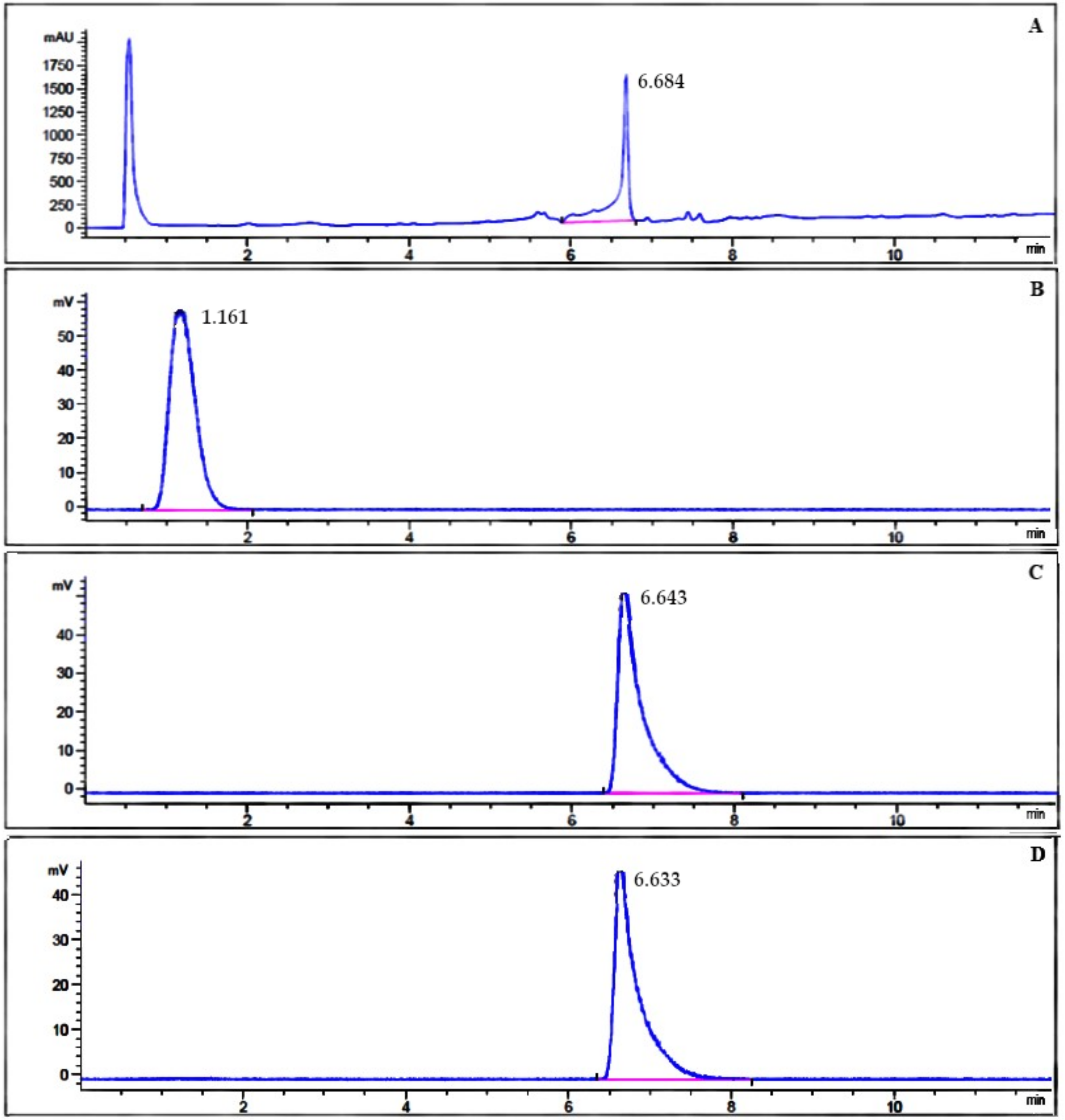
3.2. Radiochemical Purity Analysis
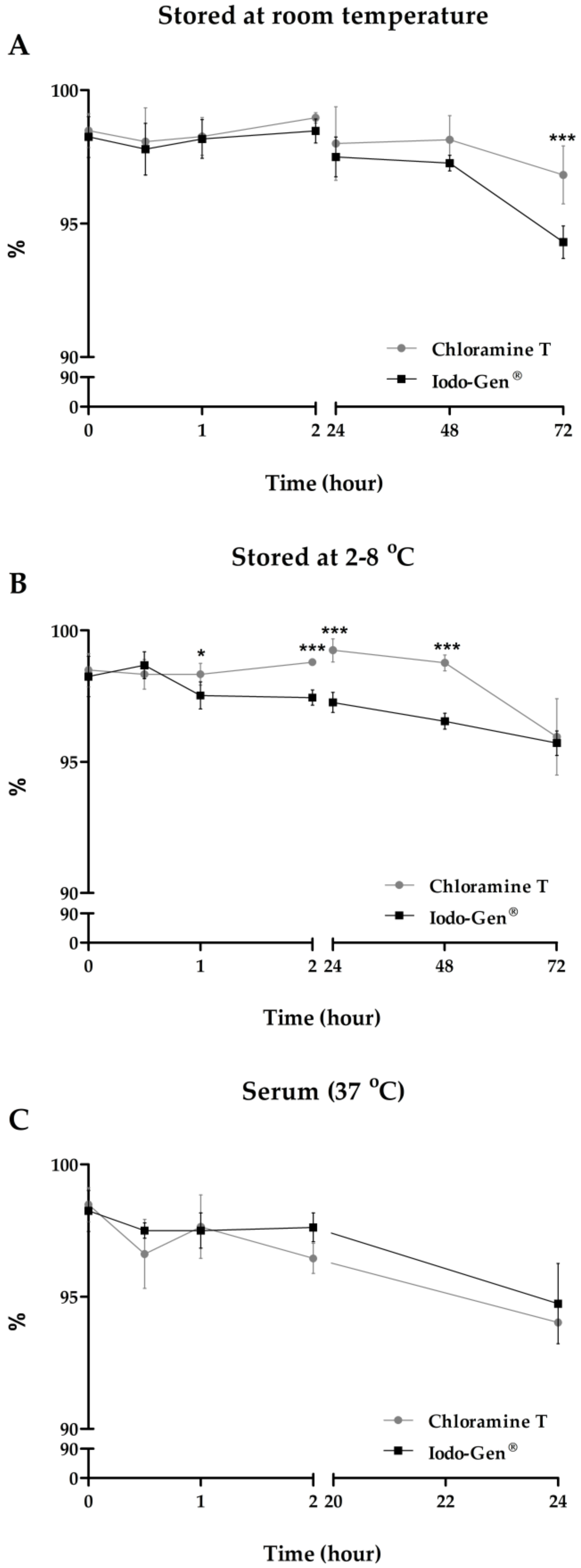
3.3. Stability Studies
3.4. Partition Coefficient
3.5. Serum Protein Binding
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Park, E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J. Nucl. Med. Technol. 2012, 40, 222–228. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23160562 (accessed on 28 March 2017). [CrossRef] [PubMed]
- Ba, F.; Martin, W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat. Disord. 2015, 21, 87–94. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25487733 (accessed on 14 April 2017). [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25904081 (accessed on 22 February 2018). [CrossRef]
- Williams-Gray, C.H.; Worth, P.F. Parkinson’s disease. Medicine 2016, 44, 542–546. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative stress in Parkinson’s disease: A systematic review and meta-analysis. Front. Mol. Neurosci. 2018, 11, 1–7. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30026688 (accessed on 10 December 2018). [CrossRef] [PubMed]
- Varrone, A.; Halldin, C. Molecular imaging of the dopamine transporter. J. Nucl. Med. 2010, 51, 1331–1334. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20720060 (accessed on 5 March 2017). [CrossRef] [PubMed]
- Cummings, J.L.; Henchcliffe, C.; Schaier, S.; Simuni, T.; Waxman, A.; Kemp, P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 2011, 134, 3146–3166. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Liao, M.; Tseng, Y. Recent advances in imaging of dopaminergic neurons for evaluation of neuropsychiatric disorders. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Li, H.; Zhang, H. SPECT molecular imaging in Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The role of DAT-SPECT in movement disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20019219 (accessed on 9 March 2017). [CrossRef]
- Bois, F.; Baldwin, R.M.; Kula, N.S.; Baldessarini, R.J.; Innis, R.B.; Tamagnan, G. Synthesis and monoamine transporter affinity of 3’-analogs of 2β-carbomethoxy-3-β-(4’-iodophenyl)tropane (β-CIT). Bioorg. Med. Chem. Lett. 2004, 14, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Eerola, J.; Tienari, P.J.; Kaakkola, S.; Nikkinen, P.; Launes, J. How useful is [123I]β-CIT SPECT in clinical practice? J. Neurol. Neurosurg. Psychiatry 2005, 76, 1211–1216. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1739796/ (accessed on 13 December 2016). [CrossRef]
- Markwell, M.A.K. A new solid-state reagent to iodinate proteins: I. Conditions for the efficient labeling of antiserum. Anal. Biochem. 1982, 125, 427–432. [Google Scholar] [CrossRef]
- Carpinelli, A.; Matarrese, M.; Moresco, R.M.; Simonelli, P.; Todde, S.; Magni, F.; Galli Kienle, M.; Fazio, F. Radiosynthesis of [123I]βCIT, a selective ligand for the study of the dopaminergic and serotoninergic systems in human brain. Appl. Radiat. Isotopes 2001, 54, 93–95. [Google Scholar] [CrossRef]
- Sihver, W.; Drewes, B.; Schulze, A.; Olsson, R.A.; Coenen, H.H. Evaluation of novel tropane analogues in comparison with the binding characteristics of [18F]FP-CIT and [131I]β-CIT. Nucl. Med. Biol. 2007, 34, 211–219. [Google Scholar] [CrossRef]
- Blois, E.; Chan, H.S.; Breeman, W.A.P. Iodination and stability of somatostatin analogues: Comparison of iodination techniques. A practical overview. Curr. Top. Med. Chem. 2012, 12, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.M.; Greenwood, F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962, 194, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Tashtoush, B.M.; Traboulsi, A.A.; Dittert, L.; Hussain, A.A. Chloramine-T in radiolabeling techniques. IV. Penta-O-acetyl-N-chloro-N-methylglucamine as an oxidizing agent in radiolabeling techniques. Anal. Biochem. 2001, 288, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; Speck, J.C., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Amin, A.M.; Gouda, A.A.; El-Sheikh, R.; Seddik, U.; Hussien, H. Radioiodination, purification and bioevaluation of Piroxicam in comparison with Meloxicam for imaging of inflammation. J. Radioanal. Nucl. Chem. 2009, 280, 589–598. [Google Scholar] [CrossRef]
- Lexa, K.W.; Dolghih, E.; Jacobson, M.P. A structure-based model for predicting serum albumin binding. PLoS ONE 2014, 9, 1–12. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24691448 (accessed on 20 June 2018). [CrossRef]
- Tavares, A.A.; Lewsey, J.; Dewar, D.; Pimlott, S.L. Radiotracer properties determined by high performance liquid chromatography: A potential tool for brain radiotracer discovery. Nucl. Med. Biol. 2012, 39, 127–135. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21958855 (accessed on 29 July 2018). [CrossRef]
- Katsifis, A.; Papazian, V.; Jackson, T.; Loc’h, C. A rapid and efficient preparation of [123I]radiopharmaceuticals using a small HPLC (Rocket) column. Appl. Radiat. Isotopes 2006, 64, 27–31. [Google Scholar] [CrossRef]
- Saji, H.; Iida, Y.; Kawashima, H.; Ogawa, M.; Kitamura, Y.; Mukai, T.; Shimazu, S.; Yoneda, F. In vivo imaging of brain dopaminergic neurotransmission system in small animals with high-resolution single photon emission computed tomography. Anal. Sci. 2003, 19, 67–71. [Google Scholar] [CrossRef]
- Fuchigami, T.; Mizoguchi, T.; Ishikawa, N.; Haratake, M.; Yoshida, S.; Magata, Y.; Nakayama, M. Synthesis and evaluation of a radioiodinated 4,6-diaryl-3-cyano-2-pyridinone derivative as a survivin targeting SPECT probe for tumor imaging. Bioorg. Med. Chem. Lett. 2016, 26, 999–1004. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26733475 (accessed on 21 January 2018). [CrossRef]
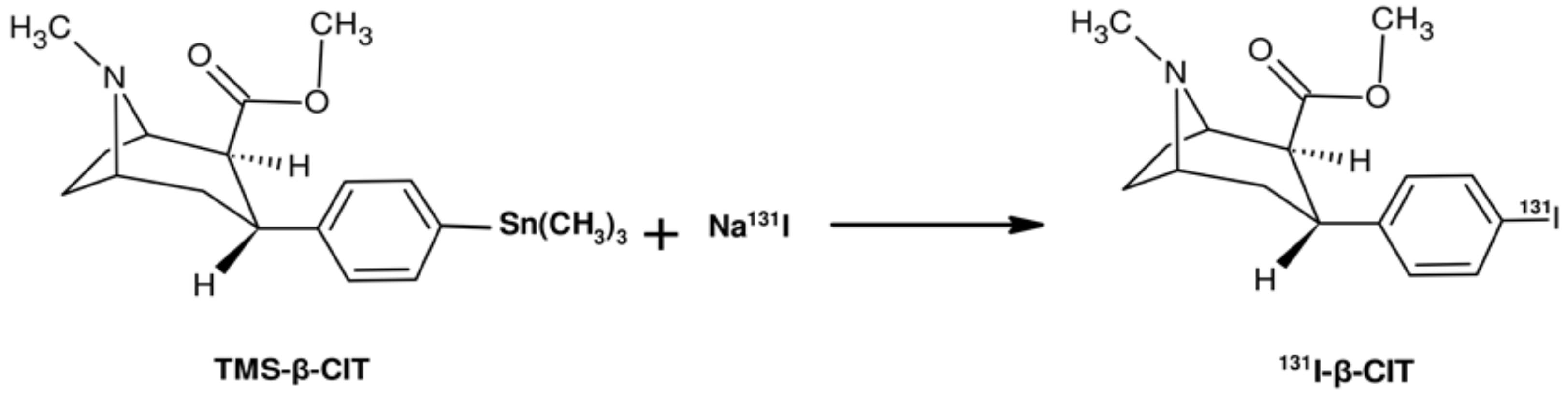
| Oxidizing Agents | Radiolabeling Yield | Radiochemical Purity |
|---|---|---|
| Chloramine T | 97.40 ± 1.17 | 98.48 ± 0.63 |
| Iodo-Gen® | 97.81 ± 0.99 | 98.24 ± 0.76 |
| Partition coefficient(P) | Chloramine T | 0.12 ± 0.02 |
| Iodo-Gen® | 0.13 ± 0.02 | |
| Serum protein binding (SPB) | Chloramine T | 47.44 ± 1.31% |
| Iodo-Gen® | 44.99 ± 3.06% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. Durante, A.C.; V. Sobral, D.; C. Miranda, A.C.; V. de Almeida, É.; L. Fuscaldi, L.; R. F. F. de Barboza, M.; Malavolta, L. Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease. Pharmaceuticals 2019, 12, 25. https://doi.org/10.3390/ph12010025
R. Durante AC, V. Sobral D, C. Miranda AC, V. de Almeida É, L. Fuscaldi L, R. F. F. de Barboza M, Malavolta L. Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease. Pharmaceuticals. 2019; 12(1):25. https://doi.org/10.3390/ph12010025
Chicago/Turabian StyleR. Durante, Ana Claudia, Danielle V. Sobral, Ana Claudia C. Miranda, Érika V. de Almeida, Leonardo L. Fuscaldi, Marycel R. F. F. de Barboza, and Luciana Malavolta. 2019. "Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease" Pharmaceuticals 12, no. 1: 25. https://doi.org/10.3390/ph12010025
APA StyleR. Durante, A. C., V. Sobral, D., C. Miranda, A. C., V. de Almeida, É., L. Fuscaldi, L., R. F. F. de Barboza, M., & Malavolta, L. (2019). Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease. Pharmaceuticals, 12(1), 25. https://doi.org/10.3390/ph12010025








