Camphor, Applied Epidermally to the Back, Causes Snout- and Chest-Grooming in Rats: A Response Mediated by Cutaneous TRP Channels
Abstract
1. Introduction
2. Results
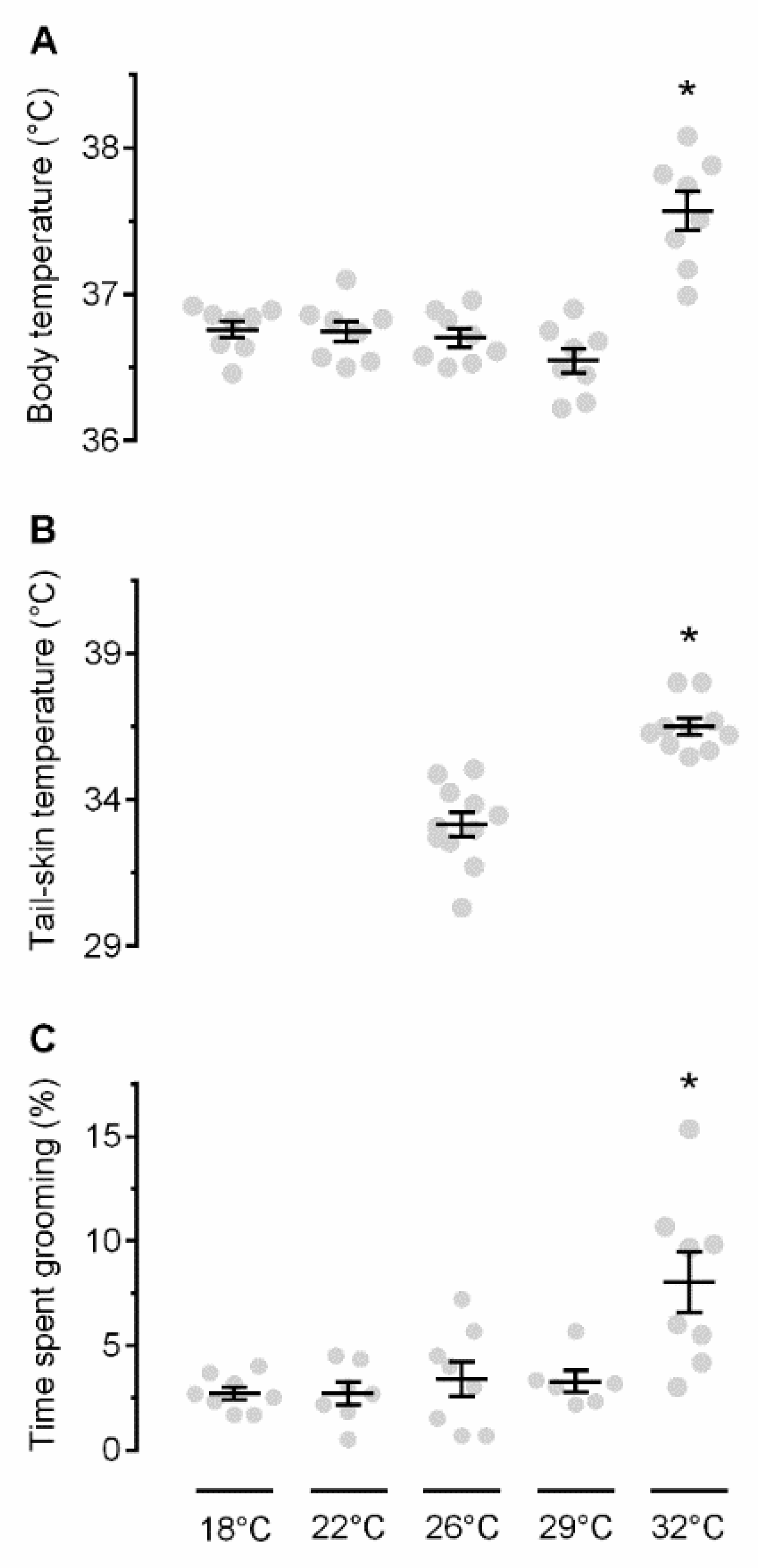
2.1. Exposure to Warmth Causes Thermoregulatory Self-Grooming
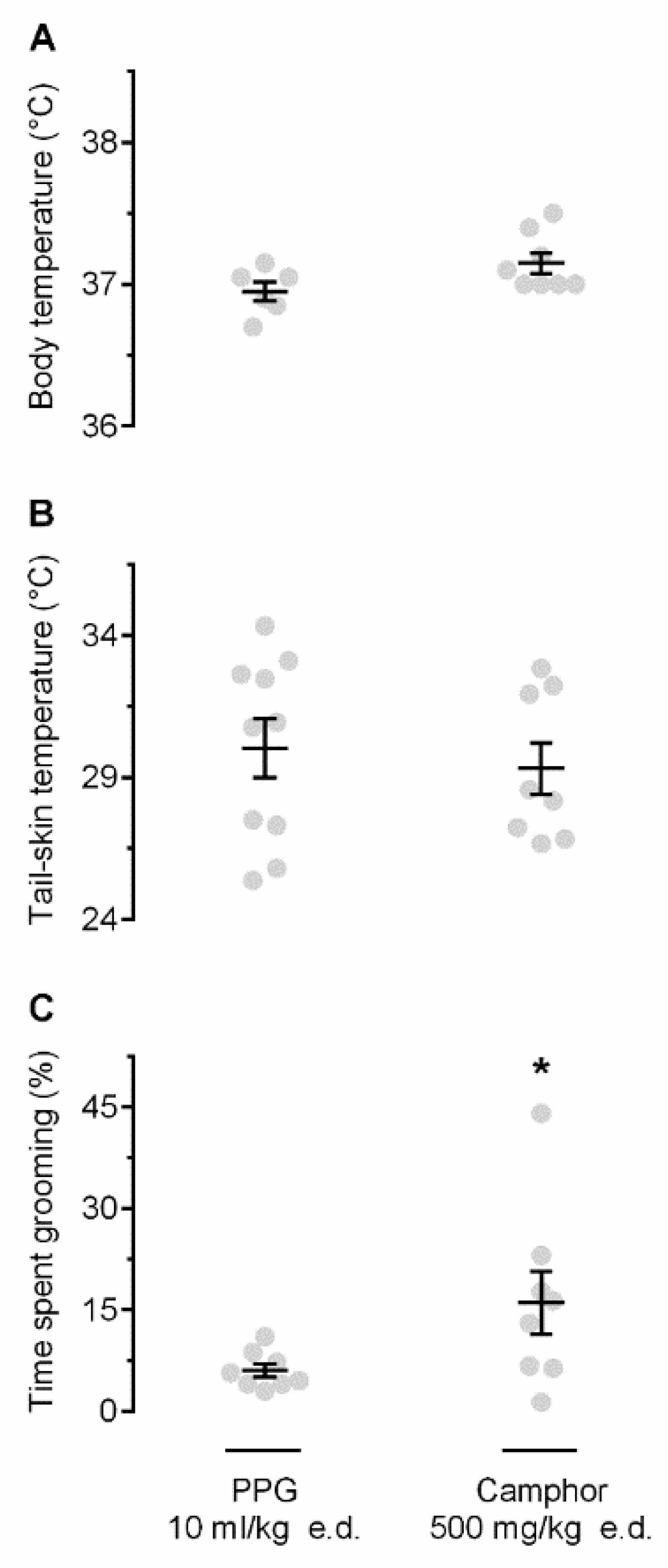
2.2. Camphor Induces Self-Grooming
2.3. Thermoregulatory Self-Grooming Is Mediated by TRP Channels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.2.1. General
4.2.2. Temperature-Measuring Device Implantation
4.2.3. Jugular Catheterization
4.3. Drugs
4.4 Experimental Setups
4.4.1. Experimental “Arena” for Behavioral Studies
4.4.2. Elevated Plus-Maze Apparatus
4.5. Parameters Measured
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| e.d. | epidermal(ly) |
| i.v. | intravenous(ly) |
| Ta | ambient temperature |
| Tb | body temperature |
| Tsk | skin temperature |
| TRP | transient receptor potential (channel) |
| PPG | polypropylene glycol |
| V | vanilloid (as in TRPV3) |
References
- Bícego, K.C.; Barros, R.C.; Branco, L.G. Physiology of temperature regulation: Comparative aspects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 616–639. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. The thermoregulation system and how it works. Handb. Clin. Neurol. 2018, 156, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.W.; Mooney, R.D. Brain areas controlling thermoregulatory grooming, prone extension, locomotion, and tail vasodilation in rats. J. Comp. Physiol. Psychol. 1974, 86, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Vizin, R.C.L.; Carrettiero, D.C. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature 2015, 2, 483–490. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of body temperature by the nervous system. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Yanase, M.; Kanosue, K.; Yasuda, H.; Tanaka, H. Salivary secretion and grooming behaviour during heat exposure in freely moving rats. J. Physiol. 1991, 432, 585–592. [Google Scholar] [CrossRef]
- Bolles, R.C. Grooming behavior in the rat. J. Comp. Physiol. Psychol. 1960, 53, 306–310. [Google Scholar] [CrossRef]
- Hainsworth, F.R.; Stricker, E.M. Evaporative cooling in the rat: Effects of partial desalivation. Am. J. Physiol. 1969, 217, 494–497. [Google Scholar] [CrossRef]
- Roberts, W.W. Differential thermosensor control of thermoregulatory grooming, locomotion, and relaxed postural extension. Ann. N. Y. Acad. Sci. 1988, 525, 363–374. [Google Scholar] [CrossRef]
- Roberts, W.W.; Martin, J.R. Peripheral thermoreceptor control of thermoregulatory responses of the rat. J. Comp. Physiol. Psychol. 1974, 87, 1109–1118. [Google Scholar] [CrossRef]
- Roberts, W.W.; Frol, A.B. Interaction of central and superficial peripheral thermosensors in control of thermoregulatory behaviors of the rat. Physiol. Behav. 1979, 23, 503–512. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Broad, L.M.; Mogg, A.J.; Eberle, E.; Tolley, M.; Li, D.L.; Knopp, K.L. TRPV3 in Drug Development. Pharmaceuticals 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Caterina, M.J. Molecular basis of peripheral innocuous warmth sensitivity. Handb. Clin. Neurol. 2018, 156, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Hew-Butler, T.; Soriano, R.N.; Rao, S.; Wang, W.; Wang, J.; Tamayo, N.; Oliveira, D.L.; Nucci, T.B.; Aryal, P.; et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J. Neurosci. 2012, 32, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Protoc. 2004, 13, 151–158. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: Potential utility for neurobehavioural stress research. J. Neurosci. Methods 2005, 143, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Aldridge, J.W.; LaPorte, J.L.; Murphy, D.L.; Tuohimaa, P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007, 2, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar] [CrossRef] [PubMed]
- Vizin, R.C.L.; Ishikawa, D.T.; de Souza, C.O.; Carrettiero, D.C.; Romanovsky, A.A.; Almeida, M.C. TRPV4 involvement in thermoregulatory grooming response. Unpublished Observation.
- Vincent, F.; Acevedo, A.; Nguyen, M.T.; Dourado, M.; DeFalco, J.; Gustafson, A.; Spiro, P.; Emerling, D.E.; Kelly, M.G.; Duncton, M.A. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009, 3, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Vennekens, R. Herbal compounds and toxins modulating TRP channels. Curr. Neuropharmacol. 2008, 6, 79–96. [Google Scholar] [CrossRef]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Nilius, B.; Biro, T.; Owsianik, G. TRPV3: Time to decipher a poorly understood family member! J. Physiol. 2014, 592, 295–304. [Google Scholar] [CrossRef]
- Green, B.G. Sensory characteristics of camphor. J. Investig. Dermatol. 1990, 94, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Fromy, B.; Josset-Lamaugarny, A.; Aimond, G.; Pagnon-Minot, A.; Marics, I.; Tattersall, G.J.; Moqrich, A.; Sigaudo-Roussel, D. Disruption of TRPV3 impairs heat-evoked vasodilation and thermoregulation: A critical role of CGRP. J. Investig. Dermatol. 2018, 138, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Marics, I.; Malapert, P.; Reynders, A.; Gaillard, S.; Moqrich, A. Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels. PLoS ONE 2014, 9, e99828. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Li, X.; Yu, Y.; Wang, J.; Caterina, M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Vizin, R.C.; Scarpellini, C.D.; Ishikawa, D.T.; Correa, G.M.; de Souza, C.O.; Gargaglioni, L.H.; Carrettiero, D.C.; Bicego, K.C.; Almeida, M.C. TRPV4 activates autonomic and behavioural warmth-defence responses in Wistar rats. Acta Physiol. 2015, 214, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Vizin, R.C.L.; Motzko-Soares, A.C.P.; Armentano, G.M.; Ishikawa, D.T.; Cruz-Neto, A.P.; Carrettiero, D.C.; Almeida, M.C. Short-term menthol treatment promotes persistent thermogenesis without induction of compensatory food consumption in Wistar rats: Implications for obesity control. J. Appl. Physiol. 2018, 124, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Ho, J.; Woo, J.H.; Lim, C.L.; Poon, D.J.; Lamba, B.; Claridge-Chang, A. Concordance and incongruence in preclinical anxiety models: Systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2016, 68, 504–529. [Google Scholar] [CrossRef] [PubMed]

| Behavioral Index | PPG (n = 7) | Camphor (n = 7) | p Value |
|---|---|---|---|
| Entries to open arms | 1 ± 0 | 1 ± 0 | 0.82 |
| Entries to closed arms | 3 ± 1 | 5 ± 1 | 0.18 |
| Time in open arms (s) | 19 ± 6 | 35 ± 13 | 0.29 |
| Time in closed arms (s) | 244 ± 10 | 234 ± 15 | 0.58 |
| Rearing events | 10 ± 1 | 11 ± 2 | 0.79 |
| Head-dipping events | 2 ± 1 | 3 ± 1 | 0.21 |
| Risk-assessment events | 6 ± 1 | 6 ± 1 | 0.82 |
| Fecal boli | 3 ± 1 | 4 ± 1 | 0.29 |
| Urine spots | 2 ± 1 | 2 ± 1 | 0.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, D.T.; Vizin, R.C.L.; Souza, C.O.d.; Carrettiero, D.C.; Romanovsky, A.A.; Almeida, M.C. Camphor, Applied Epidermally to the Back, Causes Snout- and Chest-Grooming in Rats: A Response Mediated by Cutaneous TRP Channels. Pharmaceuticals 2019, 12, 24. https://doi.org/10.3390/ph12010024
Ishikawa DT, Vizin RCL, Souza COd, Carrettiero DC, Romanovsky AA, Almeida MC. Camphor, Applied Epidermally to the Back, Causes Snout- and Chest-Grooming in Rats: A Response Mediated by Cutaneous TRP Channels. Pharmaceuticals. 2019; 12(1):24. https://doi.org/10.3390/ph12010024
Chicago/Turabian StyleIshikawa, Débora T., Robson Cristiano Lillo Vizin, Cristiane Oliveira de Souza, Daniel Carneiro Carrettiero, Andrej A. Romanovsky, and Maria Camila Almeida. 2019. "Camphor, Applied Epidermally to the Back, Causes Snout- and Chest-Grooming in Rats: A Response Mediated by Cutaneous TRP Channels" Pharmaceuticals 12, no. 1: 24. https://doi.org/10.3390/ph12010024
APA StyleIshikawa, D. T., Vizin, R. C. L., Souza, C. O. d., Carrettiero, D. C., Romanovsky, A. A., & Almeida, M. C. (2019). Camphor, Applied Epidermally to the Back, Causes Snout- and Chest-Grooming in Rats: A Response Mediated by Cutaneous TRP Channels. Pharmaceuticals, 12(1), 24. https://doi.org/10.3390/ph12010024





