Optimization of the Automated Synthesis of [11C]mHED—Administered and Apparent Molar Activities
Abstract
1. Introduction
2. Results
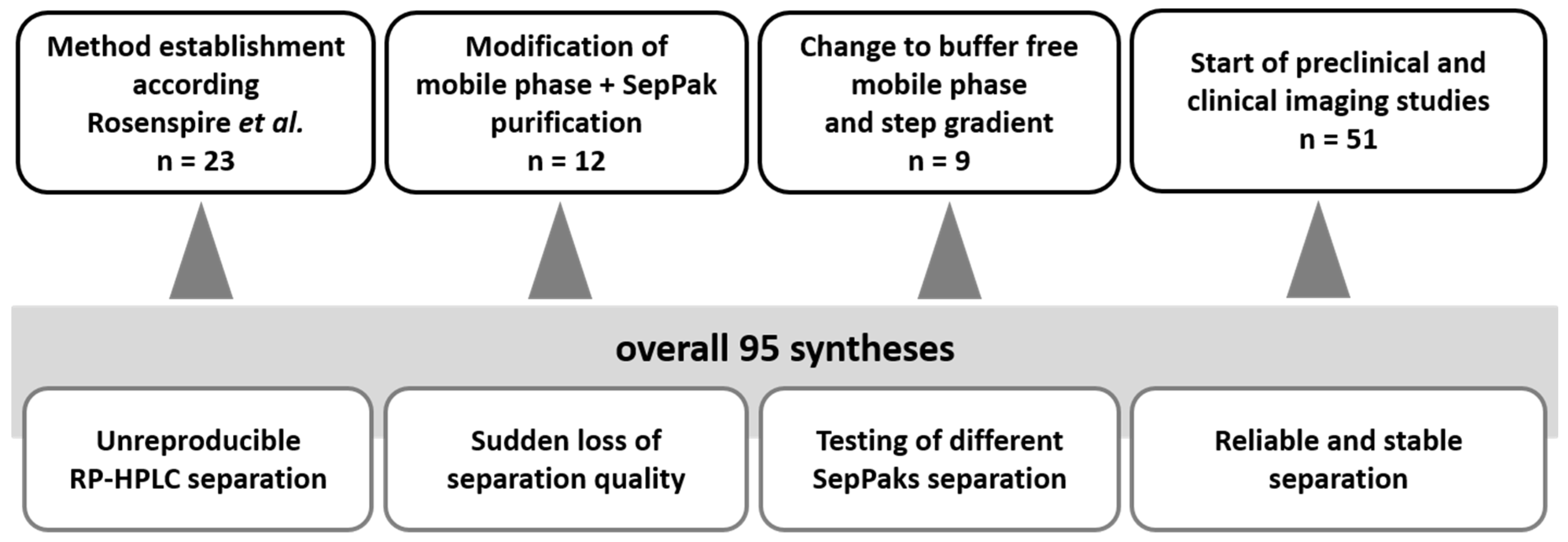
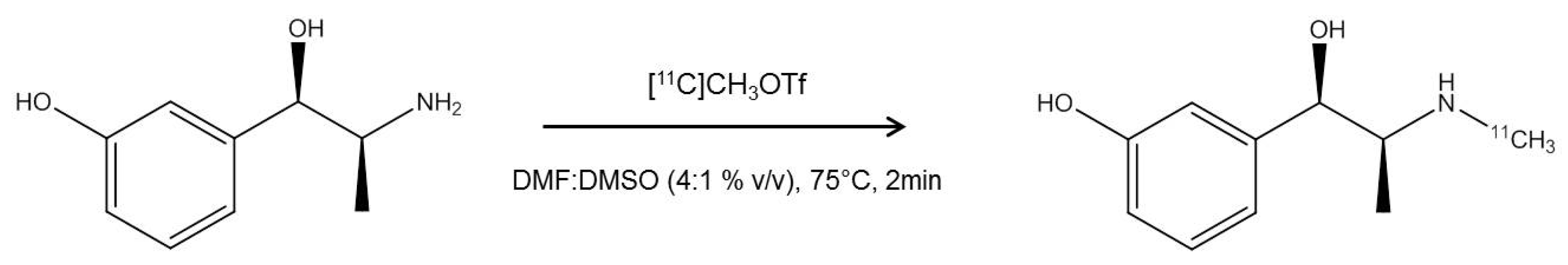
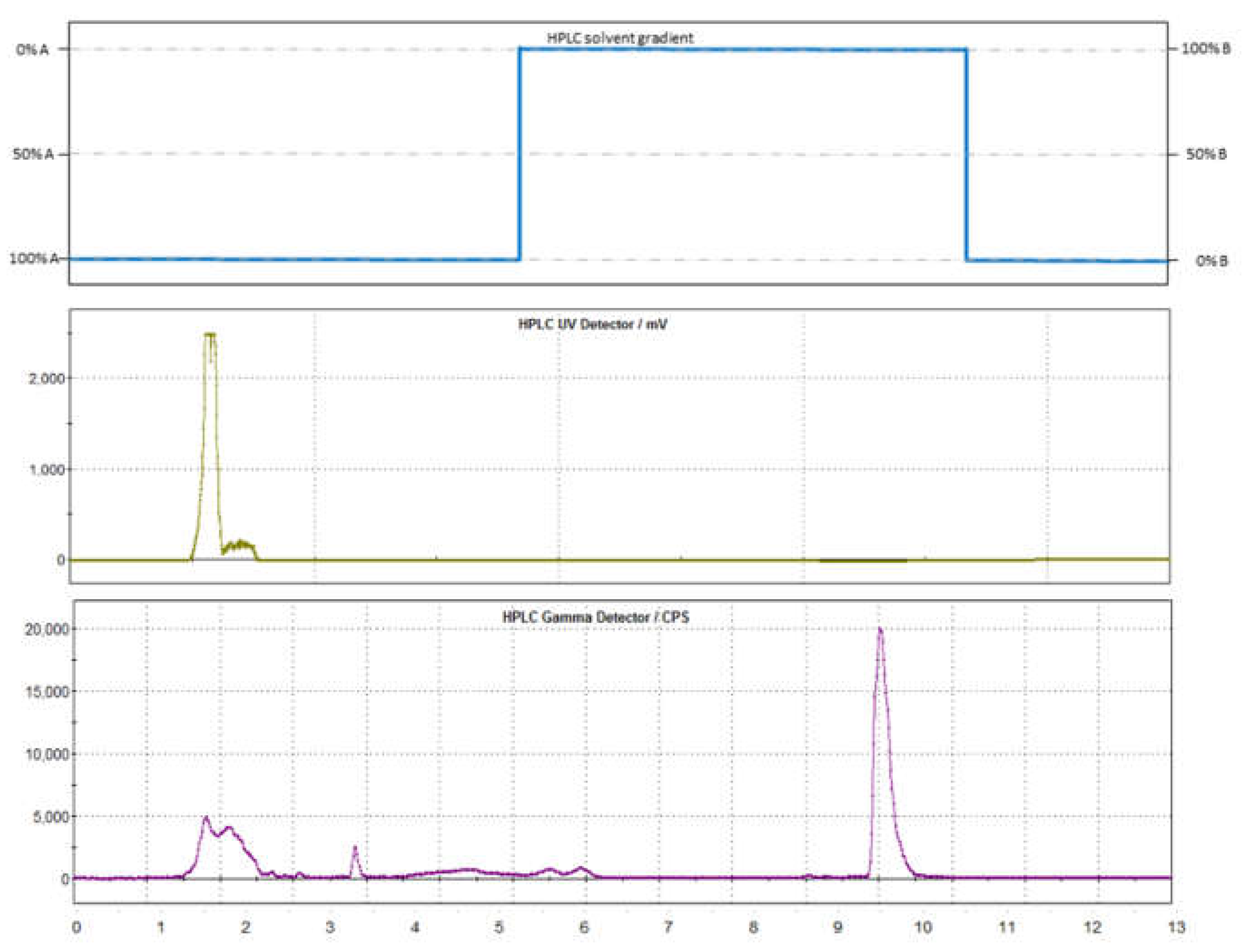
2.1. Radiosynthesis of [11C]mHED
2.2. µPET/µCT Imaging
2.3. PET/MRI Imaging
3. Discussion
4. Material and Methods
4.1. Radiosynthesis
4.2. Semi-Preparative Purification
4.3. Quality Control
4.4. Animal Preparation
4.5. Imaging of µPET/µCT
4.6. PET/MRI Imaging
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Disease. Available online: https://www.who.int/cardiovascular_diseases/en/ (accessed on 30 November 2018).
- WHO. Fact Sheet. Available online: https://www.who.int/mediacentre/factsheets/fs310.pdf (accessed on 30 November 2018).
- Någren, K.; Müller, L.; Halldin, C.; Swahn, C.G.; Lehikoinen, P. Improved synthesis of some commonly used PET radioligands by the use of [11C]methyl triflate. Nucl. Med. Biol. 1995, 22, 235–239. [Google Scholar] [CrossRef]
- Rosenspire, K.C.; Haka, M.S.; Van Dort, M.E.; Jewett, D.M.; Gildersleeve, D.L.; Schwaiger, M.; Wieland, D.M. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: A false transmitter agent for heart neuronal imaging. J. Nucl. Med. 1990, 31, 1328–1334. [Google Scholar]
- Bonfiglioli, R.; Nanni, C.; Martignani, C.; Zanoni, L.; La Donna, R.; Diemberger, I.; Boriani, G.; Pettinato, C.; Sambuceti, G.; Fanti, S.; et al. 11C-mHED for PET/CT: Principles of synthesis, methodology and first clinical applications. Curr. Radiopharm. 2014, 7, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, P.; Carpinelli, A.; Savi, A.; Manca, E.; Picchio, M.; Gianolli, L. [11C]Meta-Hydroxyephedrine PET/CT. Curr. Radiopharm. 2010, 3, 1–9. [Google Scholar] [CrossRef]
- Kunze, K.P.; Nekolla, S.G.; Rischpler, C.; Zhang, S.H.; Hayes, C.; Langwieser, N.; Ibrahim, T.; Laugwitz, K.-L.; Schwaiger, M. Myocardial perfusion quantification using simultaneously acquired 13NH3 -ammonia PET and dynamic contrast-enhanced MRI in patients at rest and stress. Magn. Reson. Med. 2018, 80, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Lodi, F.; Mazza, R.; Giannone, F.; Boschi, L.; Nanni, C.; Nisoli, E.; Boschi, S.; Pasquali, R.; Fanti, S.; et al. (11)C-meta-hydroxyephedrine PET/CT imaging allows in vivo study of adaptive thermogenesis and white-to-brown fat conversion. Mol. Metab. 2013, 2, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Izzi-Engbeaya, C.; Salem, V.; Atkar, R.S.; Dhillo, W.S. Insights into Brown Adipose Tissue Physiology as Revealed by Imaging Studies. Adipocyte 2015, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.-H. Improvement of preoperative management in patients with adrenal pheochromocytoma. Int. J. Clin. Exp. Med. 2014, 7, 5541–5546. [Google Scholar] [PubMed]
- Mann, G.N.; Link, J.M.; Pham, P.; Pickett, C.A.; Byrd, D.R.; Kinahan, P.E.; Krohn, K.A.; Mankoff, D.A. [11C]metahydroxyephedrine and [18F]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann. Surg. Oncol. 2006, 13, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Rizzello, A.; Carpinelli, A.; Di Pierro, D.; Cicoria, G.; Mesisca, V.; Marengo, M.; Boschi, S. Automated synthesis of [11C]meta hydroxyephedrine, a PET radiopharmaceutical for studying sympathetic innervation in the heart. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; Volume 35, pp. 341–343. [Google Scholar]
- Dort, M.E.V.; Tluczek, L. Synthesis and carbon-11 labeling of the stereoisomers of meta-hydroxyephedrine (HED) and meta-hydroxypseudoephedrine (HPED). Off. J. Int. Isot. Soc. 2000, 43, 603–612. [Google Scholar]
- Law, M.P.; Schäfers, K.; Kopka, K.; Wagner, S.; Schober, O.; Schäfers, M. Molecular imaging of cardiac sympathetic innervation by 11C-mHED and PET: From man to mouse? J. Nucl. Med. 2010, 51, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Rischpler, C.; Onthank, D.; Lapa, C.; Robinson, S.; Samnick, S.; Javadi, M.; Schwaiger, M.; Nekolla, S.G.; Higuchi, T. Retention Kinetics of the 18F-Labeled Sympathetic Nerve PET Tracer LMI1195: Comparison with 11C-Hydroxyephedrine and 123I-MIBG. J. Nucl. Med. 2015, 56, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Chen, X.; Maya, Y.; Eissler, C.; Hirano, M.; Nose, N.; Wakabayashi, H.; Lapa, C.; Javadi, M.S.; Higuchi, T. The Impact of Ageing on 11C-Hydroxyephedrine Uptake in the Rat Heart. Sci. Rep. 2018, 8, 11120. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.F.; Van Dort, M.E.; Sievert, M.K.; Ruoho, A.E.; Cozzi, N.V. Stereospecific inhibition of monoamine uptake transporters by meta-hydroxyephedrine isomers. J. Neural Transm. 2002, 109, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Rami-Mark, C.; Berroterán-Infante, N.; Philippe, C.; Foltin, S.; Vraka, C.; Hoepping, A.; Lanzenberger, R.; Hacker, M.; Mitterhauser, M.; Wadsak, W. Radiosynthesis and first preclinical evaluation of the novel norepinephrine transporter pet-ligand [(11)C]ME@HAPTHI. EJNMMI Res. 2015, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Nics, L.; Steiner, B.; Klebermass, E.-M.; Philippe, C.; Mitterhauser, M.; Hacker, M.; Wadsak, W. Speed matters to raise molar radioactivity: Fast HPLC shortens the quality control of C-11 PET-tracers. Nucl. Med. Biol. 2018, 57, 28–33. [Google Scholar] [CrossRef]
- Torigian, D.A.; Zaidi, H.; Kwee, T.C.; Saboury, B.; Udupa, J.K.; Cho, Z.-H.; Alavi, A. PET/MR Imaging: Technical Aspects and Potential Clinical Applications. Radiology 2013, 267, 26–44. [Google Scholar] [CrossRef]
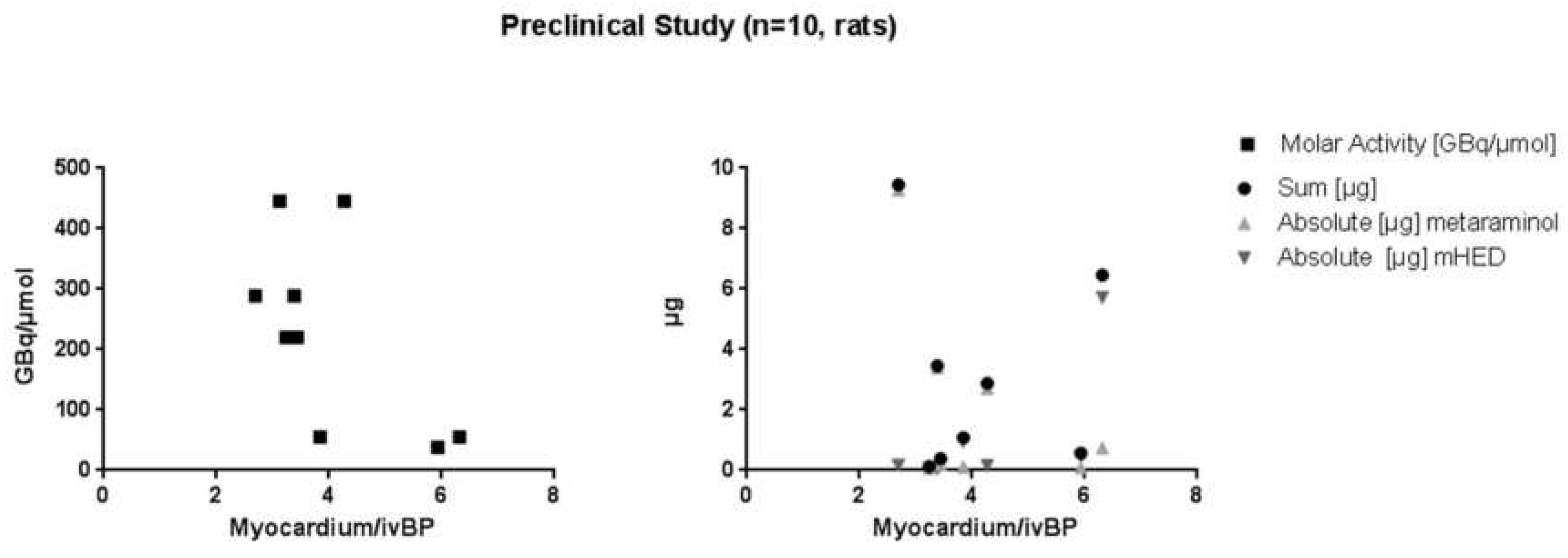



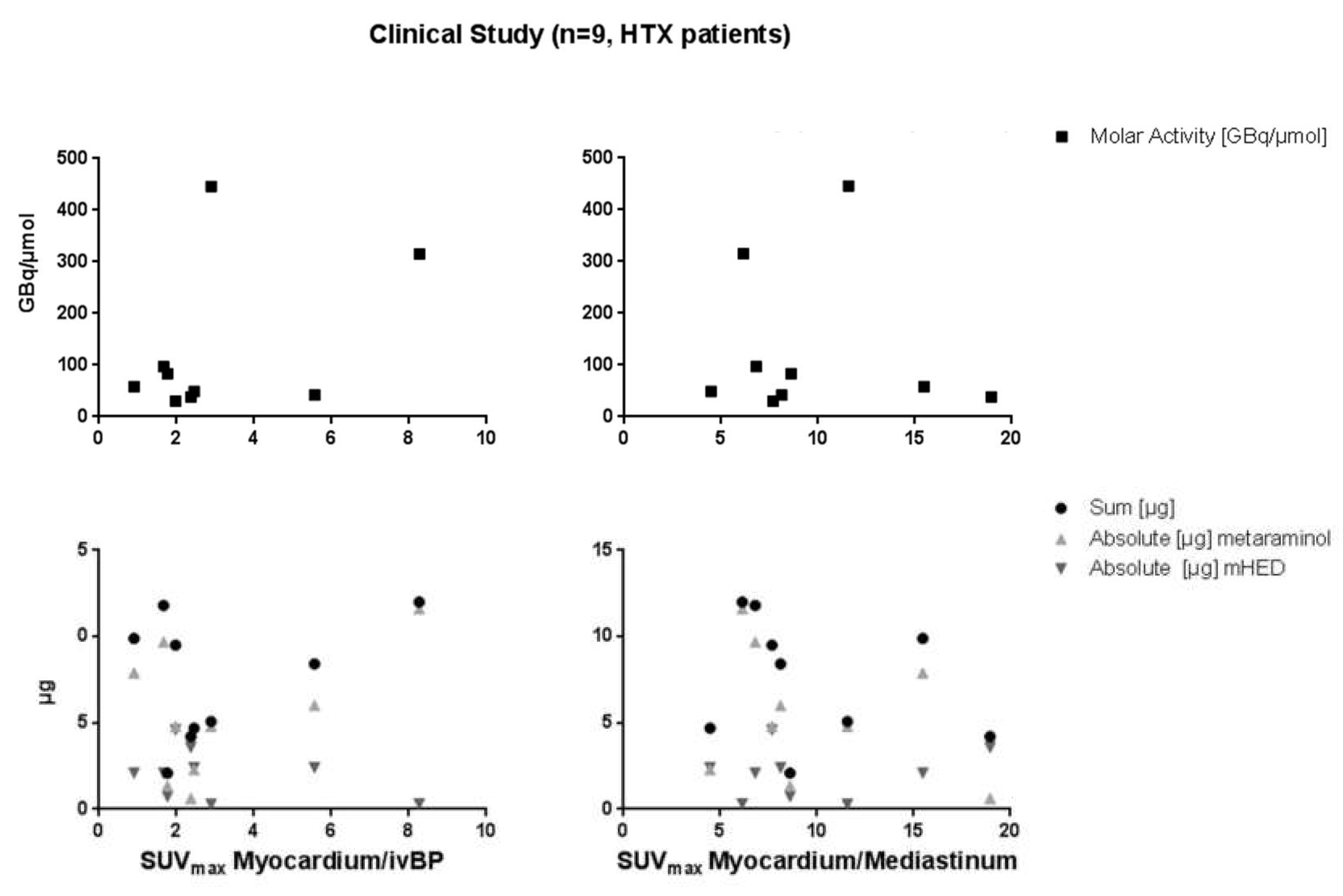

| [11C]mHED | Starting Activity [GBq] | Yield [GBq] | Yield [%EOB] | Molar Activity [GBq/µmol mHED] | Precursor Concentration [µg/mL] | mHED Concentration [µg/mL] |
|---|---|---|---|---|---|---|
| All synthesis (n = 32) | 114 ± 15 | 3 ± 2 | 2.5 ± 1.5 | 126 ± 97 | 8 ± 7 | 3 ± 8 |
| Patients: | ||||||
| Morbus Fabry (n = 5) | 124 ± 2 | 3.5 ± 0.4 | 2.9 ± 0.3 | 155 ± 85 | 6 ± 4 | 2 ± 1 |
| HTX (n = 9) | 120 ± 2 | 2.3 ± 1.3 | 2 ± 1 | 129 ± 148 | 5.5 ± 3.8 | 1.7 ± 2.8 |
| Animals (n = 11) | 122 ± 3 | 3 ± 2 | 2.4 ± 1.9 | 211 ± 152 | 4.6 ± 4.7 | 9 ± 19 |
| [11C]mHED | Metaraminol | mHED | ||
|---|---|---|---|---|
| Patients | [µg/applied volume] | [µg/kg BW] | [µg/applied volume] | [µg/kg BW] |
| Morbus Fabry (n = 5) | 5.2 ± 3.3 | 0.06 ± 0.05 | 1.6 ± 1.3 | 0.02 ± 0.02 |
| HTX (n = 9) | 5.5 ± 3.8 | 0.1 ± 0.1 | 2.1 ± 1.5 | 0.02 ± 0.02 |
| Animals (n = 11) | 1.8 ± 2.9 | 4.9 ± 8.4 | 0.8 ± 1.8 | 1.8 ± 3.8 |
| Rosenspire et al. 1990 | Nagren et al. 1995 | Van Dort et al. 2000 | Law et al. 2010 | This work | |
|---|---|---|---|---|---|
| Methylation agent | [11C]CH3I | [11C]CH3OTf | [11C]CH3I | According to Rosenspire et al. | [11C]CH3OTf |
| 250 µL DMF:DMSO (3:1) | 100 µL ACN | anhydrous DMF 150 µL | 250 µL DMF:DMSO (3:1) | DMF:DMSO (4:1, v/v%) | |
| [c] Precursor | 1 mg metaraminol free base, freshly prepared from metaraminol bitartrate | 1 mg free base | 0.6 mg free base | 1 mg free base | 1 mg free base |
| Formulation | 10% EtOH + 0.24 M NaH2PO4, ~7 mL | physiological phosphate buffer 8 mL | 0.04 M NaH2PO4 10 mL | According to Rosenspire et al. | 0.9% saline 3 mL |
| [c] Metaraminol | 21 µg mean | Not reported | Not reported | 2 µM | 8 ± 7 µg/mL |
| [c] mHED | Not reported | Not reported | 9 µM | 3 ± 8 µg/mL | |
| Radiochemical purity% | 98 2% unidentified RI | Not reported | >98 | 95 ± 5 | 98 ± 2 |
| Time of synthesis (EOB) | 45 min without evaporation | Approx. 41 min | 40 min | According to Rosenspire et al. | 30–35 min |
| Molar activity (EOS) [GBq/µmol] | 33 ± 18 (n = 19) | Not reported | 47–60 (mean = 51) (n = 8) | 10–30 | 126 ± 97 (n = 32) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vraka, C.; Pichler, V.; Berroterán-Infante, N.; Wollenweber, T.; Pillinger, A.; Hohensinner, M.; Fetty, L.; Beitzke, D.; Li, X.; Philippe, C.; et al. Optimization of the Automated Synthesis of [11C]mHED—Administered and Apparent Molar Activities. Pharmaceuticals 2019, 12, 12. https://doi.org/10.3390/ph12010012
Vraka C, Pichler V, Berroterán-Infante N, Wollenweber T, Pillinger A, Hohensinner M, Fetty L, Beitzke D, Li X, Philippe C, et al. Optimization of the Automated Synthesis of [11C]mHED—Administered and Apparent Molar Activities. Pharmaceuticals. 2019; 12(1):12. https://doi.org/10.3390/ph12010012
Chicago/Turabian StyleVraka, Chrysoula, Verena Pichler, Neydher Berroterán-Infante, Tim Wollenweber, Anna Pillinger, Maximilian Hohensinner, Lukas Fetty, Dietrich Beitzke, Xiang Li, Cecile Philippe, and et al. 2019. "Optimization of the Automated Synthesis of [11C]mHED—Administered and Apparent Molar Activities" Pharmaceuticals 12, no. 1: 12. https://doi.org/10.3390/ph12010012
APA StyleVraka, C., Pichler, V., Berroterán-Infante, N., Wollenweber, T., Pillinger, A., Hohensinner, M., Fetty, L., Beitzke, D., Li, X., Philippe, C., Pallitsch, K., Mitterhauser, M., Hacker, M., & Wadsak, W. (2019). Optimization of the Automated Synthesis of [11C]mHED—Administered and Apparent Molar Activities. Pharmaceuticals, 12(1), 12. https://doi.org/10.3390/ph12010012









