Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data
Abstract
1. Introduction
2. Results and Discussion
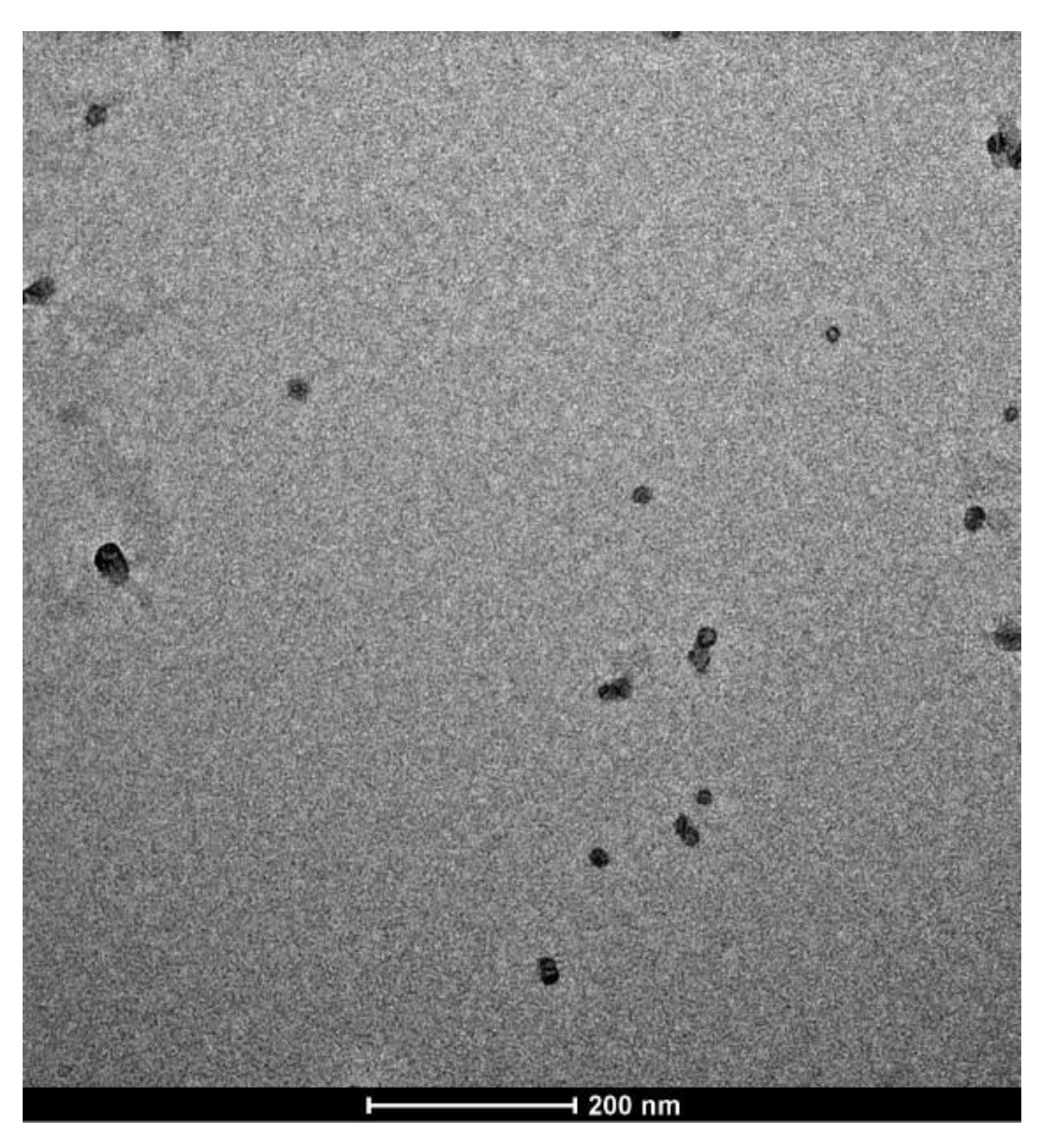
2.1. SLN Characterization
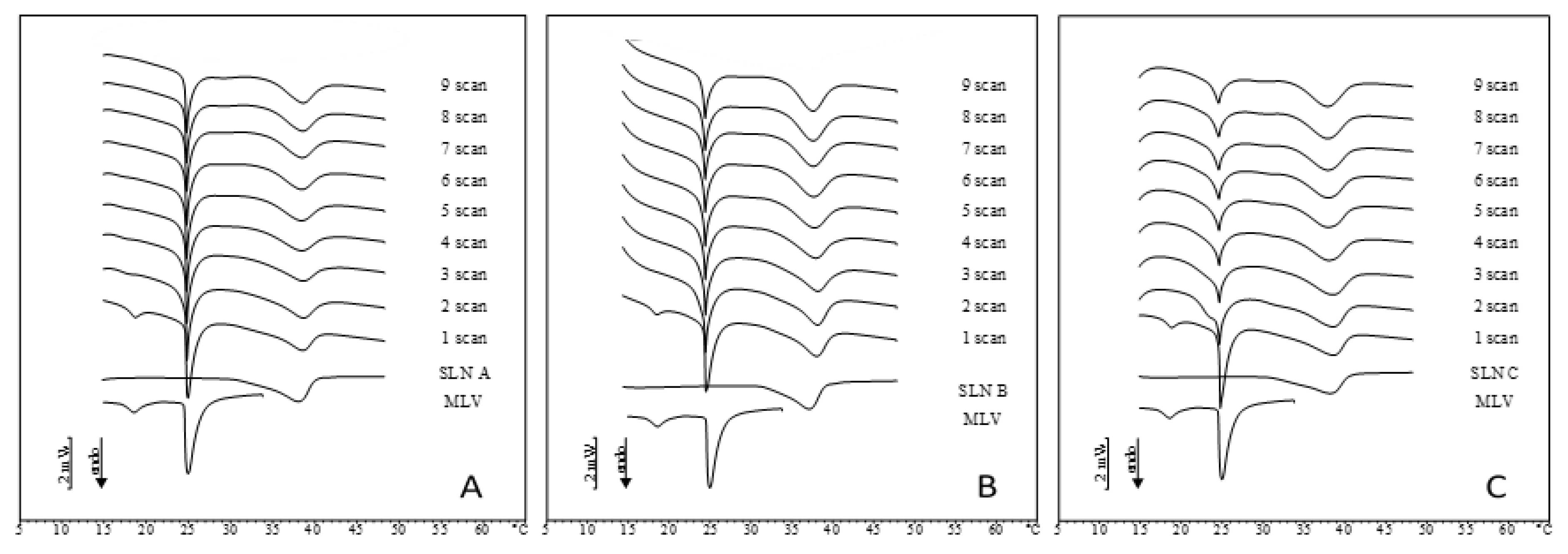
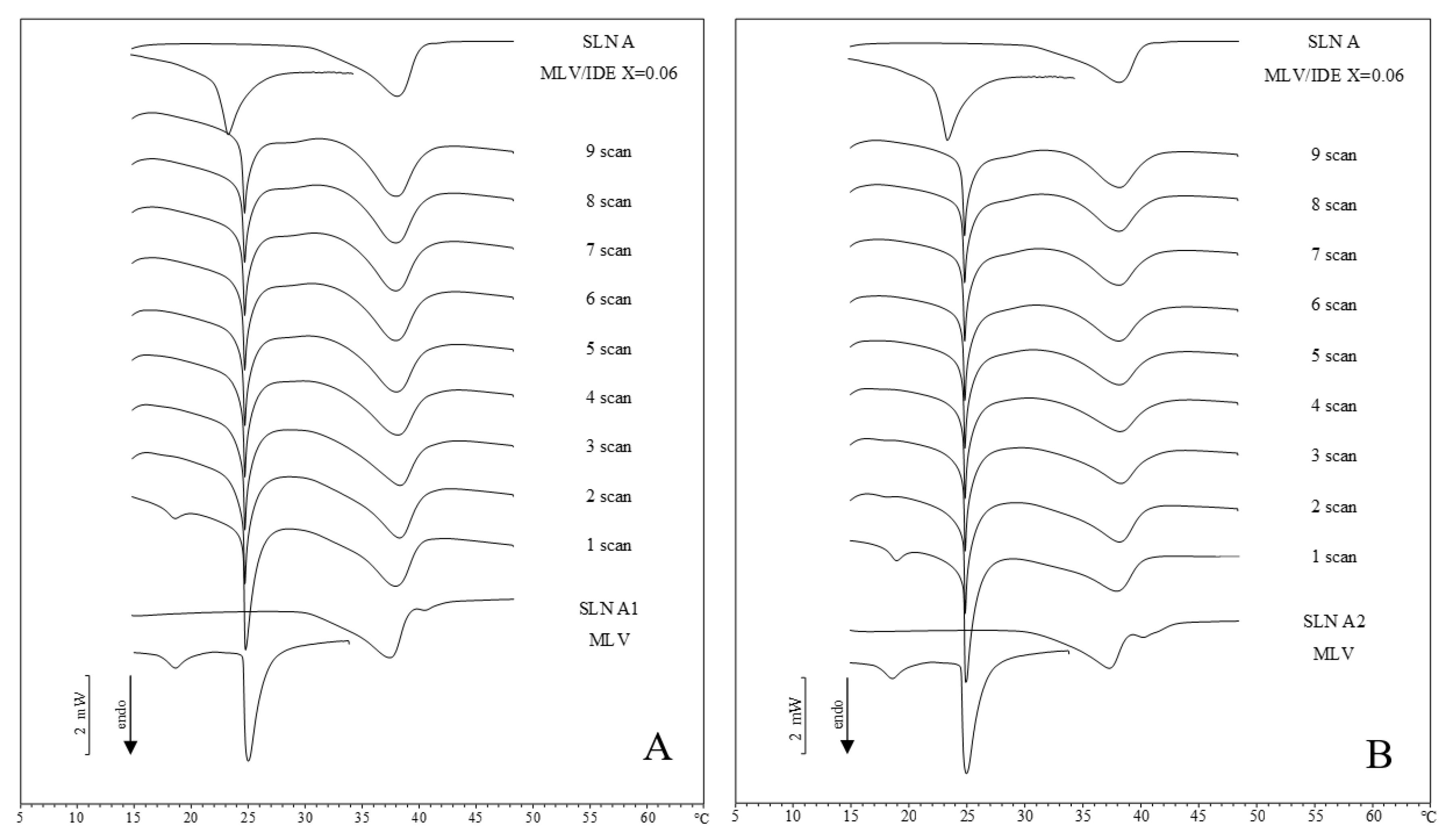
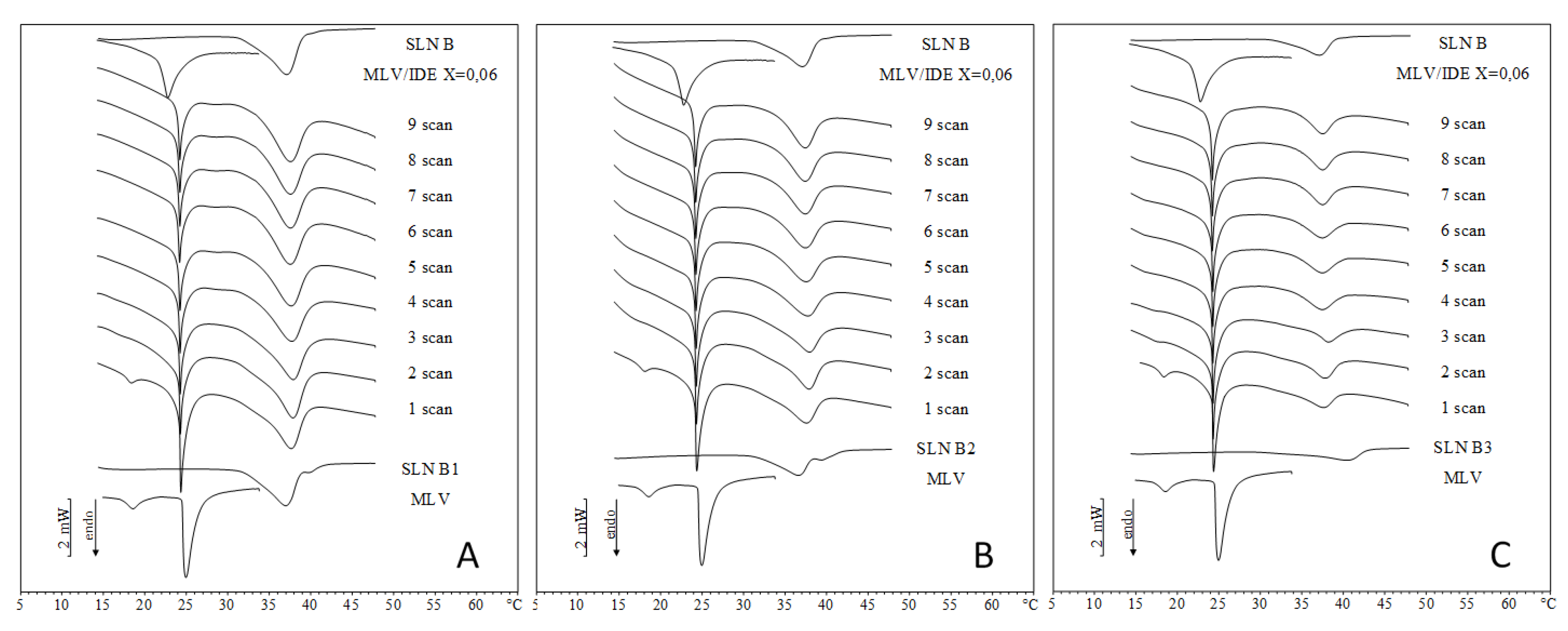
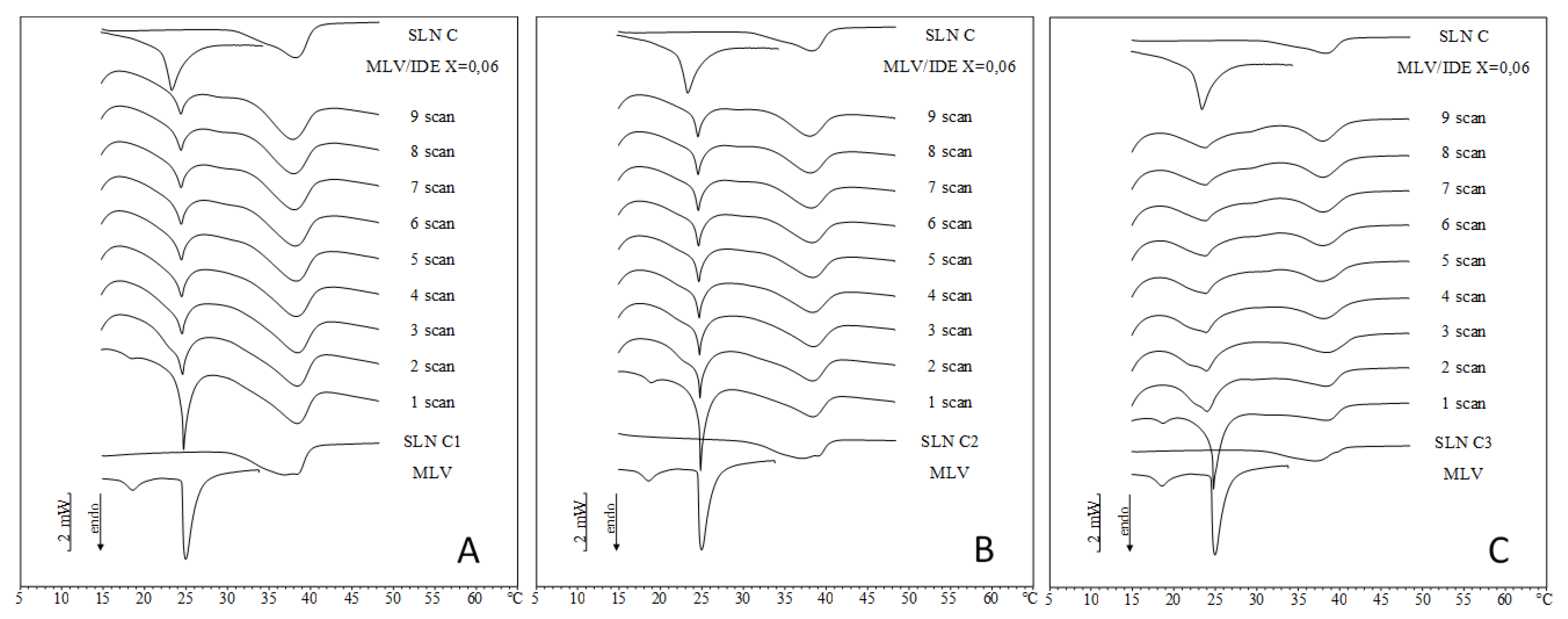
2.2. IDE-Loaded SLN Interactions with MLV Liposomes
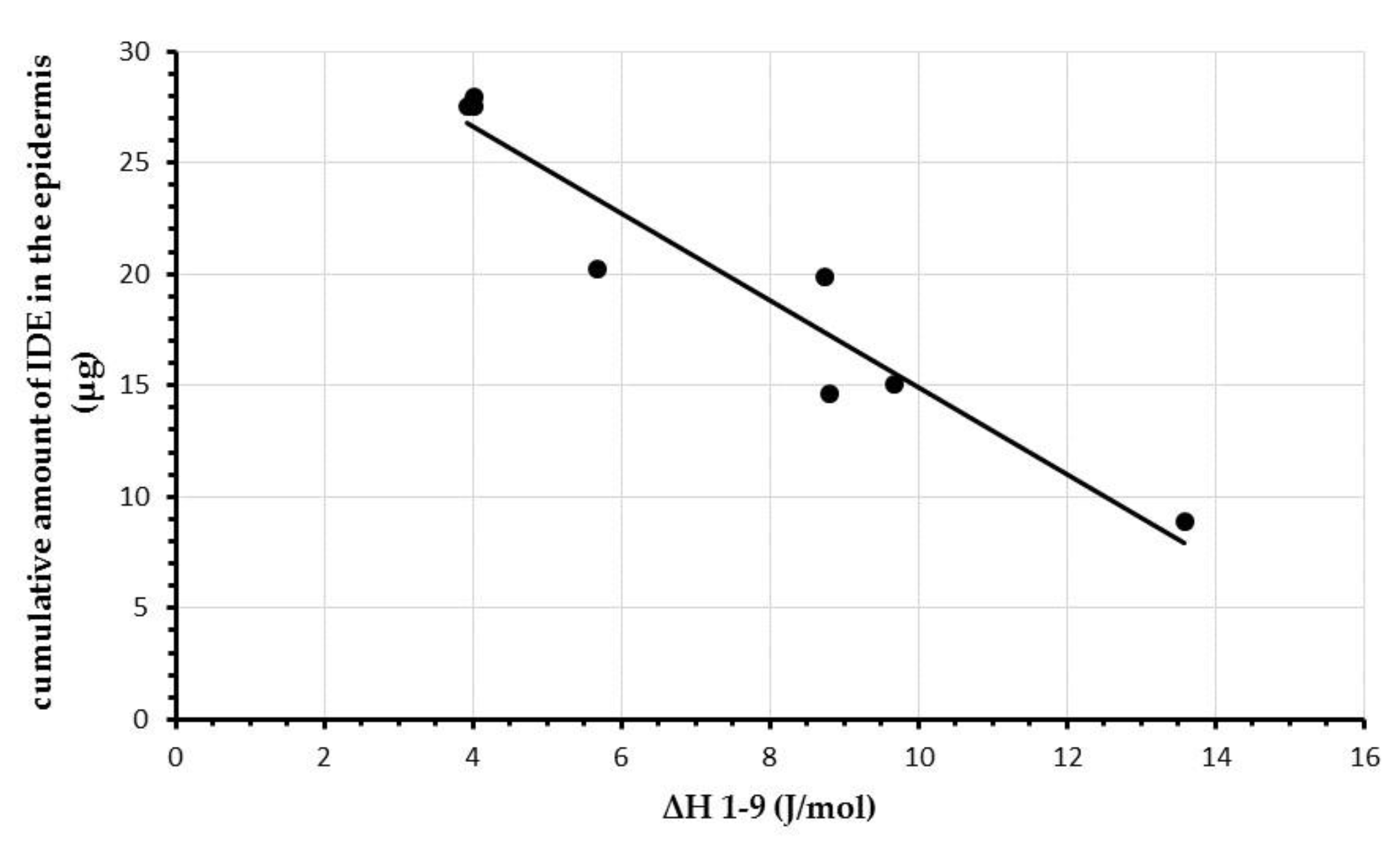
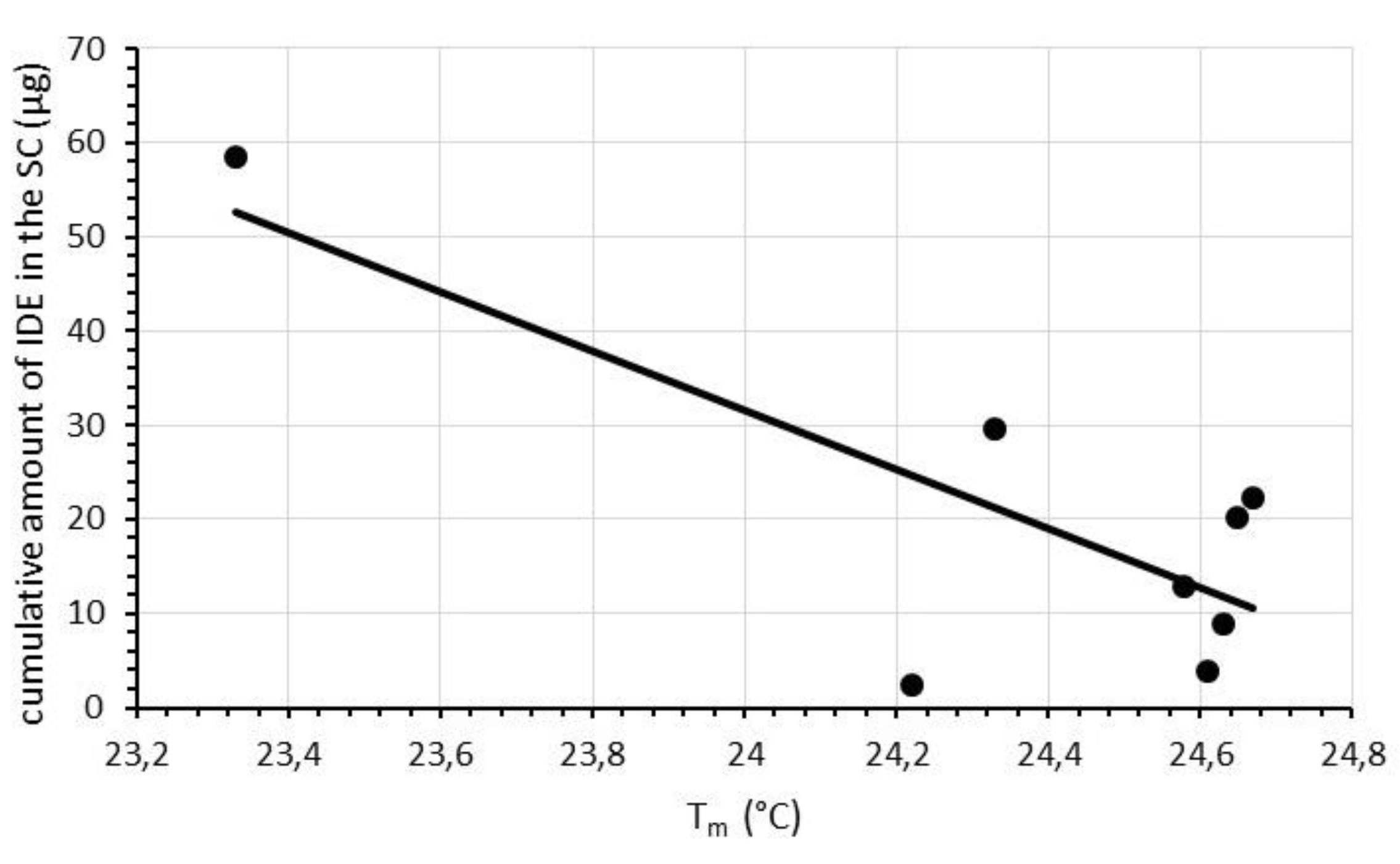
2.3. Comparison between DSC and In Vitro Skin Penetration Data
3. Materials and Methods
3.1. Materials
3.2. Preparation of SLN
3.3. Transmission Electron Microscopy (TEM)
3.4. Photon Correlation Spectroscopy (PCS)
3.5. MLV Preparation
3.6. Differential Scanning Calorimetry Analyses
3.7. In Vitro Skin Permeation Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lúcio, M.; Lima, J.L.; Reis, S. Drug-Membrane interactions: Significance for medicinal chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Lipid–protein interactions in biological membranes: A dynamic perspective. Biochim. Biophys. Acta 2012, 1818, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Petruzielo, R.S.; Goh, S.L.; Konyakhina, T.M.; Ackerman, D.G.; Amazon, J.J.; Feigenson, G.W. Liposome-based models for membrane rafts. Methodology and applications. In Liposome, Lipid Bilayers and Model Membranes. From Basic Research to Applications; Pabst, G., Kučerka, N., Nieh, M.P., Katsaras, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 143–166. ISBN 9781138198753. [Google Scholar]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Dea, P.K. Differential scanning calorimetry studies of phospholipid membranes: The interdigitated gel phase. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 407–444. ISBN 978-953-51-0947-1. [Google Scholar]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Cañadas, O.; Casals, C. Differential scanning calorimetry of protein-lipid interactions. Methods Mol. Biol. 2013, 974, 55–71. [Google Scholar] [CrossRef]
- Bruylants, G.; Wouters, J.; Michaux, C. Differential scanning calorimetry in life science: Thermodynamics, stability, molecular recognition and application in drug design. Curr. Med. Chem. 2005, 12, 2011–2020. [Google Scholar] [CrossRef]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitro evaluation. Int. J. Pharm. 2012, 43, 169–174. [Google Scholar] [CrossRef]
- Montenegro, L.; Ottimo, S.; Puglisi, G.; Castelli, F.; Sarpietro, M.G. Idebenone loaded solid lipid nanoparticles interact with biomembrane models: Calorimetric evidence. Mol. Pharm. 2012, 9, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Accolla, M.L.; Turnaturi, R.; Sarpietro, M.G.; Ronsisvalle, S.; Castelli, F.; Pasquinucci, L. Differential scanning calorimetry approach to investigate the transfer of the multitarget opioid analgesic LP1 to biomembrane model. Eur. J. Med. Chem. 2014, 77, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.G.; Morris, R.M. Thermal analysis of phase transition behaviour in liposomes. Thermochim Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Brandenburg, K.O.; Garidel, P.; Howe, J.; Andrä, J.; Hawkins, L.; Koch, M.H.J.; Seydel, U. What can calorimetry tell us about changes of three-dimensional aggregate structures of phospholipids and glycolipids? Thermochim. Acta 2006, 445, 133–143. [Google Scholar] [CrossRef]
- Montenegro, L.; Sarpietro, M.G.; Ottimo, S.; Puglisi, G.; Castelli, F. Differential scanning calorimetry studies on sunscreen loaded solid lipid nanoparticles prepared by the phase inversion temperature method. Int. J. Pharm. 2011, 415, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lim, S.J.; Kim, C.K. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 2007, 28, 2137–2146. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Accolla, M.L.; Puglisi, G.; Castelli, F.; Montenegro, L. Idebenone loaded solid lipid nanoparticles: Calorimetric studies on surfactant and drug loading effects. Int. J. Pharm. 2014, 471, 69–74. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H.I. Effects of skin occlusion on percutaneous absorption: An overview. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Almeida, A.J.; Müller, R.H. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The Influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Wissing, S.A.; Lippacher, A.; Müller, R.H. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
- Wissing, S.A.; Müller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Schlupp, P.; Blaschke, T.; Kramer, K.D.; Höltje, H.D.; Mehnert, W.; Schäfer-Korting, M. Drug release and skin penetration from solid lipid nanoparticles and a base cream: A systematic approach from a comparison of three glucocorticoids. Skin Pharmacol. Physiol. 2011, 24, 199–209. [Google Scholar] [CrossRef]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef]
- Montenegro, L.; Trapani, A.; Latrofa, A.; Puglisi, G. In vitro evaluation on a model of blood brain barrier of idebenone-loaded solid lipid nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Puglisi, G. Evaluation of sunscreen safety by in vitro skin permeation studies: Effects of vehicle composition. Pharmazie 2013, 68, 34–40. [Google Scholar] [CrossRef] [PubMed]

| SLN | Size ± S.D. (nm) | PI ± S.D. | ζ potential ± S.D. (mV) | PIT (°C) |
|---|---|---|---|---|
| A | 42.2 ± 0.8 | 0.268 ± 0.014 | −1.76 ± 0.27 | 80 |
| A1 | 41.7 ± 0.7 | 0.281 ± 0.013 | −1.66 ± 0.38 | 80 |
| A2 | 43.1 ± 0.9 | 0.272 ± 0.018 | −2.35 ± 0.37 | 80 |
| B | 45.4 ± 0.6 | 0.281 ± 0.016 | −1.90 ± 0.39 | 81 |
| B1 | 46.6 ± 0.9 | 0.290 ± 0.015 | −1.98 ± 0.45 | 80 |
| B2 | 44.9 ± 1.0 | 0.275 ± 0.021 | −2.29 ± 0.44 | 80 |
| B3 | 33.9 ± 0.8 | 0.167 ± 0.016 | −1.90 ± 0.58 | 81 |
| C | 36.1 ± 0.6 | 0.178 ± 0.017 | −1.87 ± 0.25 | 85 |
| C1 | 35.3 ± 0.4 | 0.159 ± 0.019 | −1.78 ± 0.29 | 85 |
| C2 | 36.4 ± 0.5 | 0.183 ± 0.022 | −2.41 ± 0.58 | 84 |
| C3 | 35.9 ± 0.7 | 0.155 ± 0.017 | −1.94 ± 0.29 | 84 |
| SLN | SC | E | D |
|---|---|---|---|
| A1 | 20.23 ± 2.84 | 27.95 ± 5.79 | 1.47 ± 0.24 |
| A2 | 22.20 ± 6.25 | 27.54 ± 6.87 | 1.40 ± 0.76 |
| B1 | 3.86 ± 1.36 | 14.57 ± 1.39 | 0.71 ± 0.08 |
| B2 | 8.94 ± 2.57 | 20.21 ± 7.26 | 1.64 ± 0.19 |
| B3 | 12.79 ± 1.76 | 27.51 ± 3.25 | 4.21 ± 0.66 |
| C1 | 2.50 ± 0.59 | 8.90 ± 2.31 | 0.26 ± 0.08 |
| C2 | 29.62 ± 1.19 | 15.08 ± 4.93 | 2.22 ± 0.32 |
| C3 | 58.47 ± 5.76 | 19.87 ± 2.67 | 3.44 ± 0.34 |
| Sample | ΔH (J/mol) | ΔH1–9 (J/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| scan 1 | scan 2 | scan 3 | scan 4 | scan 5 | scan 6 | scan 7 | scan 8 | scan 9 | ||
| A1 | 27.06 | 26.38 | 24.12 | 24.58 | 24.11 | 24.04 | 23.57 | 23.10 | 23.04 | 4.02 |
| A2 | 27.58 | 24.25 | 24.25 | 23.57 | 23.65 | 23.93 | 23.22 | 23.36 | 23.65 | 3.93 |
| B1 | 29.69 | 29.09 | 26.75 | 23.30 | 23.52 | 22.53 | 21.24 | 20.84 | 20.88 | 8.81 |
| B2 | 30.01 | 30.34 | 25.93 | 25.38 | 24.38 | 24.22 | 24.49 | 24.53 | 24.34 | 5.67 |
| B3 | 28.40 | 28.39 | 26.42 | 26.12 | 26.50 | 25.50 | 24.61 | 24.11 | 24.39 | 4.01 |
| C1 | 28.32 | 27.90 | 26.43 | 21.63 | 20.18 | 17.96 | 15.99 | 15.23 | 15.13 | 13.19 |
| C2 | 27.16 | 26.70 | 24.77 | 21.98 | 20.56 | 19.25 | 18.39 | 17.58 | 17.48 | 9.68 |
| C3 | 27.53 | 24.56 | 23.65 | 21.89 | 20.36 | 19.54 | 18.65 | 18.70 | 18.79 | 8.74 |
| Sample | Tm (°C) | Tm1-9 (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| scan 1 | scan 2 | scan 3 | scan 4 | scan 5 | scan 6 | scan 7 | scan 8 | scan 9 | ||
| A1 | 24.75 | 24.67 | 24.67 | 24.67 | 24.68 | 24.64 | 24.64 | 24.65 | 24.65 | 0.10 |
| A2 | 24.85 | 24.75 | 24.72 | 24.72 | 24.69 | 24.70 | 24.70 | 24.67 | 24.67 | 0.18 |
| B1 | 24.74 | 24.67 | 24.64 | 24.64 | 24.61 | 24.61 | 24.61 | 24.61 | 24.61 | 0.13 |
| B2 | 24.80 | 24.75 | 24.72 | 24.69 | 24.69 | 24.66 | 24.66 | 24.63 | 24.63 | 0.17 |
| B3 | 24.75 | 24.73 | 24.71 | 24.68 | 24.68 | 24.64 | 24.65 | 24.61 | 24.58 | 0.17 |
| C1 | 24.64 | 24.51 | 24.42 | 24.35 | 24.29 | 24.29 | 24.26 | 24.26 | 24.22 | 0.42 |
| C2 | 24.78 | 24.65 | 24.59 | 24.53 | 24.46 | 24.43 | 24.40 | 24.36 | 24.33 | 0.45 |
| C3 | 24.64 | 23.68 | 23.56 | 23.49 | 23.49 | 23.46 | 23.36 | 23.36 | 23.33 | 1.31 |
| SLN | Isoceteth-20 | Ceteth-20 | Oleth-20 | GO | CP | IDE |
|---|---|---|---|---|---|---|
| A | 10.6 | - | - | 3.5 | 7.0 | - |
| A1 | 10.6 | - | - | 3.5 | 7.0 | 0.5 |
| A2 | 10.6 | - | - | 3.5 | 7.0 | 0.7 |
| B | - | 8.7 | - | 4.4 | 7.0 | - |
| B1 | - | 8.7 | - | 4.4 | 7.0 | 0.5 |
| B2 | - | 8.7 | - | 4.4 | 7.0 | 0.7 |
| B3 | - | 8.7 | - | 4.4 | 7.0 | 1.1 |
| C | - | - | 7.5 | 3.7 | 7.0 | - |
| C1 | - | - | 7.5 | 3.7 | 7.0 | 0.5 |
| C2 | - | - | 7.5 | 3.7 | 7.0 | 0.7 |
| C3 | - | - | 7.5 | 3.7 | 7.0 | 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Castelli, F.; Sarpietro, M.G. Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals 2018, 11, 138. https://doi.org/10.3390/ph11040138
Montenegro L, Castelli F, Sarpietro MG. Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals. 2018; 11(4):138. https://doi.org/10.3390/ph11040138
Chicago/Turabian StyleMontenegro, Lucia, Francesco Castelli, and Maria Grazia Sarpietro. 2018. "Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data" Pharmaceuticals 11, no. 4: 138. https://doi.org/10.3390/ph11040138
APA StyleMontenegro, L., Castelli, F., & Sarpietro, M. G. (2018). Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals, 11(4), 138. https://doi.org/10.3390/ph11040138






