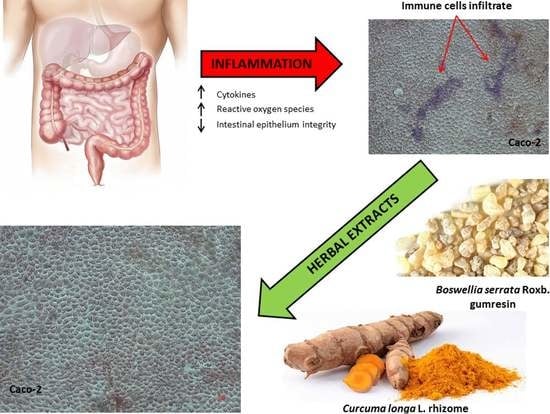
Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2
Abstract
1. Introduction
2. Results
2.1. Chemical Analyses of Dry Extracts
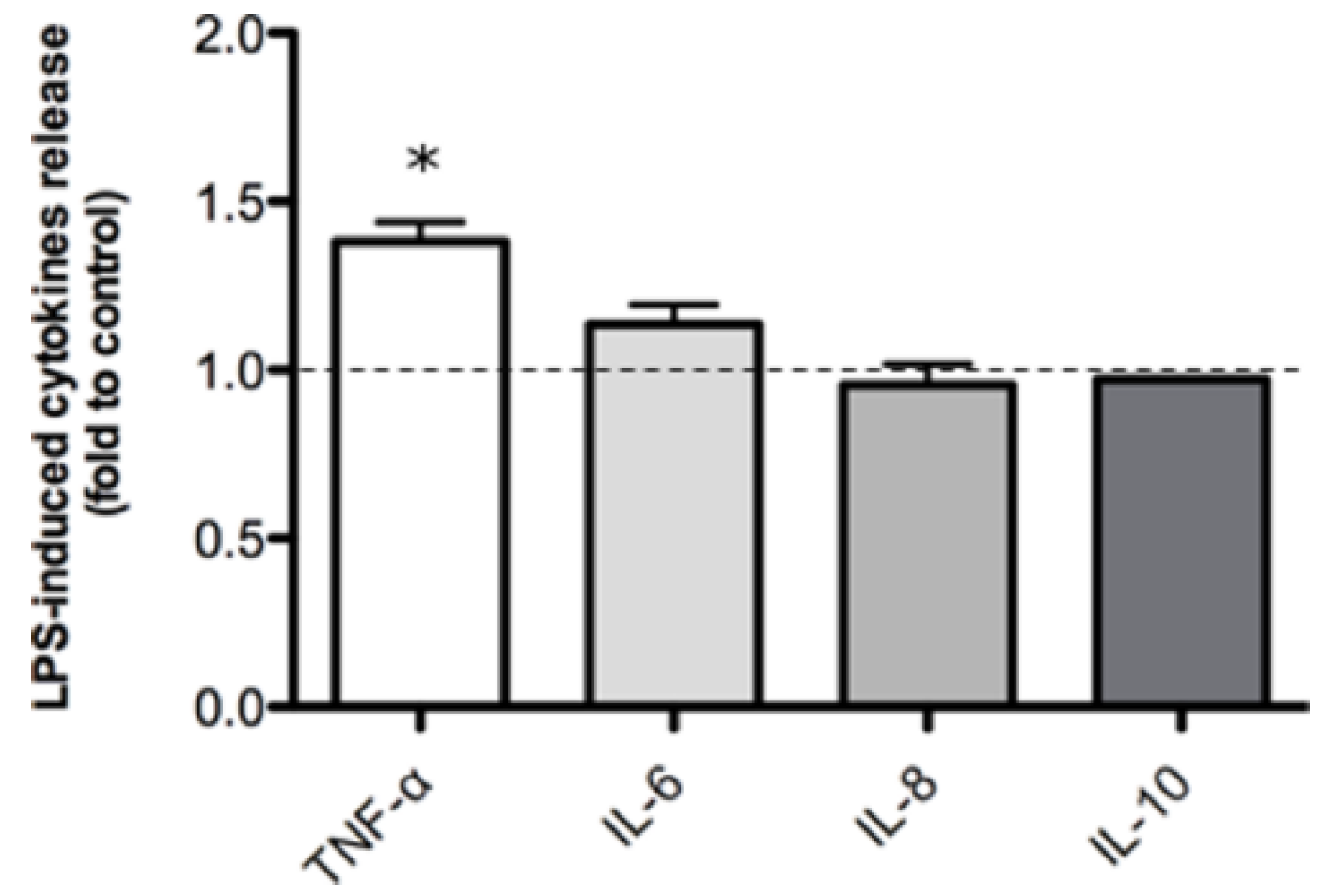
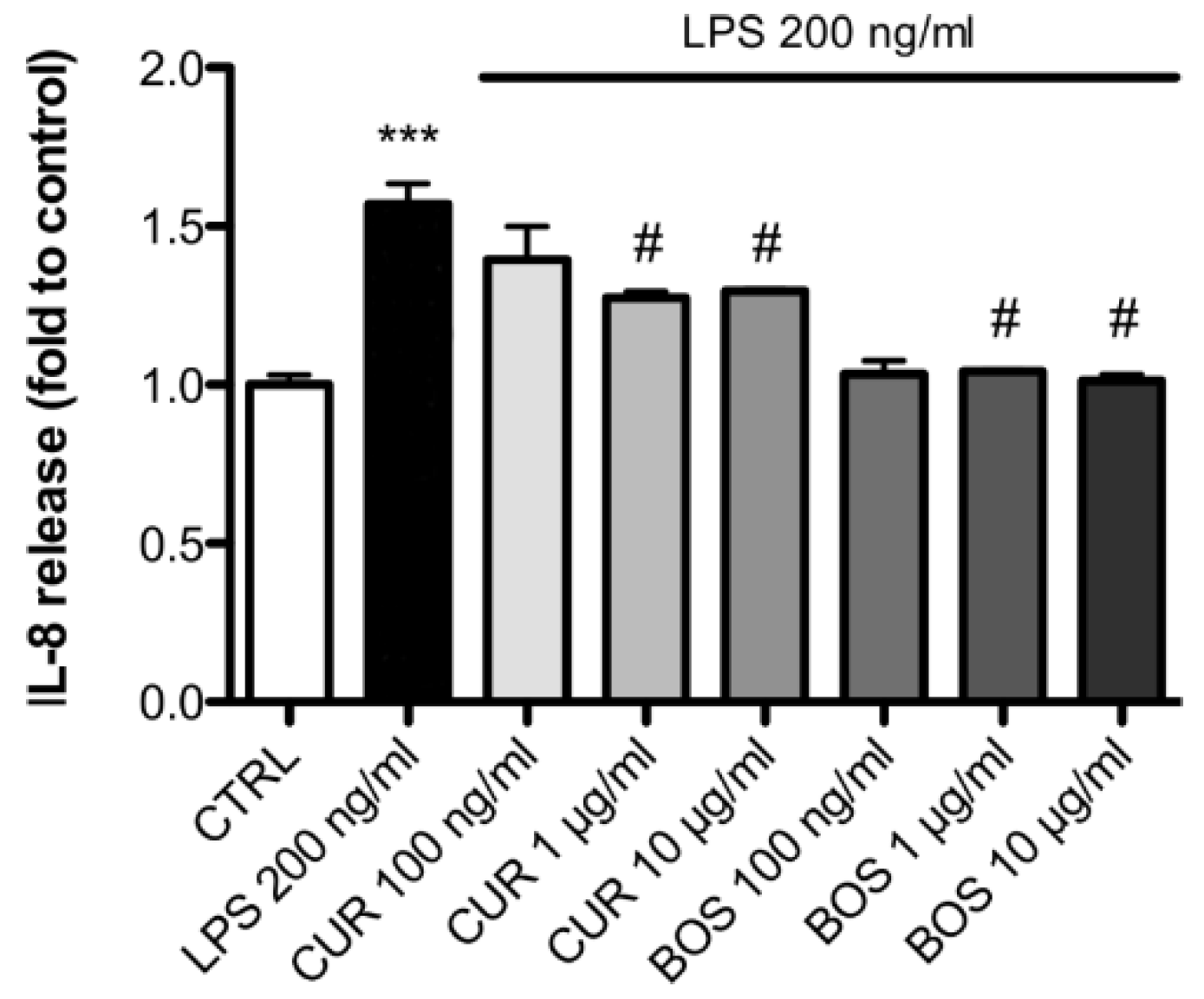
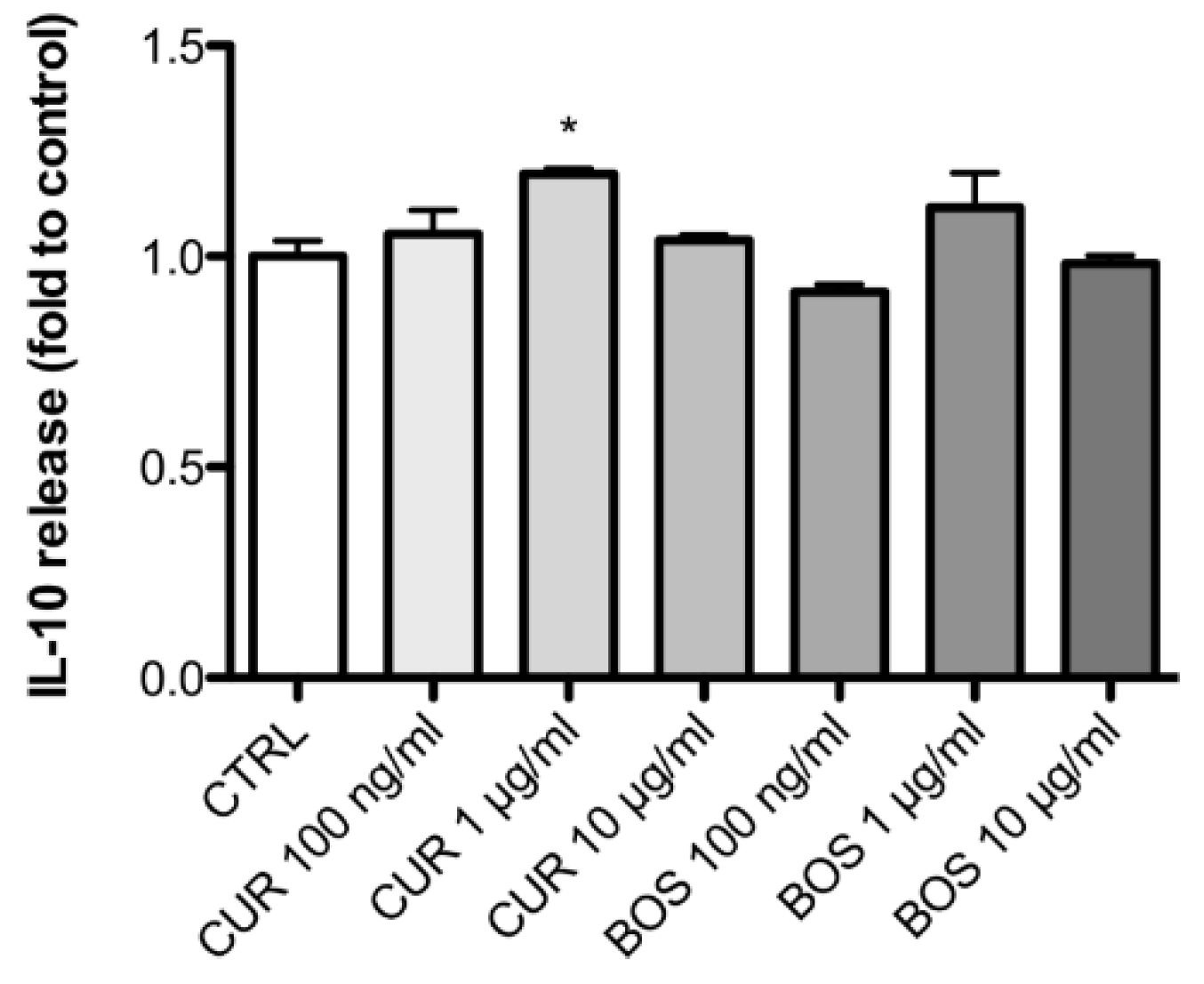
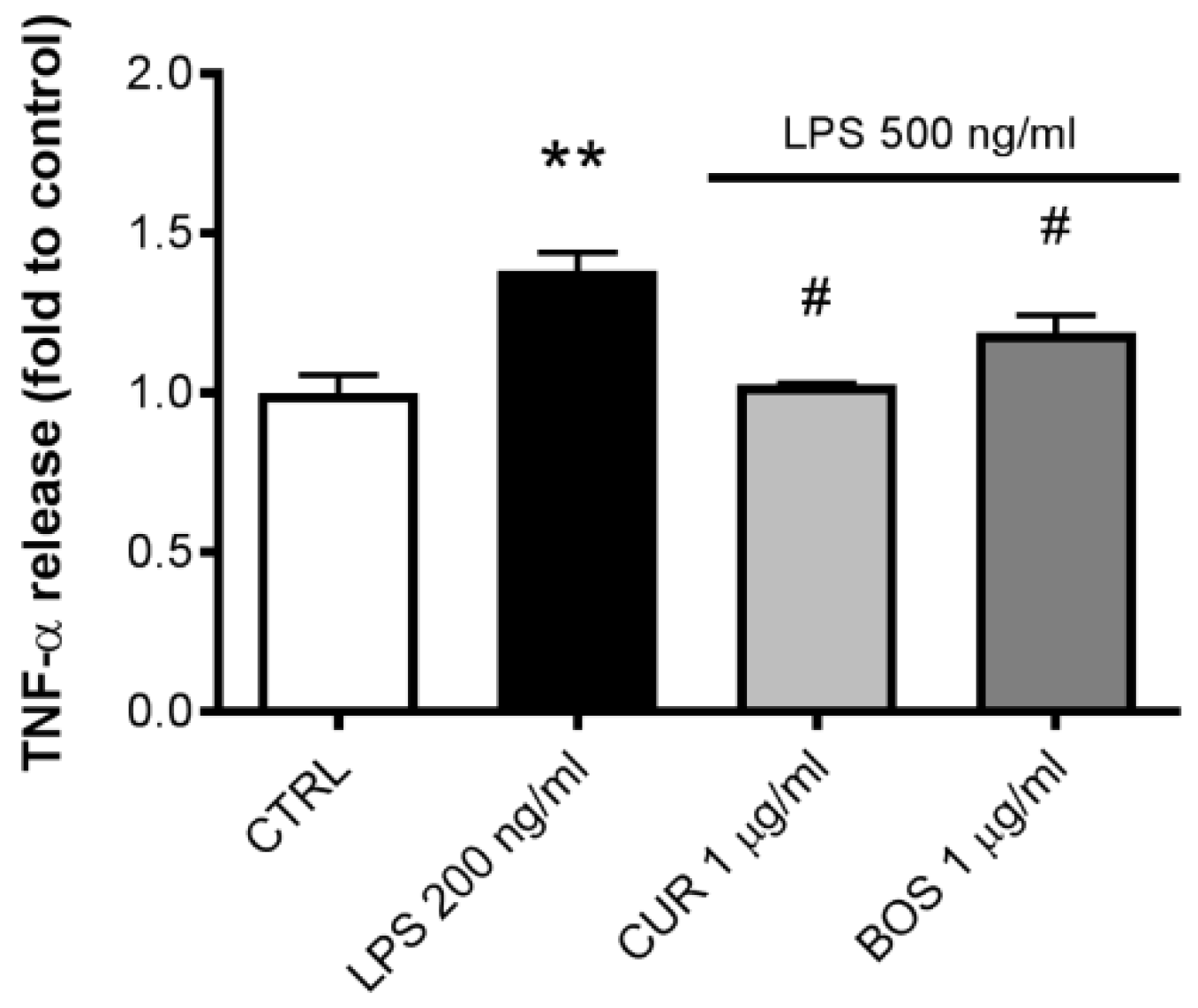
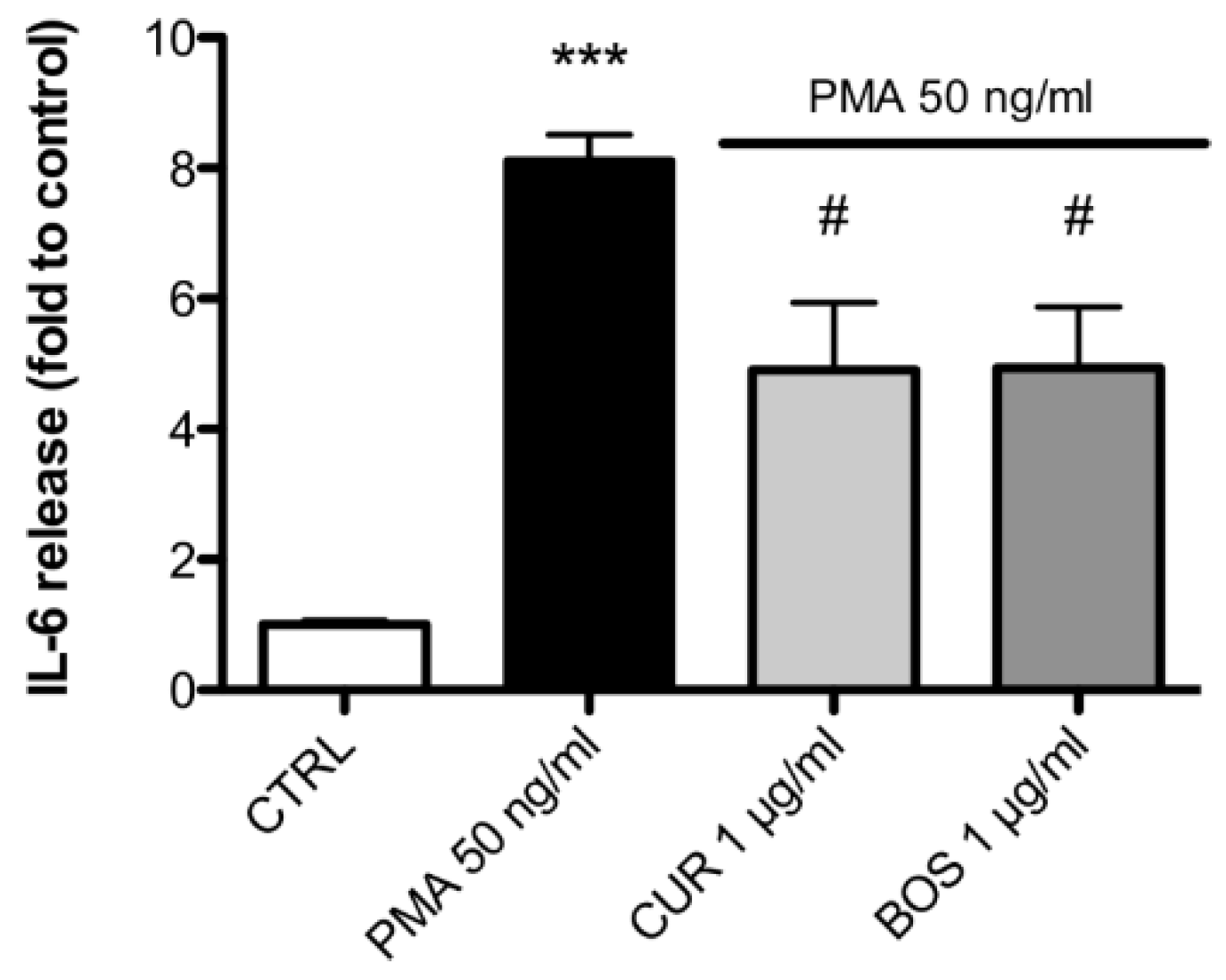
2.2. Inflammatory Model on Caco-2, PBMC and HMC-1.1: Cytokines Dosages
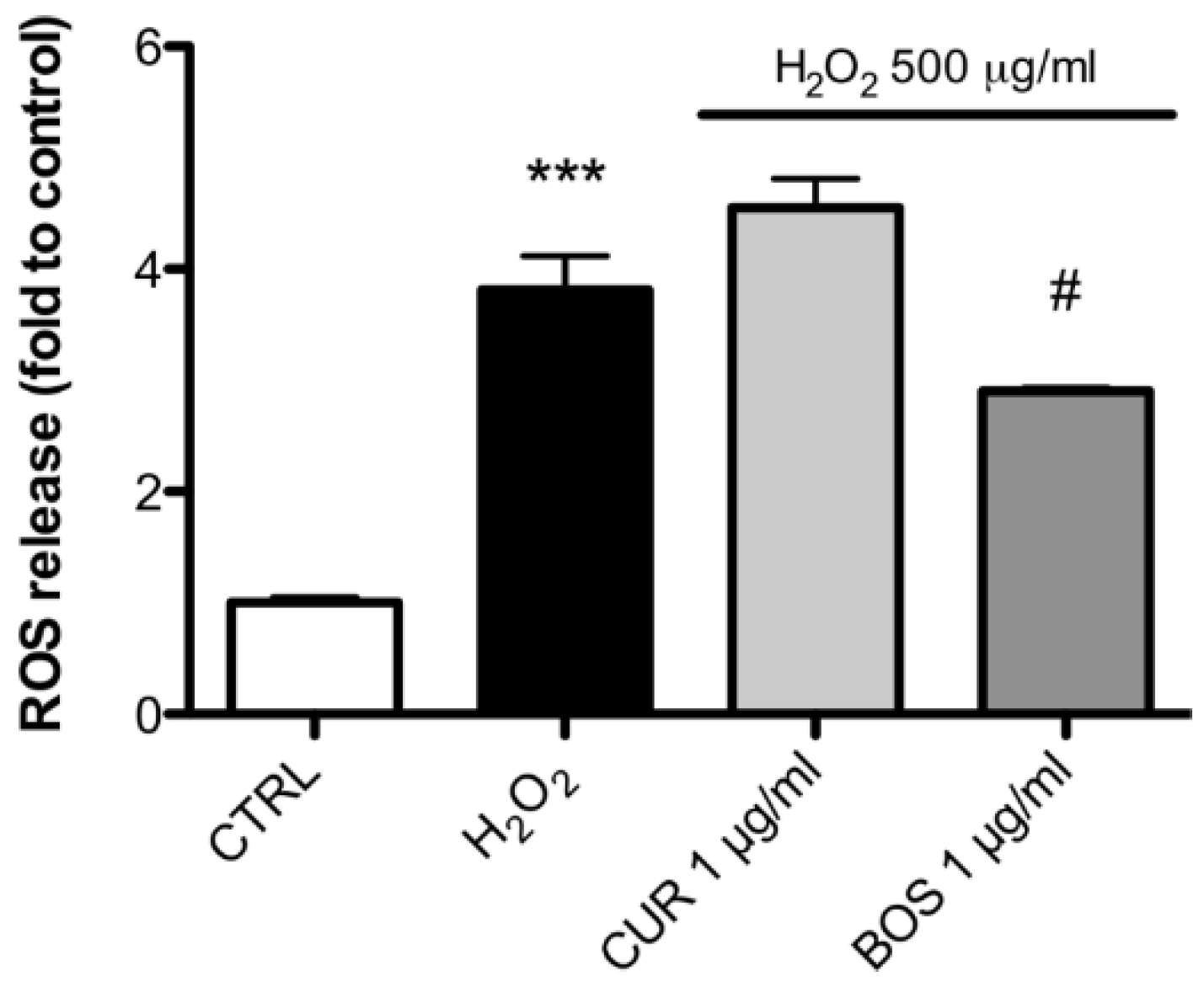
2.3. Measurement of ROS Production
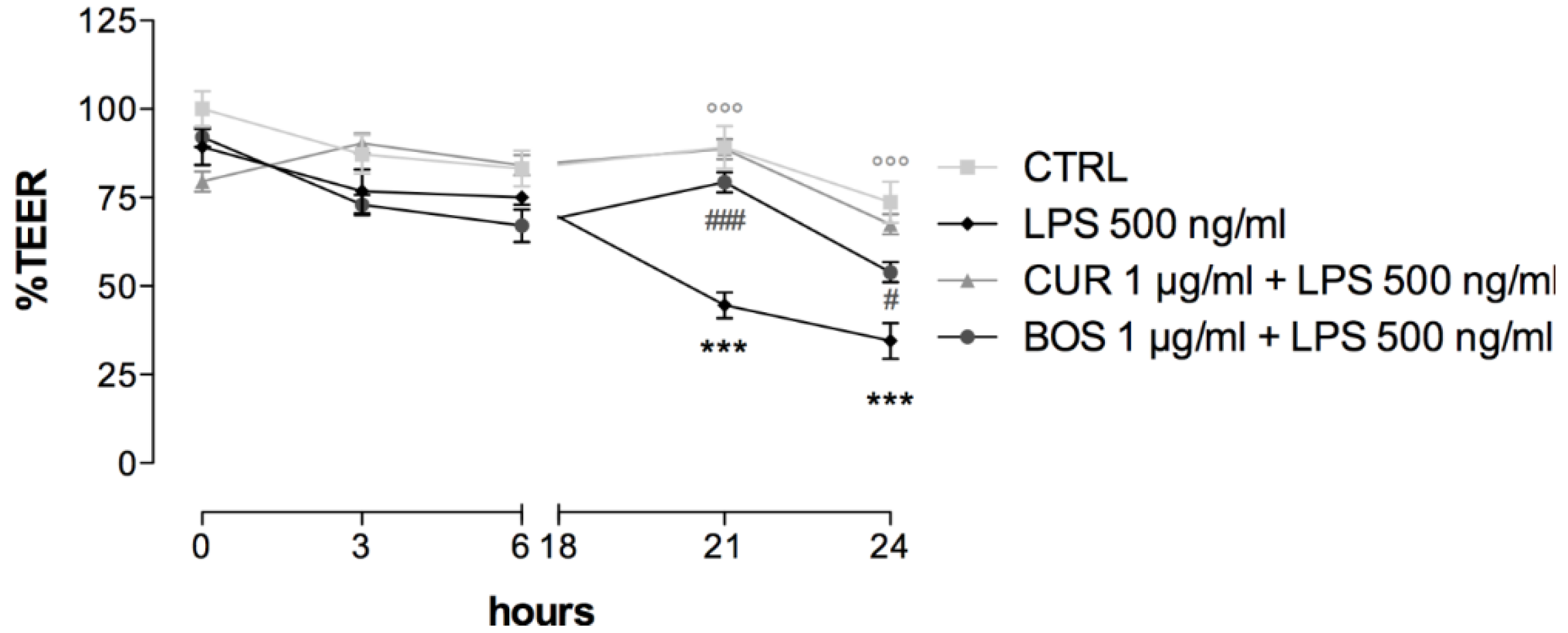
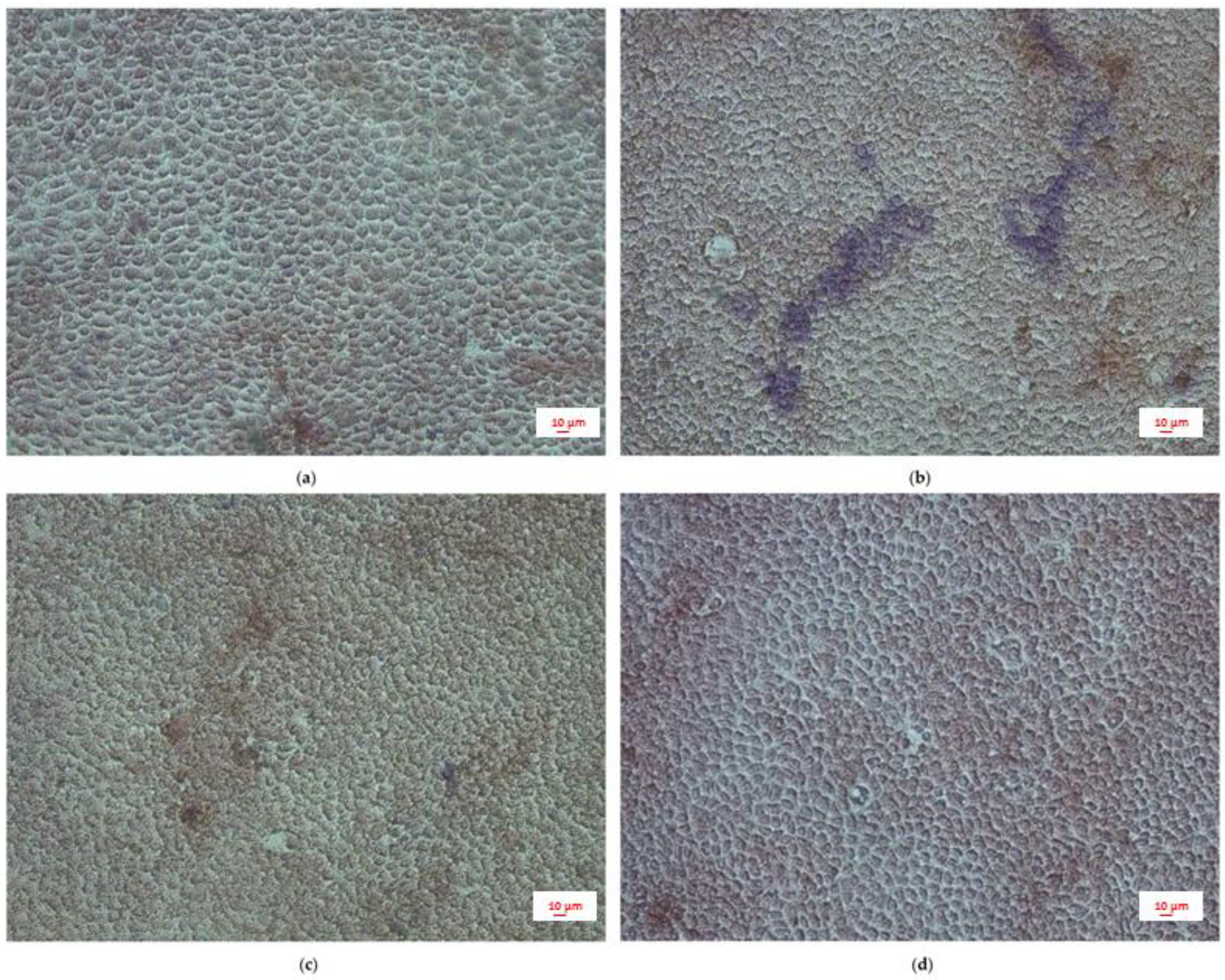
2.4. Intestinal Permeability: TEER Measurements
2.5. Leukocytes Infiltration: PBMC Adhesion Assay
3. Discussion
4. Materials and Methods
4.1. Extracts Preparation and Chemical Analysis
4.1.1. Curcumin Quantification
4.1.2. Curcumin Quantification (HPLC-DAD Method)
4.1.3. Total Triterpenes Quantification
4.2. Cell Cultures
4.2.1. Peripheral Blood Mononuclear Cells
4.2.2. Mast Cells
4.2.3. Intestinal Epithelium Cells
4.3. Evaluation of the Anti-Inflammatory Activity
4.4. Measurement of ROS Production
4.5. Measurement of Trans-Epithelial Electric Resistance (TEER)
4.6. Adhesion Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mudter, J. What’s new about inflammatory bowel diseases in 2011. World J. Gastroenterol. 2011, 17, 3177. [Google Scholar] [PubMed]
- Burisch, J.; Munkholm, P. The epidemiology of inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Crusius, J.; Meuwissen, S.; Pena, A. Inflammatory bowel disease: Definition, epidemiology, etiologic aspects and immunogenetic studies. World J. Gastroenterol. 1998, 4, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Chichlowski, M.; Westwood, G.S.; Abraham, S.N.; Hale, L.P. Role of Mast Cells in Inflammatory Bowel Disease and Inflammation-Associated Colorectal Neoplasia in IL-10-Deficient Mice. PLoS ONE 2010, 5, e12220. [Google Scholar] [CrossRef] [PubMed]
- He, S.-H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The Multifaceted Mast Cell in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, S.; Bau, E.C.; Antunes, E.; Dietrich, C.P.; Nader, H.B.; De Nucci, G. Inhibition of heparin synthesis by methotrexate in rats in vivo. Biochem. Pharmacol. 2002, 64, 169–175. [Google Scholar] [CrossRef]
- Goldsmith, P.; McGarity, B.; Walls, A.F.; Church, M.K.; Millward-Sadler, G.H.; Robertson, D.A. Corticosteroid treatment reduces mast cell numbers in inflammatory bowel disease. Dig. Dis. Sci. 1990, 35, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.C.; Moore, W.C.; Lichtenstein, L.M. Modulation of mediator release from human intestinal mast cells by sulfasalazine and 5-aminosalicylic acid. Dig. Dis. Sci. 1991, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Grevenitis, P.; Thomas, A.; Lodhia, N. Medical Therapy for Inflammatory Bowel Disease. Surg. Clin. North. Am. 2015, 95, 1159–1182. [Google Scholar] [CrossRef] [PubMed]
- Tsioutsiou, E.E.; Giachetti, D.; Miraldi, E.; Governa, P.; Magnano, A.R.; Biagi, M. Phytotherapy and skin wound healing. Acta. Vulnologica. 2016, 14, 126–139. [Google Scholar]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Lam, Y.T.; Tsoi, K.K.F.; Chan, F.K.L.; Sung, J.J.Y.; Wu, J.C.Y. Systematic review: The efficacy of herbal therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 854–863. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency EMA Assessment Report on Curcuma longa L. Rhizoma. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2010/02/WC500070703.pdf (accessed on 3 July 2018).
- Mazieiro, R.; Frizon, R.R.; Barbalho, S.M.; de Alvares Goulart, R. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.C.; Marton, L.T.; de Marqui, S.V.; Lima, T.A.; Barbalho, S.M. Curcuminoids From Curcuma Longa: New Adjuvants For The Treatment Of Crohn’S Disease And Ulcerative Colitis? Crit. Rev. Food Sci. Nutr. 2018, in press. [Google Scholar]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.J.; Johnston, S.; Reilly, K.; Men, X.; Burgess, E.J.; Perry, N.B.; Roy, N.C. The effect of turmeric (Curcuma longa) extract on the functionality of the solute carrier protein 22 A4 (SLC22A4) and interleukin-10 (IL-10) variants associated with inflammatory bowel disease. Nutrients 2014, 6, 4178–4190. [Google Scholar] [CrossRef] [PubMed]
- Billerey-Larmonier, C.; Uno, J.K.; Larmonier, N.; Midura, A.J.; Timmermann, B.; Ghishan, F.K.; Kiela, P.R. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm. Bowel Dis. 2008, 14, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, C.B.; Midura-Kiela, M.T.; Ramalingam, R.; Laubitz, D.; Janikashvili, N.; Larmonier, N.; Ghishan, F.K.; Kiela, P.R. Modulation of neutrophil motility by curcumin: Implications for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 503–515. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Monographs on Selected Medicinal Plants Volume 4; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Ocete, M.A.; Galvez, J. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediators Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.M.; Fillmann, H.S.; Martins, M.I.M.; Meurer, L.; Marroni, N.P. Boswellia serrata has beneficial anti-inflammatory and antioxidant properties in a model of experimental colitis. Phytother. Res. 2014, 28, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.M.; Morgan Martins, M.I.; Tieppo, J.; Fillmann, H.S.; Marroni, N.P. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig. Dis. Sci. 2012, 57, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.; Chan, M.; Venkataraman, S.; Brown, P.N. Curcuminoids in Turmeric Roots and Supplements: Method Optimization and Validation. Food Anal. Methods 2016, 9, 1428–1435. [Google Scholar] [CrossRef]
- Chao, I.-C.; Wang, C.M.; Li, S.P.; Lin, L.G.; Ye, W.C.; Zhang, Q.W. Simultaneous Quantification of Three Curcuminoids and Three Volatile Components of Curcuma longa Using Pressurized Liquid Extraction and High-Performance Liquid Chromatography. Molecules 2018, 23, 1568. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Occhipinti, A.; Maffei, M.E. Quantitative Determination of 3-O-Acetyl-11-Keto-β-Boswellic Acid (AKBA) and Other Boswellic Acids in Boswellia sacra Flueck (syn. B. carteri Birdw) and Boswellia serrata Roxb. Molecules 2016, 21, 1329. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Thomson, G.; Macfarlane, G.T. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 2005, 115, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Kiessling, S.; Mestermann, S.; Webb, G.; Spottl, T.; Andus, T.; Scholmerich, J.; Herfarth, H.; Ray, K.; Falk, W.; et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 2002, 122, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Sandig, H.; Bulfone-Paus, S. TLR signalling in mast cells: Common and unique features. Front. Immunol. 2012, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Balletta, A.; Lorenz, D.; Rummel, A.; Gerhard, R.; Bigalke, H.; Wegner, F. Human mast cell line-1 (HMC-1) cells exhibit a membrane capacitance increase when dialysed with high free-Ca2+ and GTPγS containing intracellular solution. Eur. J. Pharmacol. 2013, 720, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, G.; Jiang, J.; Zheng, M.; Jin, Y.; Lin, Z.; Li, G.; Choi, Y.; Yan, G. Cornuside inhibits mast cell-mediated allergic response by down-regulating MAPK and NF-κB signalling pathways. Biochem. Biophys. Res. Commun. 2016, 473, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Catanzaro, D.; Borgonetti, V.; Ragazzi, E.; Giron, M.C.; Governa, P.; Carnevali, I.; Monica, M.; Biagi, M. A Fixed Combination of Probiotics and Herbal Extracts Attenuates Intestinal Barrier Dysfunction from Inflammatory Stress in an In vitro Model Using Caco-2 Cells. Recent Pat. Food. Nutr. Agric. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, N.; Liboni, K.; Neu, J. Glutamine decreases lipopolysaccharide-induced IL-8 production in Caco-2 cells through a non-NF-kappaB p50 mechanism. Cytokine 2003, 22, 77–83. [Google Scholar] [CrossRef]
- Abreu, M.T.; Arnold, E.T.; Thomas, L.S.; Gonsky, R.; Zhou, Y.; Hu, B.; Arditi, M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 2002, 277, 20431–20437. [Google Scholar] [CrossRef] [PubMed]
- Bocker, U.; Yezerskyy, O.; Feick, P.; Manigold, T.; Panja, A.; Kalina, U.; Herweck, F.; Rossol, S.; Singer, M. V Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int. J. Colorectal Dis. 2003, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Rosenberg, I.M.; Brandwein, S.L.; Beck, P.L.; Reinecker, H.C.; Podolsky, D.K. Lipopolysaccharide activates distinct signalling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000, 164, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-{α} modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Ma, I.; Ma, T.Y. Molecular mechanism of tumor necrosis factor-α modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G496–G504. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Ma, T.Y. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-α-induced regulation of myosin light chain kinase gene activity. J. Cell. Mol. Med. 2008, 12, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, J.; Amasheh, M.; Krug, S.M.; Fromm, A.; Amasheh, S.; Hillenbrand, B.; Tavalali, S.; Fromm, M.; Schulzke, J.D. TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signalling. Cell. Tissue Res. 2009, 336, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Fromm, M.; Bentzel, C.J.; Scholz, P.; Detjen, K.; Mankertz, J.; Bode, H.; Epple, H.J.; Riecken, E.O.; Schulzke, J.D. Tumor necrosis factor-α (TNFα) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell. Sci. 1999, 112, 137–146. [Google Scholar] [PubMed]
- Gitter, A.H.; Bendfeldt, K.; Schulzke, J.D.; Fromm, M. Leaks in the epithelial barrier caused by spontaneous and TNF-α-induced single-cell apoptosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 1749–1753. [Google Scholar] [CrossRef]
- Florian, P.; Schoneberg, T.; Schulzke, J.D.; Fromm, M.; Gitter, A.H. Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J. Physiol. 2002, 545, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Marano, C.W.; Lewis, S.A.; Garulacan, L.A.; Soler, A.P.; Mullin, J.M. Tumor necrosis factor-α increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J. Membr. Biol. 1998, 161, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tazuke, Y.; Drongowski, R.A.; Teitelbaum, D.H.; Coran, A.G. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr. Surg. Int. 2003, 19, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Srinivasan, S.; Theiss, A.L.; Merlin, D.; Sitaraman, S. V Interleukin-6 induces keratin expression in intestinal epithelial cells: Potential role of keratin-8 in interleukin-6-induced barrier function alterations. J. Biol. Chem. 2007, 282, 8219–8227. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 Modulation of Intestinal Epithelial Tight Junction Permeability Is Mediated by JNK Pathway Activation of Claudin-2 Gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-J.; Hall, K.; Ha, T.; Li, C.; Krishnaswamy, G.; Chi, D.S. Baicalein inhibits IL-1β- and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-κB pathway. Clin. Mol. Allergy 2007, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Nakao, K.; Sayama, K.; Hamasaki, K.; Kato, Y.; Nakata, K.; Ishii, N.; Butterfield, J.H.; Galli, S.J. The HMC-1 human mast cell line expresses the hepatocyte growth factor receptor c-met. Biochem. Commun. 1997, 239, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Krasagakes, S.; Moller, A.; Kolde, G.; Lippert, U.; Weber, M.; Henz, B.M. Production of interleukin-6 by human mast cells and basophilic cells. J. Invest. Dermatol. 1996, 106, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ro, J.Y. Signal pathway of cytokines produced by reactive oxygen species generated from phorbol myristate acetate-stimulated HMC-1 cells. Scand. J. Immunol. 2005, 62, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jijon, H.B.; Panenka, W.J.; Madsen, K.L.; Parsons, H.G. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am. J. Physiol. Cell. Physiol. 2002, 283, C31–C41. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Hudson, J.T.; Lügering, A.; Abbas, J.A.; Bettini, M.; Lake, J.G.; Evans, M.E.; Ziegler, T.R.; Merlin, D.; Madara, J.L.; Williams, I.R. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 2005, 54, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- DeForge, L.E.; Kenney, J.S.; Jones, M.L.; Warren, J.S.; Remick, D.G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 1992, 148, 2133–2141. [Google Scholar] [PubMed]
- Osawa, Y.; Nagaki, M.; Banno, Y.; Brenner, D.A.; Asano, T.; Nozawa, Y.; Moriwaki, H.; Nakashima, S. Tumor Necrosis Factor A-Induced Interleukin-8 Production via NF-κB and Phosphatidylinositol 3-Kinase/Akt Pathways Inhibits Cell Apoptosis in Human Hepatocytes. Infect. Immun. 2002, 70, 6294–6301. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Lewis, S.A.; Tavernini, M.M.; Hibbard, J.; Fedorak, R.N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997, 113, 151–159. [Google Scholar] [CrossRef]
- Loren, V.; Cabre, E.; Ojanguren, I.; Domenech, E.; Pedrosa, E.; Garcia-Jaraquemada, A.; Manosa, M.; Manye, J. Interleukin-10 Enhances the Intestinal Epithelial Barrier in the Presence of Corticosteroids through p38 MAPK Activity in Caco-2 Monolayers: A Possible Mechanism for Steroid Responsiveness in Ulcerative Colitis. PLoS ONE 2015, 10, e0130921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hasselgren, P.-O. Heat shock response reduces intestinal permeability in septic mice: Potential role of interleukin-10. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R669–R676. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, H.; Nose, K.; Nose, S.; Haxhija, E.Q.; Koga, H.; Feng, Y.; Teitelbaum, D.H. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G139–G147. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell. Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, C.B.; Uno, J.K.; Lee, K.-M.; Karrasch, T.; Laubitz, D.; Thurston, R.; Midura-Kiela, M.T.; Ghishan, F.K.; Sartor, R.B.; Jobin, C.; et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1079–G1091. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, B.; Manjula, N.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFα, IL-1beta, NO and MAP kinases. Int. Immunopharmacol. 2007, 7, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-P.; He, C.-H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Ravindran, B. Isolation of Human PBMCs. Bio-Protocol 2013, 3, e323. [Google Scholar] [CrossRef]
- Sundström, M.; Vliagoftis, H.; Karlberg, P.; Butterfield, J.H.; Nilsson, K.; Metcalfe, D.D.; Nilsson, G. Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology 2003, 108, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell. Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Catanzaro, D.; Cocetta, V.; Igl, N.; Ragazzi, E.; Giron, M.C.; Cecconello, L.; Montopoli, M. Protective effects of psi taraxasterol 3-O-myristate and arnidiol 3-O-myristate isolated from Calendula officinalis on epithelial intestinal barrier. Fitoterapia 2016, 109, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Borgonetti, V.; Ragazzi, E.; Cecilia Giron, M.; Catanzaro, D.; Cecilia, M.; Governa, P.; Carnevali, I.; Biagi, M.; Montopoli, M. A Fixed Combination of Probiotics and Herbal Extracts Attenuates Intestinal Barrier Dysfunction from Inflammatory Stress. Preprints 2017, 2017120199. [Google Scholar]
- Liu, T.; Chang, L.-J.; Uss, A.; Chu, I.; Morrison, R.A.; Wang, L.; Prelusky, D.; Cheng, K.-C.; Li, C. The impact of protein on Caco-2 permeability of low mass balance compounds for absorption projection and efflux substrate identification. J. Pharm. Biomed. Anal. 2010, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Brunet, J.-L.; Badiou, A.; Belzunces, L.P. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicol. In Vitro 2009, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 cell culture practices. Toxicol. In Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.Y.; Youn, G.S.; Choi, S.Y.; Park, J. Butein, a tetrahydroxychalcone, suppresses pro-inflammatory responses in HaCaT keratinocytes. BMB Rep. 2015, 48, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin–Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef] [PubMed]
| Sample | Chemical Marker | Method | % |
|---|---|---|---|
| CUR | total curcuminoids | Ph. Eur. 9th method | 56.85 ± 2.79 |
| total curcuminoids | HPLC-DAD | 56.06 ± 0.76 | |
| curcumin | 49.04 ± 0.40 | ||
| demethoxycurcumin | 5.98 ± 0.11 | ||
| bisdemethoxycurcumin | 1.04 ± 0.03 | ||
| BOS | total triterpenes | Colorimetric method | 68.41 ± 3.33 |
| total boswellic acids* | HPLC-MS HPLC-DAD-ELSD | 39 | |
| KBA* | 5.02 ± 0.09 | ||
| AKBA* | 2.71 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Governa, P.; Marchi, M.; Cocetta, V.; De Leo, B.; Saunders, P.T.K.; Catanzaro, D.; Miraldi, E.; Montopoli, M.; Biagi, M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals 2018, 11, 126. https://doi.org/10.3390/ph11040126
Governa P, Marchi M, Cocetta V, De Leo B, Saunders PTK, Catanzaro D, Miraldi E, Montopoli M, Biagi M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals. 2018; 11(4):126. https://doi.org/10.3390/ph11040126
Chicago/Turabian StyleGoverna, Paolo, Maddalena Marchi, Veronica Cocetta, Bianca De Leo, Philippa T. K. Saunders, Daniela Catanzaro, Elisabetta Miraldi, Monica Montopoli, and Marco Biagi. 2018. "Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2" Pharmaceuticals 11, no. 4: 126. https://doi.org/10.3390/ph11040126
APA StyleGoverna, P., Marchi, M., Cocetta, V., De Leo, B., Saunders, P. T. K., Catanzaro, D., Miraldi, E., Montopoli, M., & Biagi, M. (2018). Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals, 11(4), 126. https://doi.org/10.3390/ph11040126