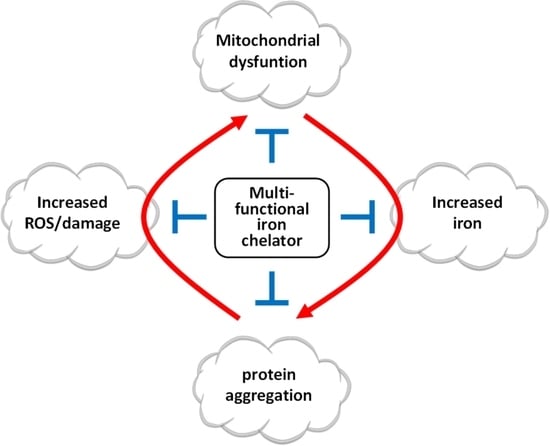
New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases
Abstract
Share and Cite
Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. https://doi.org/10.3390/ph11040109
Nuñez MT, Chana-Cuevas P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals. 2018; 11(4):109. https://doi.org/10.3390/ph11040109
Chicago/Turabian StyleNuñez, Marco T., and Pedro Chana-Cuevas. 2018. "New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases" Pharmaceuticals 11, no. 4: 109. https://doi.org/10.3390/ph11040109
APA StyleNuñez, M. T., & Chana-Cuevas, P. (2018). New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals, 11(4), 109. https://doi.org/10.3390/ph11040109