Iron Release from Soybean Seed Ferritin Induced by Cinnamic Acid Derivatives
Abstract
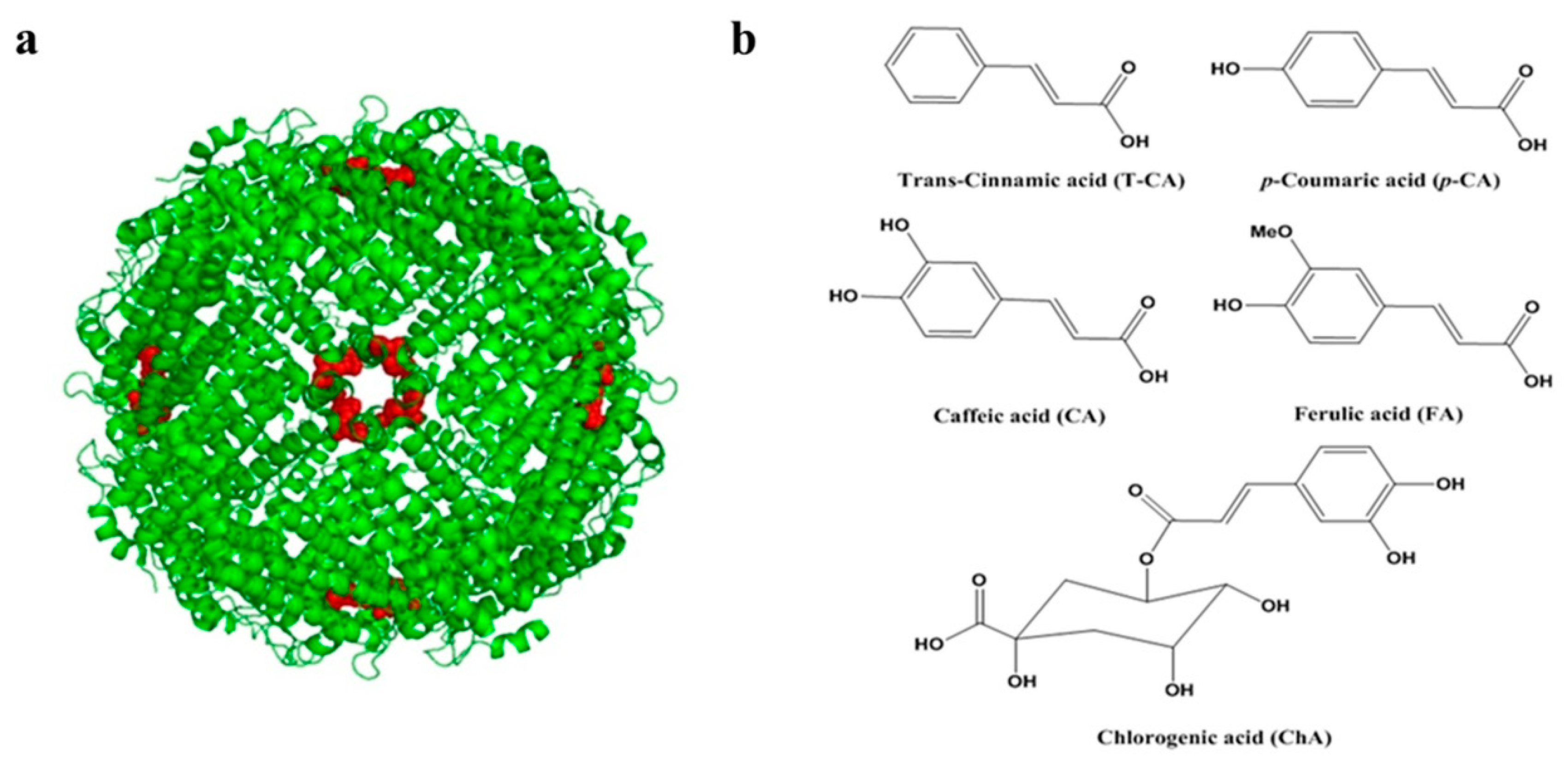
:1. Introduction
2. Results and Discussion
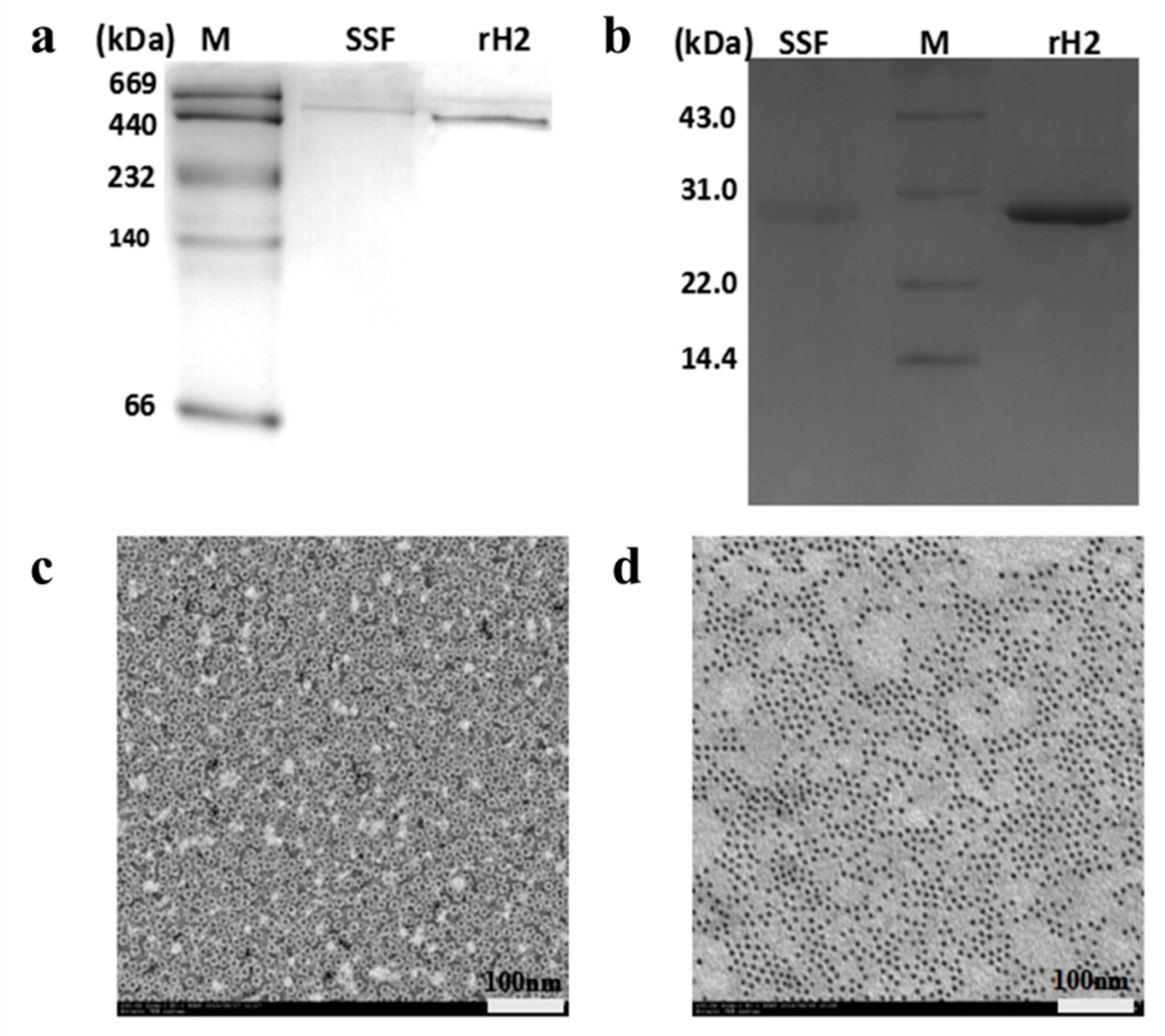
2.1. Isolation and Characterization of SSF and rH-2
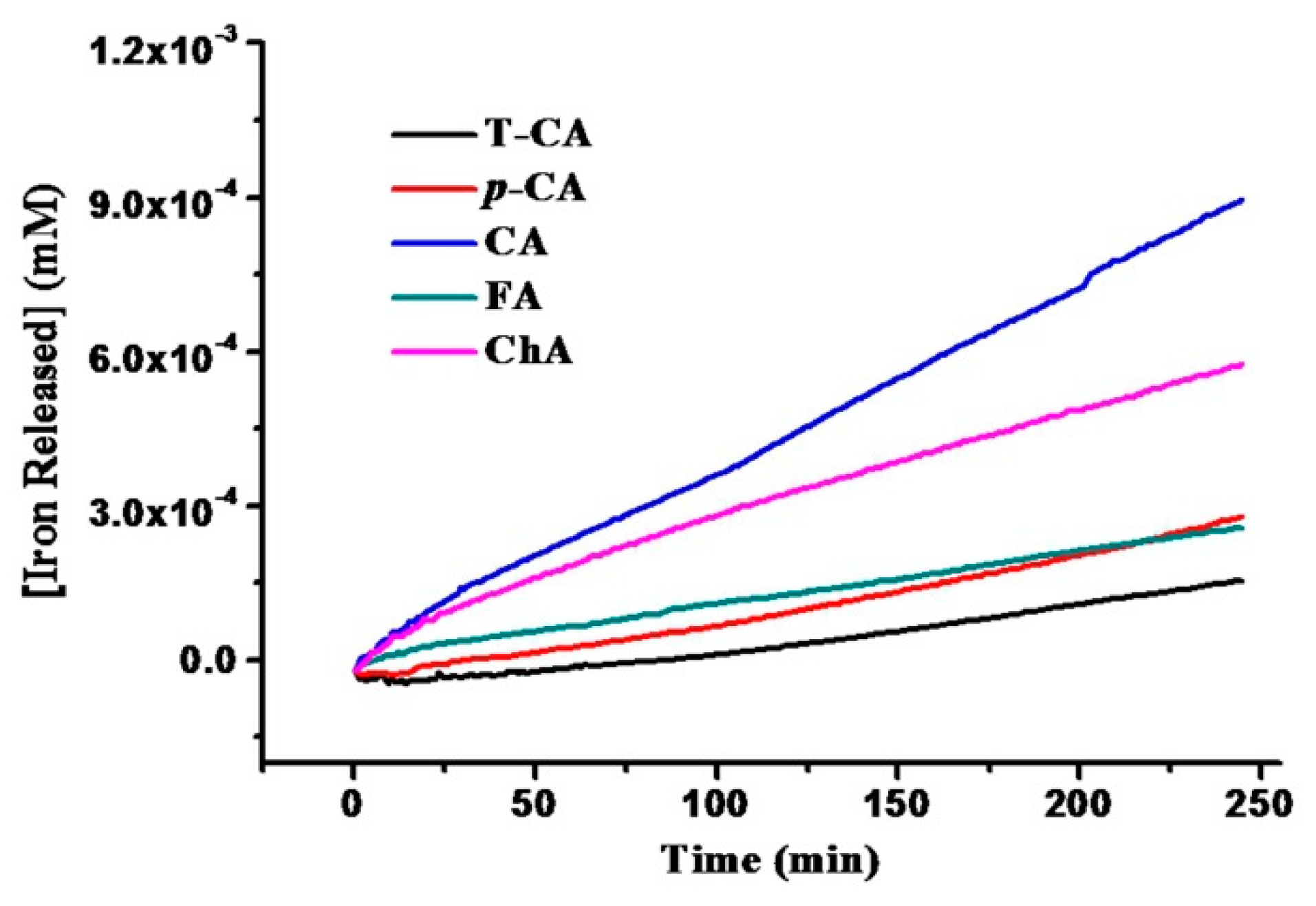
2.2. Iron Release from SSF Induced by Cinnamic Acid Derivatives
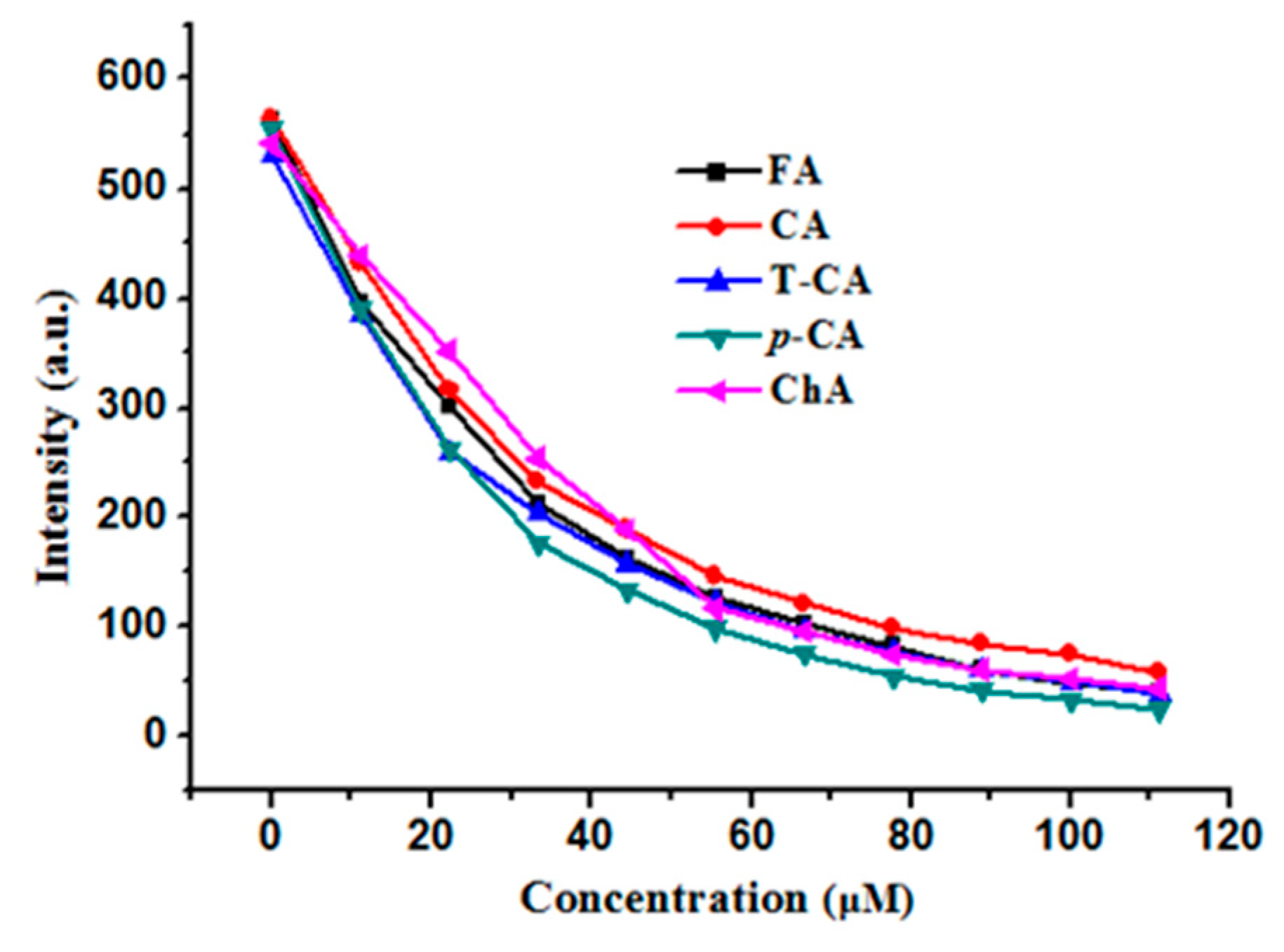
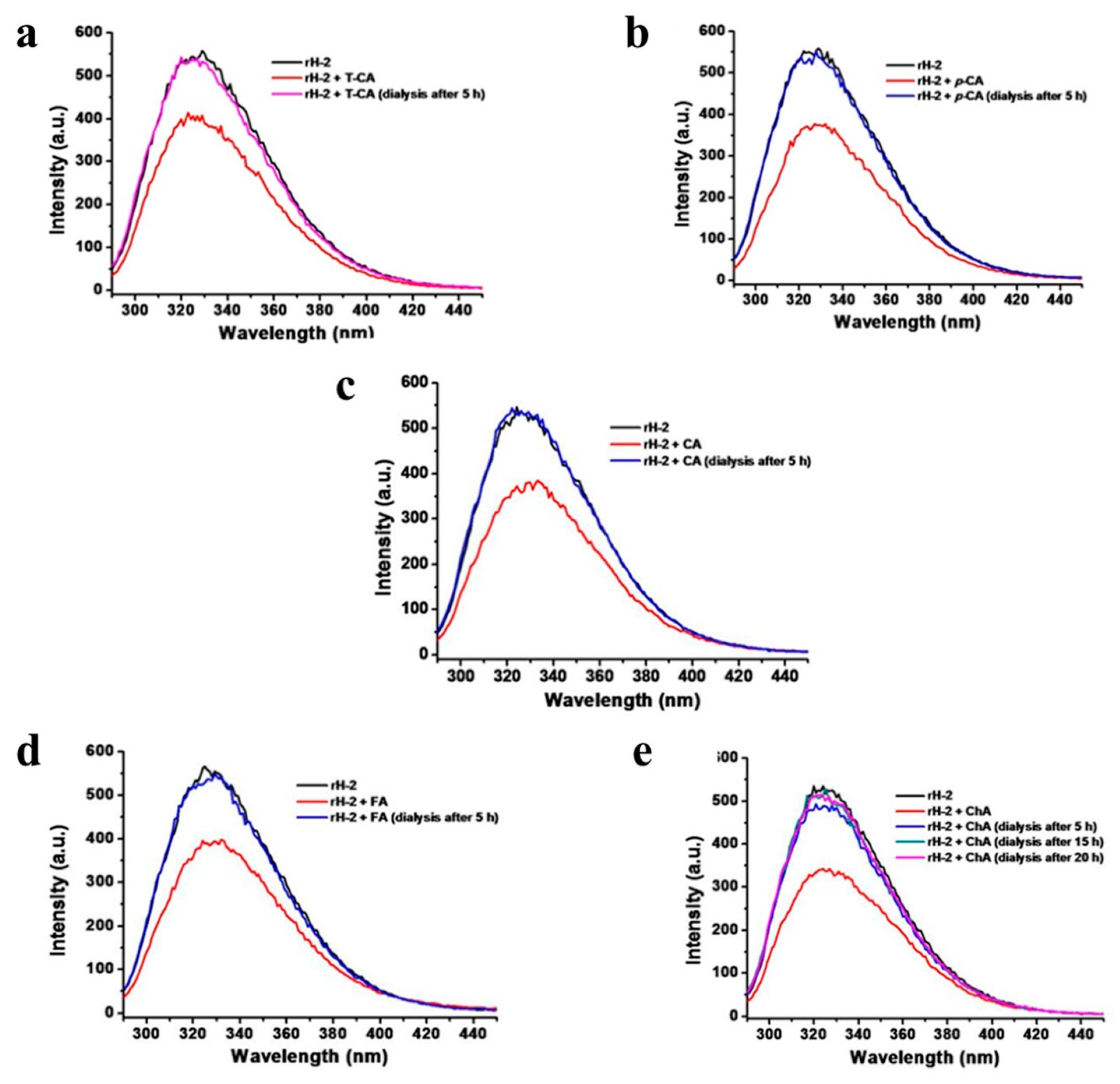
2.3. Fluorescence Quenching Analyses
2.4. Effect of the Fe2+ Chelating Activity of Cinnamic Acid Derivatives on the Iron Release from Ferritin
2.5. Effect of Reducibility of Cinnamic Acid Derivatives on the Iron Release from Ferritin
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Soybean Seed Ferritin (SSF) and Recombinant Soybean Seed H-2 Ferritin (rH-2)
3.3. Kinetic Measurement of Iron Release from Holo Soybean Seed Ferritin
3.4. Fluorescence Titration Analysis
3.5. Chelating Activity of Cinnamic Acid Derivatives on Ferrous Ion
3.6. Cyclic Voltammetry
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, G. Phytoferritin and its implications for human health and nutrition. BBA-Gen. Subj. 2010, 1800, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. BBA-Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Zhao, G.; Bou-Abdallah, F.; Arosio, P.; Levi, S.; Janus-Chandler, C.; Chasteen, N.D. Multiple pathways for mineral core formation in mammalian apoferritin. The role of hydrogen peroxide. Biochemistry 2003, 42, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cheng, J.; Liao, X.; Zhang, T.; Leng, X.; Zhao, G. Comparative study on iron release from Soybean (Glycine max) seed ferritin induced by anthocyanins and ascorbate. J. Agric. Food Chem. 2010, 58, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Bai, Y.; Yang, S.; Zhao, G.; Chen, B. NADH induces iron release from pea seed ferritin: A model for interaction between coenzyme and protein components in foodstuffs. Food Chem. 2013, 14, 3851–3858. [Google Scholar] [CrossRef] [PubMed]
- Castruita, M.; Elmegreen, L.A.; Shaked, Y.; Stiefel, E.I.; Morel, F.M.M. Comparison of the kinetics of iron release from a marine (Trichodesmium erythraeum) Dps protein and mammalian ferritin in the presence and absence of ligands. J. Inorg. Biochem. 2007, 101, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Roman, F.; Roland, F. Iron mobilization from ferritin by chelating agents. J. Inorg. Biochem. 1980, 13, 305–316. [Google Scholar] [CrossRef]
- Laulhere, J.P.; Briat, J.F. Iron release and uptake by plant ferritin: Effects of pH, reduction and chelation. Biochem. J. 1993, 290, 693–699. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents—A review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.; Fischer, T.; Cortes, J.; Garcia-Manero, G.; Beck, J.; Ravandi, F.; Masson, E.; Rae, P.; Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar]
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Lv, C.; Zhao, G.; Lönnerdal, B. Bioavailability of iron from plant and animal ferritins. J. Nutr. Biochem. 2015, 26, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Deng, J.; Yang, H.; Masuda, T.; Goto, F.; Yoshiara, T.; Zhao, G. A novel EP-involved pathway for iron release from soya bean seed ferritin. Biochem. J. 2010, 427, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Goto, F.; Yoshihara, T. A novel plant ferritin subunit from soybean that is related to a mechanism in iron release. J. Biol. Chem. 2001, 276, 19575–19579. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.J.; Coinceanainn, M.Ó. Investigation of the release of iron from ferritin by naturally occuring antioxidants. J. Inorg. Biochem. 2002, 90, 18–21. [Google Scholar] [CrossRef]
- Masuda, T.; Goto, F.; Yoshihara, T.; Mikami, B. Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin. J. Biol. Chem. 2010, 285, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, K.; Ning, Y.; Zhao, G. Effect of the structure of gallic acid and its derivatives on their interaction with plant ferritin. Food Chem. 2016, 213, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jia, X.; Yang, J.; Deng, J.; Zhao, G. Effect of tannic acid on properties of soybean (Glycine max) seed ferritin: A model for interaction between naturallyoccurring components in foodstuffs. Food Chem. 2012, 133, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Boyer, R.F.; McCleary, C.J. Superoxide ion as a primary reductant in ascorbate mediated ferritin iron release. Free Radic. Biol. Med. 1987, 3, 389–395. [Google Scholar] [CrossRef]
- Madrakian, T.; Soleimani, M.; Afkhami, A. Simultaneous determination of mycophenolate mofetil and its active metabolite, mycophenolic acid, by differential pulse voltammetry using multi-walled carbon nanotubes modified glassy carbon electrode. Mat. Sci. Eng. C 2014, 42, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Laulhere, J.P.; Lescure, A.M.; Briat, J.F. Purification and characterization of ferritins from maize, pea, and soyabean seeds. J. Biol. Chem. 1988, 263, 10289–10294. [Google Scholar] [PubMed]
- Masuda, T.; Goto, F.; Yoshihara, T.; Ezure, T.; Suzuki, T.; Kobayashi, S.; Shikata, M.; Utsumi, S. Construction of homo- and heteropolymers of plant ferritin subunits using an in vitro protein expression system. Protein Expr. Purif. 2007, 56, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]

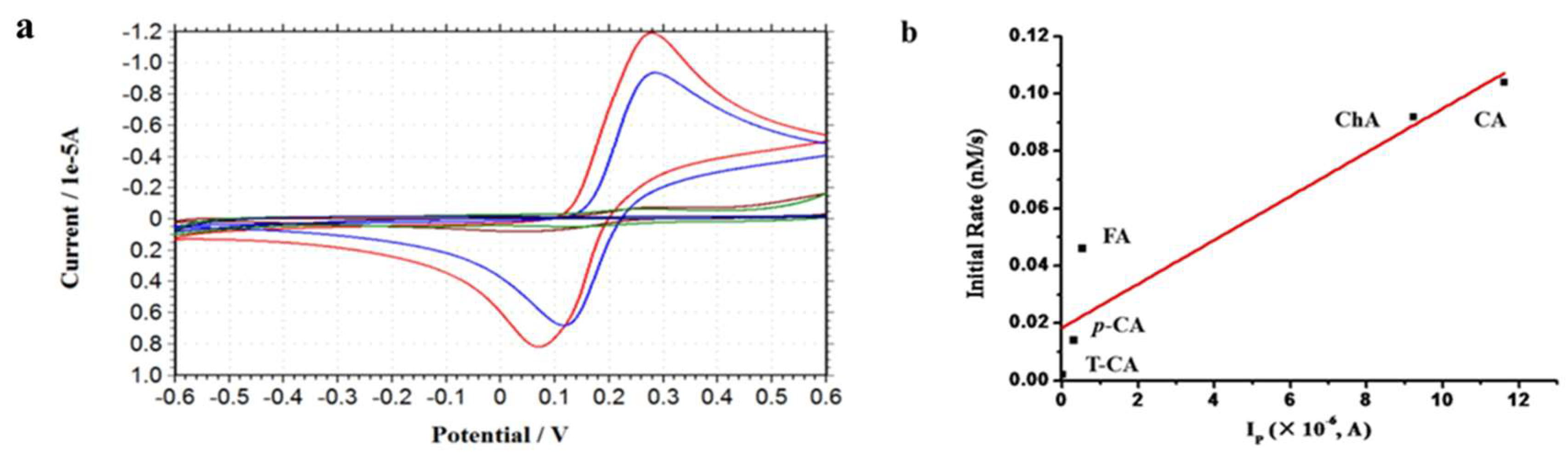
| Phenolic Compound | Ep | Ip (×10−6) |
|---|---|---|
| T-CA | 0 | 0 |
| p-CA | 0.27 | 0.31 ± 0.12 |
| CA | 0.28 | 11.61 ± 0.35 |
| FA | 0.24 | 0.53 ± 0.07 |
| ChA | 0.31 | 9.23 ± 0.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, X.; Chen, H.; Zhang, J.; Zhao, G. Iron Release from Soybean Seed Ferritin Induced by Cinnamic Acid Derivatives. Pharmaceuticals 2018, 11, 39. https://doi.org/10.3390/ph11020039
Sha X, Chen H, Zhang J, Zhao G. Iron Release from Soybean Seed Ferritin Induced by Cinnamic Acid Derivatives. Pharmaceuticals. 2018; 11(2):39. https://doi.org/10.3390/ph11020039
Chicago/Turabian StyleSha, Xuejiao, Hai Chen, Jingsheng Zhang, and Guanghua Zhao. 2018. "Iron Release from Soybean Seed Ferritin Induced by Cinnamic Acid Derivatives" Pharmaceuticals 11, no. 2: 39. https://doi.org/10.3390/ph11020039
APA StyleSha, X., Chen, H., Zhang, J., & Zhao, G. (2018). Iron Release from Soybean Seed Ferritin Induced by Cinnamic Acid Derivatives. Pharmaceuticals, 11(2), 39. https://doi.org/10.3390/ph11020039





