Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis
Abstract
:1. Introduction
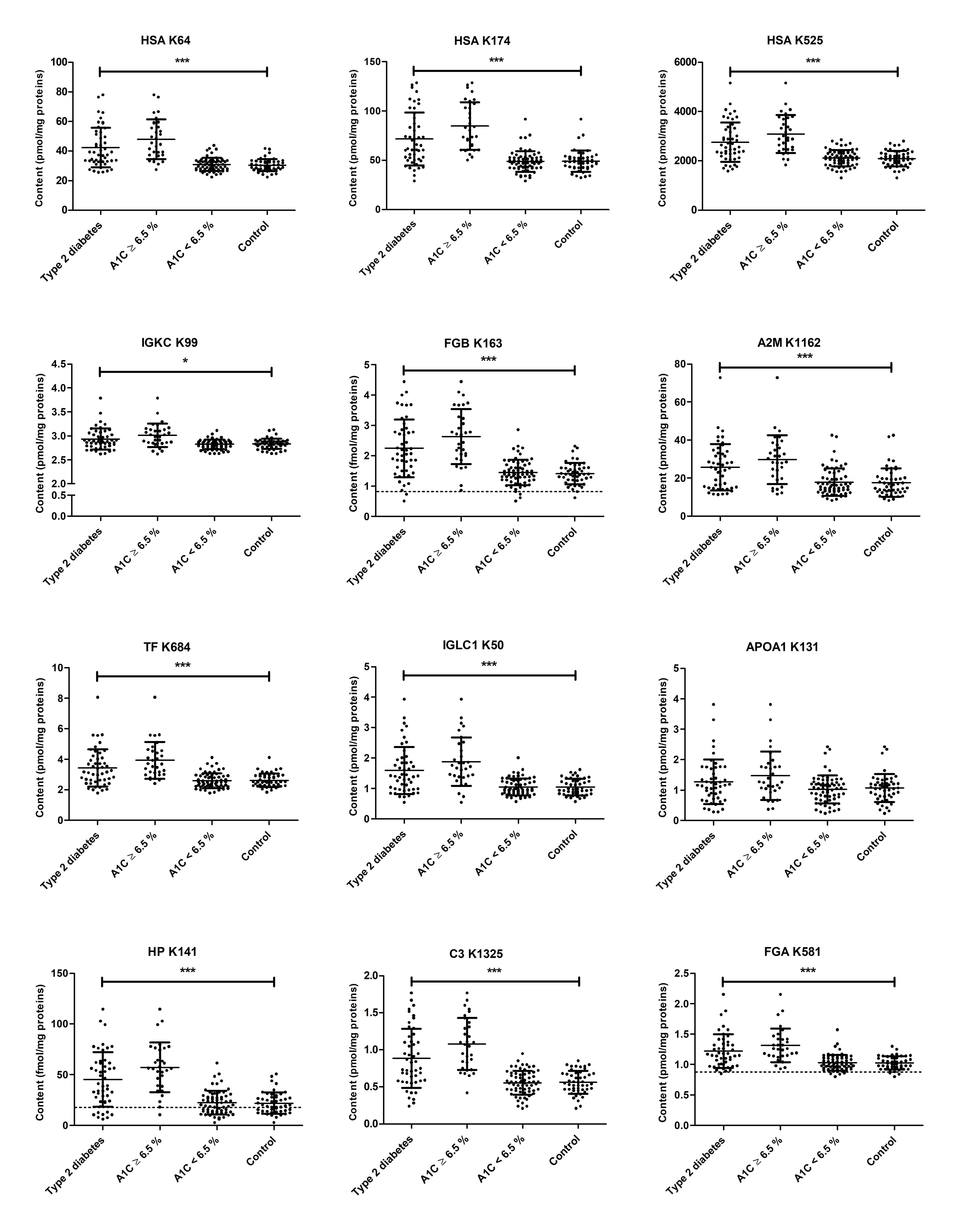
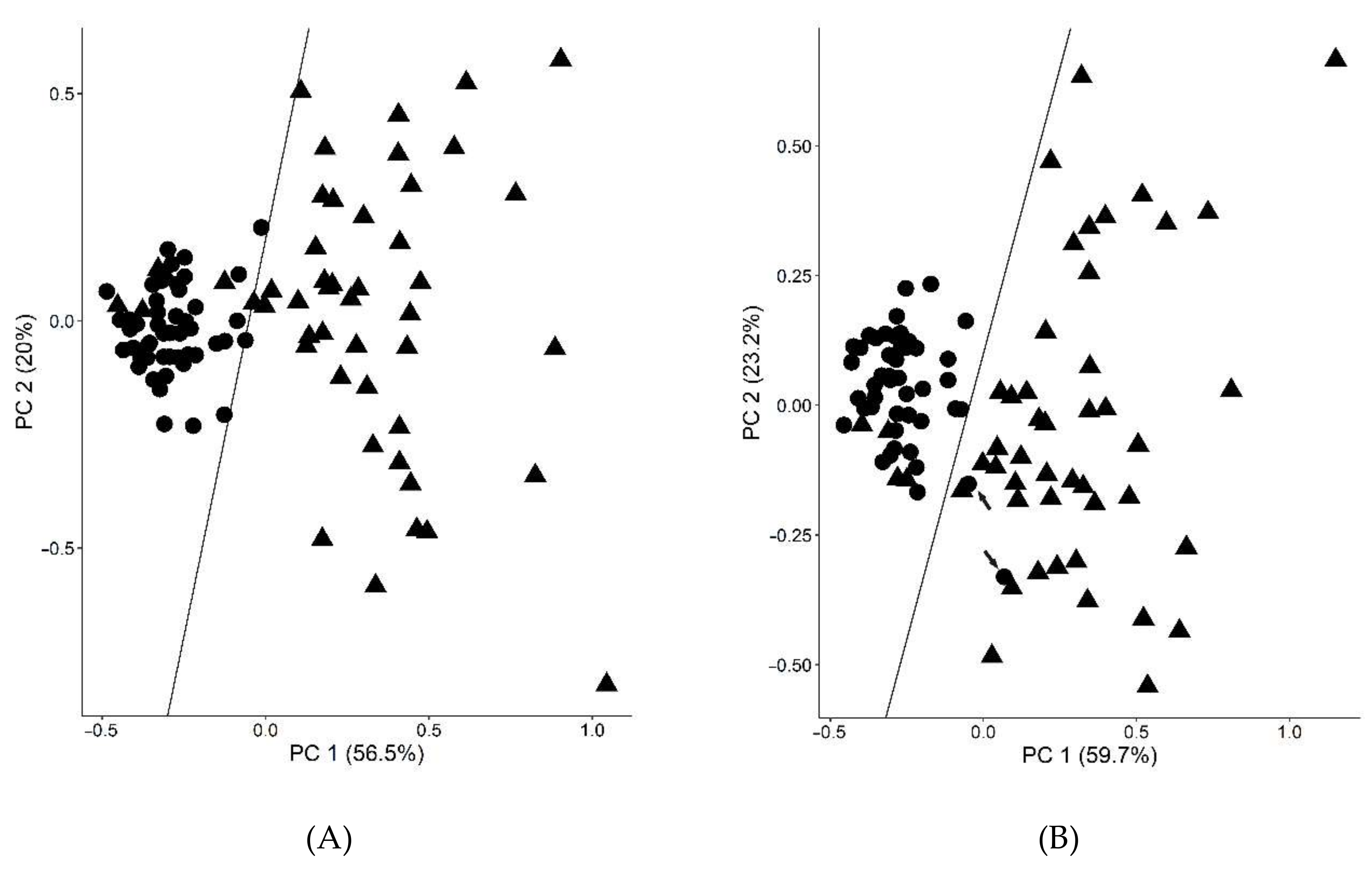
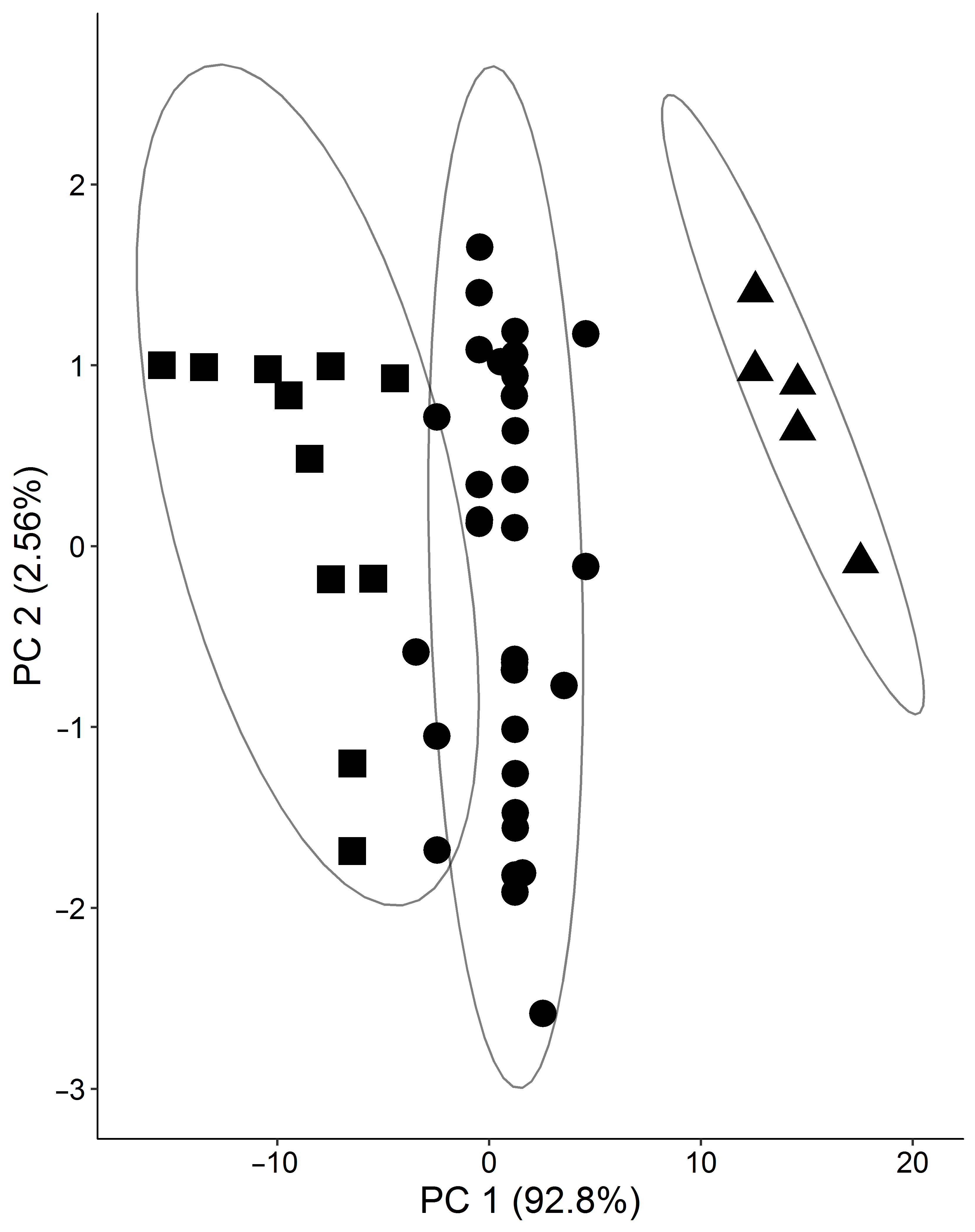
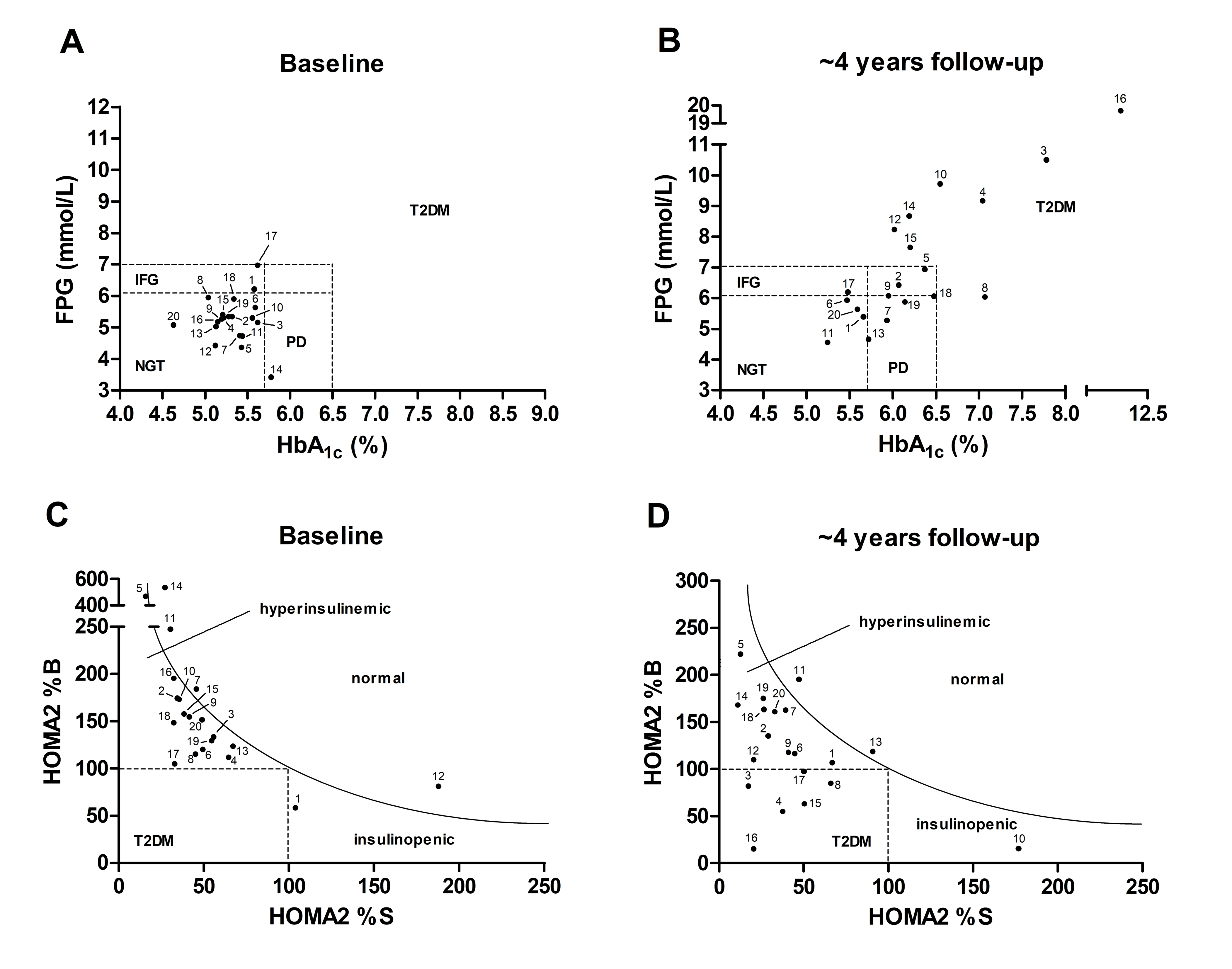
2. Results
2.1. Long-Term Controlled Diabetic Patients
2.2. Prediabetic Patients
3. Discussion
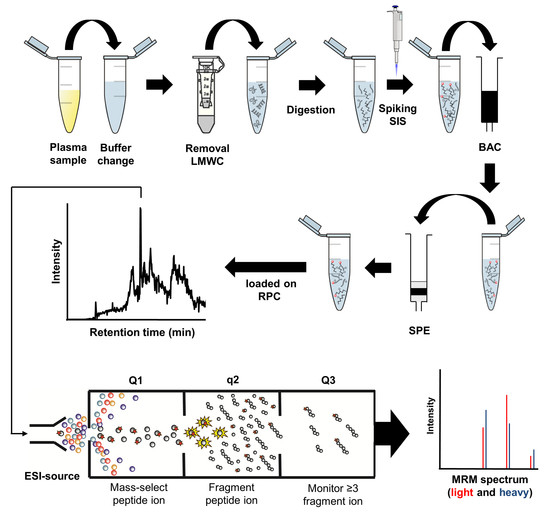
4. Materials and Methods
4.1. Study Participants
4.2. Peptide Quantification
4.3. Statistics and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 7th ed.; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Bullard, K.M.; Saydah, S.H.; Imperatore, G.; Cowie, C.C.; Gregg, E.W.; Geiss, L.S.; Cheng, Y.J.; Rolka, D.B.; Williams, D.E.; Caspersen, C.J. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care 2013, 36, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G.; Tanner, R.J.; Baker, R.; Zayas, C.E.; Harle, C.A. Prevalence of prediabetes in England from 2003 to 2011: Population-based, cross-sectional study. BMJ Open 2014, 4, e005002. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report in Diabetes; WHO: Brussels, Belgium, 2016. [Google Scholar]
- American Diabetes Association (ADA). Classification and Diagnosis of Diabetes. Sec. 2. Diabetes Care 2017, 40 (Suppl. 1), S11–S24. [Google Scholar]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for developing diabetes. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Dunkley, A.J.; Bodicoat, D.H.; Greaves, C.J.; Russell, C.; Yates, T.; Davies, M.J.; Khunti, K. Diabetes prevention in the real world: Effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: A systematic review and meta-analysis. Diabetes Care 2014, 37, 922–933. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Consultation, Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization (WHO). Abbreviated Report of a WHO Consultation, Use of Glycated Hemoglobin (HbA1c) in the Diagnosis If Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Buell, C.; Kermah, D.; Davidson, M.B. Utility of A1C for Diabetes Screening in the 1999–2004 NHANES Population. Diabetes Care 2007, 30, 2233–2235. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Araneta, M.R.G.; Barrett-Connor, E. A1C and Diabetes Diagnosis: The Rancho Bernardo Study. Diabetes Care 2010, 33, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.P.; Reynolds, K.; Fonseca, V.A.; Muntner, P. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010, 33, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Wagenknecht, L.E.; Hanley, A.J.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pang, Z.; Gao, W.; Wang, S.; Zhang, L.; Ning, F.; Qiao, Q. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 2010, 33, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Gosmanov, A.R.; Wan, J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. Am. J. Med. Sci. 2014, 348, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Moellering, D.R.; Garvey, W.T. Use of HbA1c for diagnoses of diabetes and prediabetes: Comparison with diagnoses based on fasting and 2-hr glucose values and effects of gender, race, and age. Metab. Syndr. Relat. Disord. 2014, 12, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Van’t Riet, E.; Alssema, M.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Nijpels, G.; Dekker, J.M. Relationship between A1C and glucose levels in the general Dutch population: The new Hoorn study. Diabetes Care 2010, 33, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Kahn, R. A Step Backward—Or Is it Forward? Diabetes Care 2008, 31, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.M.; Raina, P.S.; Gerstein, H.C.; Santaguida, P.L.; Morrison, K.M.; Booker, L.; Hunt, D.L. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: A systematic review. Clin. Chem. Lab. Med. 2007, 45, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.M.; Barinas-Mitchell, E.; Bartucci, A.; Robertson, R.; Arslanian, S. Reproducibility of the oral glucose tolerance test in overweight children. J. Clin. Endocrinol. Metab. 2008, 93, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A. Fructosamine: Structure, analysis, and clinical usefulness. Clin. Chem. 1987, 33, 2153–2163. [Google Scholar] [PubMed]
- Hill, R.P.; Hindle, E.J.; Howey, J.E.; Lemon, M.; Lloyd, D.R. Recommendations for adopting standard conditions and analytical procedures in the measurement of serum fructosamine concentration. Ann. Clin. Biochem. 1990, 27 Pt 5, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Furusyo, N.; Hayashi, J. Glycated albumin and diabetes mellitus. Biochim. Biophys. Acta 2013, 1830, 5509–5514. [Google Scholar] [CrossRef] [PubMed]
- Anguizola, J.; Matsuda, R.; Barnaby, O.S.; Hoy, K.S.; Wa, C.; DeBolt, E.; Koke, M.; Hage, D.S. Review: Glycation of human serum albumin. Clin. Chim. Acta 2013, 425, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Arasteh, A.; Farahi, S.; Habibi-Rezaei, M.; Moosavi-Movahedi, A.A. Glycated albumin: An overview of the In Vitro models of an In Vivo potential disease marker. J. Diabetes Metab. Disord. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.E.; Little, R.R.; Lorenz, R.A.; Malone, J.I.; Nathan, D.; Peterson, C.M.; Sacks, D.B. Tests of glycemia in diabetes. Diabetes Care 2004, 27, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of Glycated Proteins in the Diagnosis and Management of Diabetes: Research Gaps and Future Directions. Diabetes Care 2016, 39, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Otokozawa, S.; Schaefer, E.J.; Asztalos, B.F.; Nakajima, K.; Shrader, P.; Kathiresan, S.; Meigs, J.B.; Williams, G.; Nathan, D.M. Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin. Chim. Acta 2009, 406, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Steffes, M.W.; Selvin, E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin. Chem. 2012, 58, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; McGee, P.; Steffes, M.W.; Lachin, J.M. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014, 63, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Rawlings, A.M.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: A prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014, 2, 279–288. [Google Scholar] [CrossRef]
- Beck, R.; Steffes, M.; Xing, D.; Ruedy, K.; Mauras, N.; Wilson, D.M.; Kollman, C. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediat. Diabetes 2011, 12, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, C.M.; Selvin, E. Beyond HbA1c and glucose: The role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr. Diabetes Rep. 2014, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Hoffmann, R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal. Bioanal. Chem. 2010, 397, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Monroe, M.E.; Schepmoes, A.A.; Clauss, T.R.W.; Gritsenko, M.A.; Meng, D.; Petyuk, V.A.; Smith, R.D.; Metz, T.O. Comprehensive Identification of Glycated Peptides and Their Glycation Motifs in Plasma and Erythrocytes of Control and Diabetic Subjects. J. Proteome Res. 2011, 10, 3076–3088. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Blüher, M.; Hoffmann, R. Glycation sites of human plasma proteins are affected to different extents by hyperglycemic conditions in type 2 diabetes mellitus. Anal. Bioanal. Chem. 2014, 406, 5755–5763. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Frolov, A.; Hoffmann, R. Quantification of specific glycation sites in human serum albumin as prospective type 2 diabetes mellitus biomarkers. Protein Pept. Lett. 2017, 24, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Spiller, S.; Li, Y.; Blüher, M.; Welch, L.; Hoffmann, R. Glycated lysine-141 in haptoglobin improves the diagnostic accuracy for type 2 diabetes mellitus in combination with glycated hemoglobin HbA(1c) and fasting plasma glucose. Clin. Proteom 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.; Worwood, M. Haptoglobin: A review of the major allele frequencies worldwide and their association with diseases. Int. J. Lab. Hematol. 2007, 29, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B. Multiple Biomarker Prediction of Type 2 Diabetes. Diabetes Care 2009, 32, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schon, M.R.; Kern, M.; Stumvoll, M.; Bluher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Pfenninger, A.; Teichert, L.; Tonjes, A.; Dietrich, A.; Schon, M.R.; Kloting, N.; Bluher, M. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 2015, 58, 799–808. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association (ADA). Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. 1), 67–74. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Hunter, C.L. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteom. 2006, 5, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Hoffmann, R. Analysis of Amadori Peptides Enriched by Boronic Acid Affinity Chromatography. Ann. N. Y. Acad. Sci. 2008, 1126, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Chen, L.; Xuan, J.; Wang, C.; Shih Ie, M.; Wang, Y.; Zhang, Z.; Hoffman, E.; Clarke, R. Knowledge-guided multi-scale independent component analysis for biomarker identification. BMC Bioinform. 2008, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Granitto, P.M.; Furlanello, C.; Biasioli, F.; Gasperi, F. Recursive feature elimination with random forest for PTR-MS analysis of agroindustrial products. Chemom. Intell. Lab. Syst. 2006, 83, 83–90. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Von Luxburg, U. Clustering Stability: An Overview. Found. Trends Mach. Learn. 2009, 2, 235–274. [Google Scholar]
- Ketchen, D.J.; Shook, C.L. The application of cluster analysis in strategic management research: An analysis and critique. Strateg. Manag. J. 1996, 17, 441–458. [Google Scholar] [CrossRef]
- Florkowski, C.M. Sensitivity, Specificity, Receiver-Operating Characteristic (ROC) Curves and Likelihood Ratios: Communicating the Performance of Diagnostic Tests. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S83–S87. [Google Scholar] [PubMed]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiller, S.; Li, Y.; Blüher, M.; Welch, L.; Hoffmann, R. Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis. Pharmaceuticals 2018, 11, 38. https://doi.org/10.3390/ph11020038
Spiller S, Li Y, Blüher M, Welch L, Hoffmann R. Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis. Pharmaceuticals. 2018; 11(2):38. https://doi.org/10.3390/ph11020038
Chicago/Turabian StyleSpiller, Sandro, Yichao Li, Matthias Blüher, Lonnie Welch, and Ralf Hoffmann. 2018. "Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis" Pharmaceuticals 11, no. 2: 38. https://doi.org/10.3390/ph11020038
APA StyleSpiller, S., Li, Y., Blüher, M., Welch, L., & Hoffmann, R. (2018). Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis. Pharmaceuticals, 11(2), 38. https://doi.org/10.3390/ph11020038