Intravenous Irons: From Basic Science to Clinical Practice
Abstract
1. Introduction
2. Iron Deficiency Anaemia
2.1. Pathophysiology of IDA
2.2. Iron Supplementation Strategies
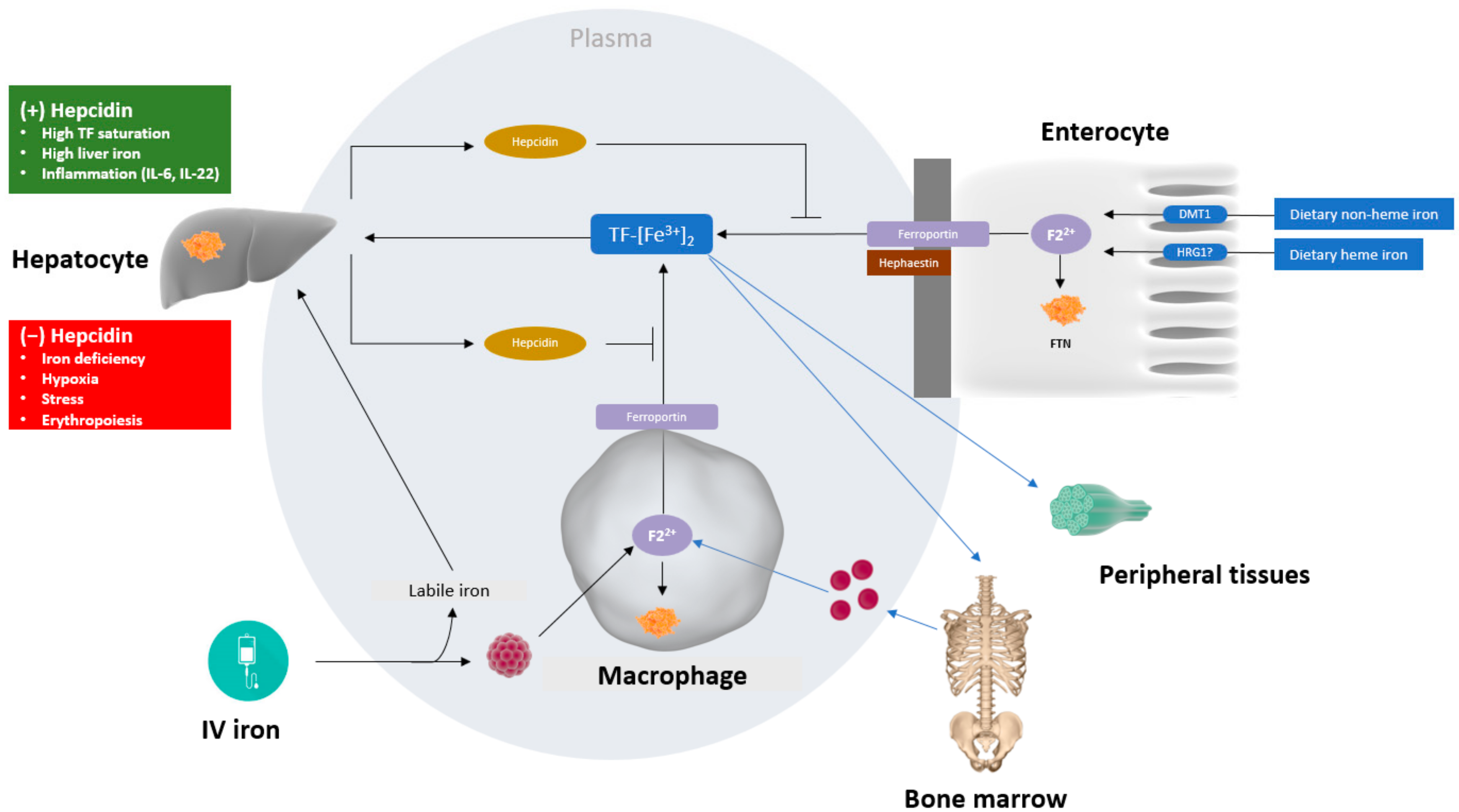
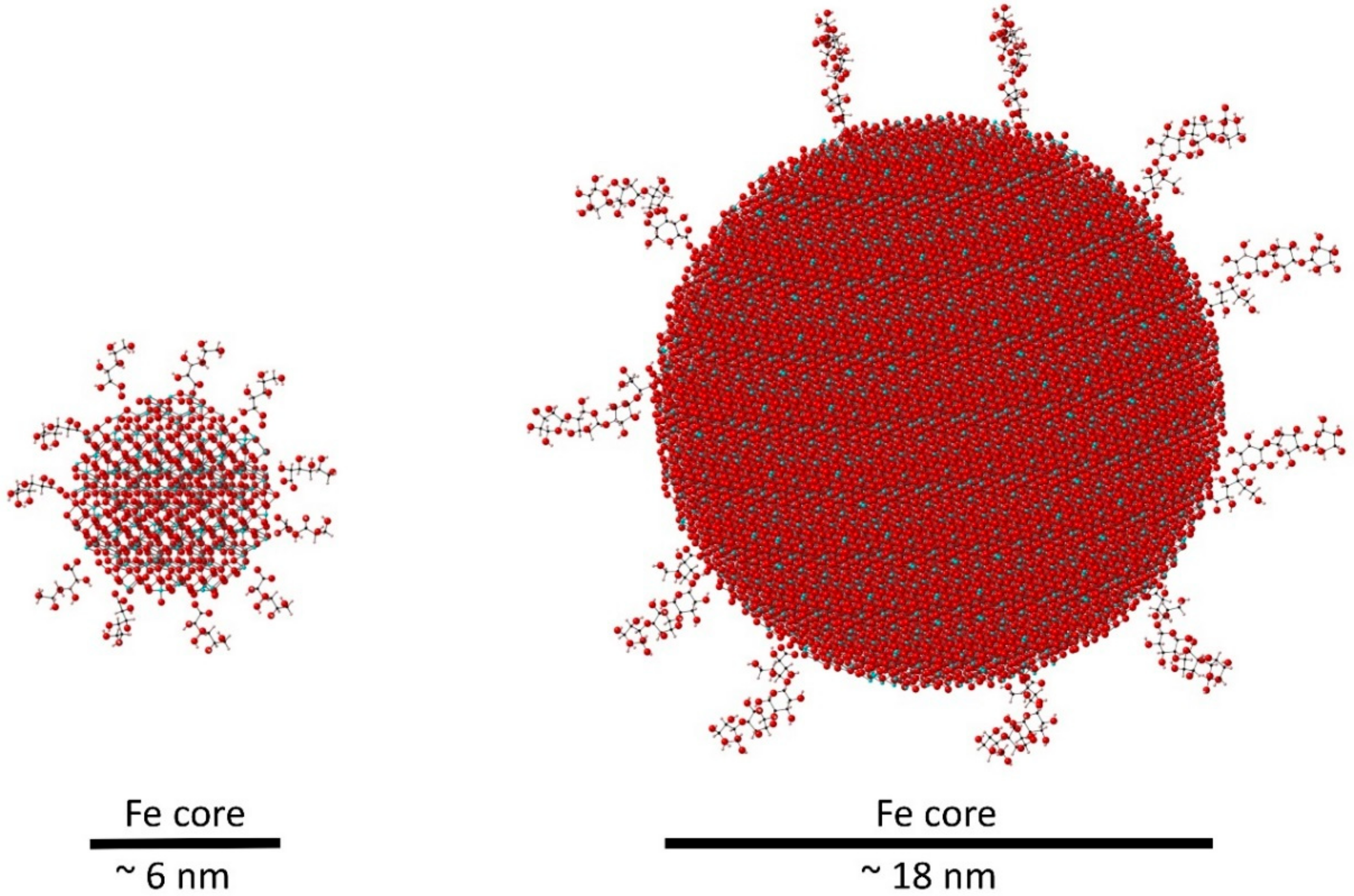
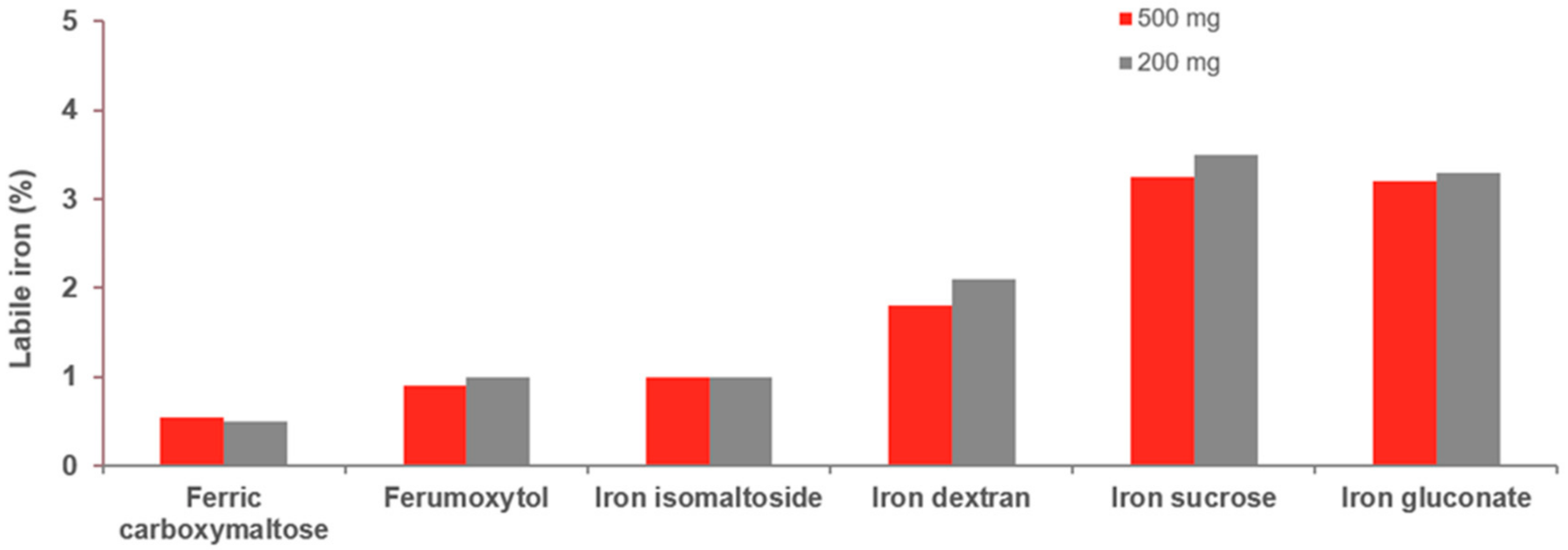
3. Bioengineering and Metabolism of IV Iron
Regulatory View of IV Iron Formulations and Bioequivalence
4. Clinical Use of IV Iron
Evolving Evidence Base Identifies Differences between Third-Generation IV Irons
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and iron: Current questions. Expert Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.P.; Shen, M.; Eisenstein, R.S.; Leibold, E.A. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta 2012, 1823, 1468–1483. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 2015, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Butcher, A.; Richards, T. Cornerstones of patient blood management in surgery. Transfus. Med. 2017, 28, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; Xiao, J.; Ho, K.M. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: Systematic review and meta-analysis of randomised clinical trials. BMJ 2013, 347. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Finberg, K.E.; Heeney, M.M.; Campagna, D.R.; Aydinok, Y.; Pearson, H.A.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in tmprss6 cause iron-refractory iron deficiency anemia (irida). Nat. Genet. 2008, 40, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Block, G.A.; Loram, L.; Neylan, J.; Pergola, P.E.; Uhlig, K.; Chertow, G.M. Effects of ferric citrate in patients with nondialysis-dependent ckd and iron deficiency anemia. J. Am. Soc. Nephrol. 2017, 28, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron and hepcidin: A story of recycling and balance. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J. Regulation of iron metabolism by hepcidin under conditions of inflammation. J. Biol. Chem. 2015, 290, 18975–18983. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Spinowitz, B. Update on anemia in esrd and earlier stages of ckd: Core curriculum 2018. Am. J. Kidney Dis. 2018, 71, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S. Balance of benefit and risk in intravenous iron treatment in chronic kidney disease. Semin. Nephrol. 2016, 36, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Munoz, M.; Macdougall, I.C. Intravenous iron: Out of sight, out of mind. Lancet Haematol. 2018, 5, e10–e12. [Google Scholar] [CrossRef]
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine 2014, 10, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in ckd patients: A. randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Shafie, E.H.; Keshavarz, S.A.; Kefayati, M.E.; Taheri, F.; Sarbakhsh, P.; Vafa, M.R. The effects of nanoparticles containing iron on blood and inflammatory markers in comparison to ferrous sulfate in anemic rats. Int. J. Prev. Med. 2016, 7. [Google Scholar] [CrossRef]
- Garces, V.; Rodriguez-Nogales, A.; Gonzalez, A.; Galvez, N.; Rodriguez-Cabezas, M.E.; Garcia-Martin, M.L.; Gutierrez, L.; Rondon, D.; Olivares, M.; Galvez, J.; et al. Bacteria-carried iron oxide nanoparticles for treatment of anemia. Bioconjug. Chem. 2018, 29, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(iii) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine 2014, 10, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Ahmad, T.; Tulassay, Z.; Baumgart, D.C.; Bokemeyer, B.; Buning, C.; Howaldt, S.; Stallmach, A.; Group, A.S. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: Results from a phase-3 clinical trial program. Inflamm. Bowel Dis. 2015, 21, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, K.A.; Brown, F.; Hawley, C.M.; Leary, D.; Noble, E.; Campbell, S.B.; Isbel, N.M.; Mudge, D.W.; van Eps, C.L.; Johnson, D.W. A randomized controlled trial of oral heme iron polypeptide versus oral iron supplementation for the treatment of anaemia in peritoneal dialysis patients: Hematocrit trial. Nephrol. Dial. Transplant. 2012, 27, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, S.P.; Cohn, A.; Akbari, A.; Davis, J.L.; Zimmerman, D.L. Heme iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: A randomized controlled trial. BMC Nephrol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Macdougall, I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial. Int. 2017, 21 (Suppl. 1), S83–S92. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.R.; Andreasen, H.B.; Futterer, S.; Nawroth, T.; Schunemann, V.; Kolb, U.; Hofmeister, W.; Munoz, M.; Bock, K.; Meldal, M.; et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (monofer), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011, 78, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Lubas, A.; Fiedor, P.; Fiedor, M.; Niemczyk, S. Safety and efficacy of intravenous administration of iron preparations. Acta Pol. Pharm. 2017, 74, 13–24. [Google Scholar] [PubMed]
- Neiser, S.; Koskenkorva, T.S.; Schwarz, K.; Wilhelm, M.; Burckhardt, S. Assessment of dextran antigenicity of intravenous iron preparations with enzyme-linked immunosorbent assay (elisa). Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Angerosa, M. Optimizing iron delivery in the management of anemia: Patient considerations and the role of ferric carboxymaltose. Drug Des. Dev. Ther. 2014, 8, 2475–2491. [Google Scholar] [CrossRef] [PubMed]
- Neiser, S.; Rentsch, D.; Dippon, U.; Kappler, A.; Weidler, P.G.; Gottlicher, J.; Steininger, R.; Wilhelm, M.; Braitsch, M.; Funk, F.; et al. Physico-chemical properties of the new generation iv iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. Biometals 2015, 28, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.B. Complexity of intravenous iron nanoparticle formulations: Implications for bioequivalence evaluation. Ann. N. Y. Acad. Sci. 2017, 1407, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Geisser, P.; Burckhardt, S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Chappell, H.F.; Thom, W.; Bowron, D.T.; Faria, N.; Hasnip, P.J.; Powell, J.J. Structure of naturally hydrated ferrihydrite revealed through neutron diffraction and first-principles modeling. Phys. Rev. Mater. 2017, 1. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sontum, P.C.; Oulie, I. In vitro stability analyses as a model for metabolism of ferromagnetic particles (clariscan), a contrast agent for magnetic resonance imaging. J. Pharm. Biomed. Anal. 2002, 28, 323–329. [Google Scholar] [CrossRef]
- Maneeprakorn, W.; Maurizi, L.; Siriket, H.; Wutikhun, T.; Dharakul, T.; Hofmann, H. Superparamagnetic nanohybrids with cross-linked polymers providing higher in vitro stability. J. Mater. Sci. 2017, 52, 9249–9261. [Google Scholar] [CrossRef]
- Corna, G.; Campana, L.; Pignatti, E.; Castiglioni, A.; Tagliafico, E.; Bosurgi, L.; Campanella, A.; Brunelli, S.; Manfredi, A.A.; Apostoli, P.; et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 2010, 95, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch. 2017, 469, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Vernon, K. Complement activation-related pseudo-allergy: A fresh look at hypersensitivity reactions to intravenous iron. Am. J. Nephrol. 2017, 45, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Gomez-Ramirez, S.; Bhandari, S. The safety of available treatment options for iron-deficiency anemia. Expert Opin. Drug Saf. 2018, 17, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Slotki, I.; Cabantchik, Z.I. The labile side of iron supplementation in ckd. J. Am. Soc. Nephrol. 2015, 26, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Lamplugh, A.; Naudeer, S.; Edey, M.; Bhandari, S. Efficacy and tolerability of accelerated-dose low-molecular-weight iron dextran (cosmofer) in patients with chronic kidney disease. Am. J. Nephrol. 2012, 35, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chiu, D.; Peebles, G.; Swoboda, P.; Kolakkat, S.; Lamerton, E.; Fenwick, S.; Bhandari, S.; Kalra, P.A. Accelerated total dose infusion of low molecular weight iron dextran is safe and efficacious in chronic kidney disease patients. QJM 2011, 104, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Mason, P.D.; Vaage-Nilsen, O.; Ahlmen, J. Update on adverse drug events associated with parenteral iron. Nephrol. Dial. Transplant. 2006, 21, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Pappadakis, J.A.; Bahrain, H.; Auerbach, S.A.; Ballard, H.; Dahl, N.V. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (infed) for the treatment of iron deficient anemia. Am. J. Hematol. 2011, 86, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Rampton, D.; Folkersen, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Szebeni, J.; Weiss, G. Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 2014, 99, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, T.; Philipp, E.; Borchard, G. Iron sucrose: Assessing the similarity between the originator drug and its intended copies. Ann. N. Y. Acad. Sci. 2017, 1407, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; Giani, J.F.; Dominici, F.P.; Angerosa, M. Nitrosative stress and apoptosis by intravenous ferumoxytol, iron isomaltoside 1000, iron dextran, iron sucrose, and ferric carboxymaltose in a nonclinical model. Drug Res. 2015, 65, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Rottembourg, J.; Guerin, A.; Diaconita, M.; Kadri, A. The complete study of the switch from iron-sucrose originator to iron-sucrose similar and vice versa in hemodialysis patients. J. Kidney 2016, 2. [Google Scholar] [CrossRef]
- Rottembourg, J.; Kadri, A.; Leonard, E.; Dansaert, A.; Lafuma, A. Do two intravenous iron sucrose preparations have the same efficacy? Nephrol. Dial. Transplant. 2011, 26, 3262–3267. [Google Scholar] [CrossRef] [PubMed]
- Aguera, M.L.; Martin-Malo, A.; Alvarez-Lara, M.A.; Garcia-Montemayor, V.E.; Canton, P.; Soriano, S.; Aljama, P. Efficiency of original versus generic intravenous iron formulations in patients on haemodialysis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Dignass, A.; Chow, K.U. Clinical case reports raise doubts about the therapeutic equivalence of an iron sucrose similar preparation compared with iron sucrose originator. Curr. Med. Res. Opin. 2012, 28, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Martin-Malo, A.; Merino, A.; Carracedo, J.; Alvarez-Lara, M.A.; Ojeda, R.; Soriano, S.; Crespo, R.; Ramirez, R.; Aljama, P. Effects of intravenous iron on mononuclear cells during the haemodialysis session. Nephrol. Dial. Transplant. 2012, 27, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Park, B.R.; Kim, J.S.; Choi, G.Y.; Lee, J.J.; Lee, I.S. Comparison of adverse event profile of intravenous iron sucrose and iron sucrose similar in postpartum and gynecologic operative patients. Curr. Med. Res. Opin. 2013, 29, 141–147. [Google Scholar] [CrossRef] [PubMed]
- FDA. Draft Guidance on Iron Sucrose. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM297630.pdf (accessed on 15 March 2018).
- FDA. Draft Guidance on Sodium Ferric Gluconate Complex. Available online: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358142.pdf (accessed on 15 March 2018).
- EMA. Reflection Paper on the Data Requirements for Intravenous Iron-Based Nano-Colloidal Products Developed with Reference to an Innovator Medicinal Product. Ema/chmp/swp/620008/2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184922 (accessed on 23 February 2018).
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- EU Clinical Trials Register. UK Multicentre Open-Label Randomised Controlled Trial of Iv Iron Therapy in Incident Haemodialysis Patients. 2013-002267-25. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search (accessed on 07 June 2018).
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Meier, Y.; Larroque, S.; Roger, S.D.; FIND-CKD Study investigators. Renal function in patients with non-dialysis chronic kidney disease receiving intravenous ferric carboxymaltose: An analysis of the randomized find-ckd trial. BMC Nephrol. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.A.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Van Wyck, D.B.; Bansal, S.S.; Cronin, M.; Meier, Y.; Larroque, S.; Roger, S.D.; et al. Hepcidin response to iron therapy in patients with non-dialysis dependent ckd: An analysis of the find-ckd trial. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.A.; Bhandari, S.; Saxena, S.; Agarwal, D.; Wirtz, G.; Kletzmayr, J.; Thomsen, L.L.; Coyne, D.W. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol. Dial. Transplant. 2016, 31, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Bhandari, S. Correction of iron deficiency anaemia using iv cosmofer in ckd patients with asthma: A prospective study. QJM 2016, 109, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Di Gennaro, F. Switching patients with non-dialysis chronic kidney disease from oral iron to intravenous ferric carboxymaltose: Effects on erythropoiesis-stimulating agent requirements, costs, hemoglobin and iron status. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D.; Investigators, F.-C.S. Find-ckd: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Kalra, P.A.; Kothari, J.; Ambuhl, P.M.; Christensen, J.H.; Essaian, A.M.; Thomsen, L.L.; Macdougall, I.C.; Coyne, D.W. A randomized, open-label trial of iron isomaltoside 1000 (monofer(r)) compared with iron sucrose (venofer(r)) as maintenance therapy in haemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Kuji, T.; Toya, Y.; Fujikawa, T.; Kakimoto-Shino, M.; Nishihara, M.; Shibata, K.; Tamura, K.; Hirawa, N.; Satta, H.; Kawata, S.; et al. Acceleration of iron utilization after intravenous iron administration during activated erythropoiesis in hemodialysis patients: A randomized study. Ther. Apher. Dial. 2015, 19, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Strauss, W.E.; McLaughlin, J.; Li, Z.; Dellanna, F.; Hertel, J. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with ckd. Clin. J. Am. Soc. Nephrol. 2014, 9, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.E.; Bregman, D.B.; Harrington, R.A.; Morris, D.; Buerkert, J.; Hamerski, D.; Iftikhar, H.; Mangoo-Karim, R.; Martin, E.R.; Martinez, C.O.; et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: The repair-ida trial. Nephrol. Dial. Transplant. 2014, 29, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Arogundade, F.A.; Soyinka, F.O.; Sanusi, A.A.; Ojo, O.E.; Akinsola, A. Iron status and benefit of the use of parenteral iron therapy in pre-dialysis chronic kidney disease patients. Niger. Postgrad. Med. J. 2013, 20, 299–304. [Google Scholar] [PubMed]
- Charytan, C.; Bernardo, M.V.; Koch, T.A.; Butcher, A.; Morris, D.; Bregman, D.B. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: A randomized, active-controlled, multi-center study. Nephrol. Dial. Transplant. 2013, 28, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, B.; Bhandari, S.; Barany, P.; Kalra, P.A.; Ladefoged, S.; Wilske, J.; Thomsen, L.L. Iron isomaltoside 1000: A new intravenous iron for treating iron deficiency in chronic kidney disease. J. Nephrol. 2011, 24, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, L.; Acharya, S. Efficacy of iv iron compared to oral iron for increment of haemoglobin level in anemic chronic kidney disease patients on erythropoietin therapy. J. Nepal Med. Assoc. 2011, 51, 133–136. [Google Scholar]
- Qunibi, W.Y.; Martinez, C.; Smith, M.; Benjamin, J.; Mangione, A.; Roger, S.D. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol. Dial. Transplant. 2011, 26, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Covic, A.; Mircescu, G. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: A multi-centre, open-label, clinical study. Nephrol. Dial. Transplant. 2010, 25, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Bailie, G.R.; Mason, N.A.; Valaoras, T.G. Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial. Int. 2010, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Spinowitz, B.S.; Kausz, A.T.; Baptista, J.; Noble, S.D.; Sothinathan, R.; Bernardo, M.V.; Brenner, L.; Pereira, B.J. Ferumoxytol for treating iron deficiency anemia in ckd. J. Am. Soc. Nephrol. 2008, 19, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Rizkala, A.R.; Bastani, B.; Kaskas, M.O.; Leehey, D.J.; Besarab, A. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am. J. Nephrol. 2006, 26, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Mircescu, G.; Garneata, L.; Capusa, C.; Ursea, N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol. Dial. Transplant. 2006, 21, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Van Wyck, D.B.; Roppolo, M.; Martinez, C.O.; Mazey, R.M.; McMurray, S.; United States Iron Sucrose Clinical Trials, G. A randomized, controlled trial comparing iv iron sucrose to oral iron in anemic patients with nondialysis-dependent ckd. Kidney Int. 2005, 68, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Charytan, C.; Qunibi, W.; Bailie, G.R.; Venofer Clinical Studies, G. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin. Pract. 2005, 100, C55–C62. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.L.; Morris, D.; Warady, B.A. Comparison of the safety and efficacy of 3 iron sucrose iron maintenance regimens in children, adolescents, and young adults with ckd: A randomized controlled trial. Am. J. Kidney Dis. 2013, 61, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kshirsagar, A.V.; Brookhart, M.A. Safety of intravenous iron in hemodialysis patients. Hemodial. Int. 2017, 21 (Suppl. 1), S93–S103. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, G.R.; Bennett, W.M.; Blumenthal, S.; Charytan, C.; Pennell, J.P.; Reed, J.; Rothstein, M.; Strom, J.; Wolfe, A.; Van Wyck, D.; et al. Iron sucrose in hemodialysis patients: Safety of replacement and maintenance regimens. Kidney Int. 2004, 66, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Chandler, G.; Harchowal, J.; Macdougall, I.C. Intravenous iron sucrose: Establishing a safe dose. Am. J. Kidney Dis. 2001, 38, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Charytan, C.; Levin, N.; Al-Saloum, M.; Hafeez, T.; Gagnon, S.; Van Wyck, D.B. Efficacy and safety of iron sucrose for iron deficiency in patients with dialysis-associated anemia: North American clinical trial. Am. J. Kidney Dis. 2001, 37, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Van Wyck, D.B.; Cavallo, G.; Spinowitz, B.S.; Adhikarla, R.; Gagnon, S.; Charytan, C.; Levin, N. Safety and efficacy of iron sucrose in patients sensitive to iron dextran: North American clinical trial. Am. J. Kidney Dis. 2000, 36, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.D.; Gaillard, C.A.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Van Wyck, D.B.; Cronin, M.; Meier, Y.; Larroque, S.; Macdougall, I.C.; et al. Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent ckd: An analysis of the 1-year find-ckd trial. Nephrol. Dial. Transplant. 2017, 32, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Wyck, D.V.; Meier, Y.; Larroque, S.; Perrin, A.; Roger, S.D. Erythropoietic response to oral iron in patients with nondialysis-dependent chronic kidney disease in the find-ckd trial. Clin. Nephrol. 2017, 88, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Bhandari, S. Anemia in peritoneal dialysis patients; iron repletion, current and future therapies. Perit. Dial. Int. 2017, 37, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Bohm, M.; Doletsky, A.; Voors, A.A.; Macdougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Di Gennaro, F.; Rivas, C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ. 2015, 24, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Farmakis, D.; Colet, J.C.; Dickstein, K.; Luscher, T.F.; Willenheimer, R.; Parissis, J.; Gaudesius, G.; Mori, C.; von Eisenhart Rothe, B.; et al. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: A subanalysis of the fair-hf trial. Eur. J. Heart Fail. 2013, 15, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Beck-da-Silva, L.; Piardi, D.; Soder, S.; Rohde, L.E.; Pereira-Barretto, A.C.; de Albuquerque, D.; Bocchi, E.; Vilas-Boas, F.; Moura, L.Z.; Montera, M.W.; et al. Iron-hf study: A randomized trial to assess the effects of iron in heart failure patients with anemia. Int. J. Cardiol. 2013, 168, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, E.M.; Conraads, V.M.; Greenlaw, N.; Gaudesius, G.; Mori, C.; Ponikowski, P.; Anker, S.D. The effect of intravenous ferric carboxymaltose on red cell distribution width: A subanalysis of the fair-hf study. Eur. J. Heart Fail. 2013, 15, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Comin-Colet, J.; Lainscak, M.; Dickstein, K.; Filippatos, G.S.; Johnson, P.; Luscher, T.F.; Mori, C.; Willenheimer, R.; Ponikowski, P.; Anker, S.D. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: A subanalysis of the fair-hf study. Eur. Heart J. 2013, 34, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency ferric-hf: A randomized, controlled, observer-blinded trial. J. Am. Coll. Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.P.; Bartlett, F.R.; Penston, H.S.; O’Leary, J.; Pollock, N.; Kaprielian, R.; Chapman, C.M. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J. Am. Coll. Cardiol. 2006, 48, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Minana, G.; Cardells, I.; Palau, P.; Llacer, P.; Facila, L.; Almenar, L.; Lopez-Lereu, M.P.; Monmeneu, J.V.; Amiguet, M.; Gonzalez, J.; et al. Changes in myocardial iron content following administration of intravenous iron (myocardial-iron): Study design. Clin. Cardiol. 2018, 41, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Dahlerup, J.F.; Jacobsen, B.A.; van der Woude, J.; Bark, L.A.; Thomsen, L.L.; Lindgren, S. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand. J. Gastroenterol. 2016, 51, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Altorjay, I.; Zsigmond, F.; Primas, C.; Vogelsang, H.; Novacek, G.; Reinisch, S.; Thomsen, L.L. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Staun, M.; Tandon, R.K.; Altorjay, I.; Thillainayagam, A.V.; Gratzer, C.; Nijhawan, S.; Thomsen, L.L. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (monofer) compared with oral iron for treatment of anemia in ibd (proceed). Am. J. Gastroenterol. 2013, 108, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Nordfjeld, K.; Andreasen, H.; Thomsen, L.L. Pharmacokinetics of iron isomaltoside 1000 in patients with inflammatory bowel disease. Drug Des. Dev. Ther. 2012, 6, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C.; et al. Fergicor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e2. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Oustamanolakis, P.; Karakoidas, C.; Mantzaris, G.J.; Kouroumalis, E.A. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Digest. Dis. Sci. 2010, 55, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Wikman, O.; Befrits, R.; Blom, H.; Eriksson, A.; Granno, C.; Ung, K.A.; Hjortswang, H.; Lindgren, A.; Unge, P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in ibd patients: A randomized, controlled, evaluator-blind, multicentre study. Scand. J. Gastroenterol. 2009, 44, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (ferinject) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Schroder, O.; Mickisch, O.; Seidler, U.; de Weerth, A.; Dignass, A.U.; Herfarth, H.; Reinshagen, M.; Schreiber, S.; Junge, U.; Schrott, M.; et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—A randomized, controlled, open-label, multicenter study. Am. J. Gastroenterol. 2005, 100, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, K.; Ulvik, R.J.; Nysaeter, G.; Johansen, J.; Ostborg, J.; Berstad, A.; Berge, R.K.; Hausken, T. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2005, 40, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Mahey, R.; Kriplani, A.; Mogili, K.D.; Bhatla, N.; Kachhawa, G.; Saxena, R. Randomized controlled trial comparing ferric carboxymaltose and iron sucrose for treatment of iron deficiency anemia due to abnormal uterine bleeding. Int. J. Gynaecol. Obstet. 2016, 133, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tariq, N.; Ayub, R.; Khan, W.U.; Ijaz, S.; Alam, A.Y. Parenteral iron therapy in the treatment of iron deficiency anemia during pregnancy: A randomized controlled trial. J. Coll. Physicians Surg. Pak. 2015, 25, 193–197. [Google Scholar] [PubMed]
- Kochhar, P.K.; Kaundal, A.; Ghosh, P. Intravenous iron sucrose versus oral iron in treatment of iron deficiency anemia in pregnancy: A randomized clinical trial. J. Obstet. Gynaecol. Res. 2013, 39, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ayub, R.; Tariq, N.; Adil, M.M.; Iqbal, M.; Junaid, A.; Jaferry, T. Efficacy and safety of total dose infusion of low molecular weight iron dextran in the treatment of iron deficiency anemia during pregnancy. J. Coll. Physicians Surg. Pak. 2008, 18, 424–427. [Google Scholar] [PubMed]
- Al, R.A.; Unlubilgin, E.; Kandemir, O.; Yalvac, S.; Cakir, L.; Haberal, A. Intravenous versus oral iron for treatment of anemia in pregnancy: A randomized trial. Obstet. Gynecol. 2005, 106, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Bencaiova, G.; von Mandach, U.; Zimmermann, R. Iron prophylaxis in pregnancy: Intravenous route versus oral route. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Keeler, B.D.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Group, I.T. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br. J. Surg. 2017, 104, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Birgegard, G.; Henry, D.; Glaspy, J.; Chopra, R.; Thomsen, L.L.; Auerbach, M. A randomized noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: The profound trial. Pharmacotherapy 2016, 36, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Borstlap, W.A.A.; Buskens, C.J.; Tytgat, K.; Tuynman, J.B.; Consten, E.C.J.; Tolboom, R.C.; Heuff, G.; van Geloven, N.; van Wagensveld, B.A.; Wientjes, C.A.C.A.; et al. Multicentre randomized controlled trial comparing ferric(iii)carboxymaltose infusion with oral iron supplementation in the treatment of preoperative anaemia in colorectal cancer patients. BMC Surg. 2015, 15. [Google Scholar] [CrossRef]
- Hedenus, M.; Karlsson, T.; Ludwig, H.; Rzychon, B.; Felder, M.; Roubert, B.; Birgegard, G. Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Dangsuwan, P.; Manchana, T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol. Oncol. 2010, 116, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Cocchiaro, C.; Saadat-Gilani, K.; Hodyl, N.; Dekker, G. Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: A randomized trial. J. Matern. Fetal. Neonatal Med. 2013, 26, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Michels, W.M.; Jaar, B.G.; Ephraim, P.L.; Liu, Y.; Miskulin, D.C.; Tangri, N.; Crews, D.C.; Scialla, J.J.; Shafi, T.; Sozio, S.M.; et al. Intravenous iron administration strategies and anemia management in hemodialysis patients. Nephrol. Dial. Transplant. 2017, 32, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Freburger, J.K.; Ellis, A.R.; Winkelmayer, W.C.; Wang, L.; Kshirsagar, A.V. Comparative short-term safety of sodium ferric gluconate versus iron sucrose in hemodialysis patients. Am. J. Kidney Dis. 2016, 67, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Airy, M.; Mandayam, S.; Mitani, A.A.; Chang, T.I.; Ding, V.Y.; Brookhart, M.A.; Goldstein, B.A.; Winkelmayer, W.C. Comparative outcomes of predominant facility-level use of ferumoxytol versus other intravenous iron formulations in incident hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Hazara, A.M.; Bhandari, S. Intravenous iron administration is associated with reduced platelet counts in patients with chronic kidney disease. J. Clin. Pharm. Ther. 2015, 40, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Sturm, G.; Kronenberg, F.; Neyer, U.; Knoll, F.; Lhotta, K.; Weiss, G. Iron supplementation and mortality in incident dialysis patients: An observational study. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Schiller, B.; Bhat, P.; Sharma, A. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the united states over a 12-month period. Clin. Ther. 2014, 36, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.H.; Marafino, B.J.; McCulloch, C.E.; Dalrymple, L.S.; Dudley, R.A.; Grimes, B.A.; Johansen, K.L. Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin. J. Am. Soc. Nephrol. 2015, 10, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Miskulin, D.C.; Zhou, J.; Bandeen-Roche, K.; Michels, W.M.; Ephraim, P.L.; McDermott, A.; Crews, D.C.; Scialla, J.J.; Sozio, S.M.; et al. Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the decide-esrd study. Nephrol. Dial. Transplant. 2015, 30, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Miskulin, D.C.; Tangri, N.; Bandeen-Roche, K.; Zhou, J.; McDermott, A.; Meyer, K.B.; Ephraim, P.L.; Michels, W.M.; Jaar, B.G.; Crews, D.C.; et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.V.; Freburger, J.K.; Ellis, A.R.; Wang, L.; Winkelmayer, W.C.; Brookhart, M.A. Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, S.; Bocos, J.M.; Gisbert, J.P.; Bajador, E.; Chaparro, M.; Castano, C.; Garcia-Erce, J.A.; Gomollon, F. High-dose intravenous treatment in iron deficiency anaemia in inflammatory bowel disease: Early efficacy and impact on quality of life. Blood Transfus. 2016, 14, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cortes, X.; Borras-Blasco, J.; Moles, J.R.; Bosca, M.; Cortes, E. Safety of ferric carboxymaltose immediately after infliximab administration, in a single session, in inflammatory bowel disease patients with iron deficiency: A pilot study. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Befrits, R.; Wikman, O.; Blomquist, L.; Hjortswang, H.; Hammarlund, P.; Bajor, A.; Klintman, D.; Blom, H. Anemia and iron deficiency in inflammatory bowel disease: An open, prospective, observational study on diagnosis, treatment with ferric carboxymaltose and quality of life. Scand. J. Gastroenterol. 2013, 48, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Collingwood, J.; Hodyl, N.A.; Dekker, G. Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Preg. Childbirth 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Calleja, J.L.; Delgado, S.; del Val, A.; Hervas, A.; Larraona, J.L.; Teran, A.; Cucala, M.; Mearin, F.; Colon Cancer Study Group. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int. J. Colorectal Dis. 2016, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Toledano, A.; Luporsi, E.; Morere, J.F.; Scotte, F.; Laribi, K.; Barriere, J.; Huot-Marchand, P.; Duvillie, L.; Concas, V.H.; Bugat, R. Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support. Care Cancer 2016, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. Kidney disease: Improving global outcomes (kdigo) anemia work group. Kdigo clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc). Developed with the special contribution of the heart failure association (hfa) of the esc. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascon, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: Esmo clinical practice guidelines. Ann. Oncol. 2018. [Google Scholar] [CrossRef]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Klip, I.T.; Jankowska, E.A.; Enjuanes, C.; Voors, A.A.; Banasiak, W.; Bruguera, J.; Rozentryt, P.; Polonski, L.; van Veldhuisen, D.J.; Ponikowski, P.; et al. The additive burden of iron deficiency in the cardiorenal-anaemia axis: Scope of a problem and its consequences. Eur. J. Heart Fail. 2014, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Filippatos, G.; Colet, J.C.; Willenheimer, R.; Dickstein, K.; Luscher, T.; Gaudesius, G.; von Eisenhart Rothe, B.; Mori, C.; Greenlaw, N.; et al. The impact of intravenous ferric carboxymaltose on renal function: An analysis of the fair-hf study. Eur. J. Heart Fail. 2015, 17, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.Gov. Intravenous Iron Treatment in Patients with Heart Failure and Iron Deficiency: Ironman. NCT02642562. Available online: https://clinicaltrials.gov/ct2/show/NCT02642562 (accessed on 07 June 2018).
- Alves, R.A.; Miszputen, S.J.; Figueiredo, M.S. Anemia in inflammatory bowel disease: Prevalence, differential diagnosis and association with clinical and laboratory variables. Sao Paulo Med. J. 2014, 132, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; Morena Fde, L.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in european countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Kaitha, S.; Bashir, M.; Ali, T. Iron deficiency anemia in inflammatory bowel disease. World J. Gastrointest. Pathophysiol. 2015, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Aksan, A.; Isik, H.; Radeke, H.H.; Dignass, A.; Stein, J. Systematic review with network meta-analysis: Comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Wurtinger, P.; Finkenstedt, A.; Braithwaite, V.; Viveiros, A.; Effenberger, M.; Sulzbacher, I.; Moschen, A.; Griesmacher, A.; Tilg, H.; et al. Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Adkinson, N.F.; Strauss, W.E.; Macdougall, I.C.; Bernard, K.E.; Auerbach, M.; Kaper, R.F.; Chertow, G.M.; Krop, J.S. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: A randomized trial. Am. J. Hematol. 2018, 93, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Hvas, C.L.; Dahlerup, J.F. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br. J. Clin. Pharmacol. 2017, 83, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Graham, D.J.; Kane, R.C.; Xie, D.; Wernecke, M.; Levenson, M.; MaCurdy, T.E.; Houstoun, M.; Ryan, Q.; Wong, S.; et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 2015, 314, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.A.; Bhandari, S. Safety of intravenous iron use in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
| Cause | Details |
|---|---|
| Insufficient uptake | Malnutrition or diet-related (low-iron, vegetarian, vegan) |
| Increased physiological demand | Rapid growth during infancy/adolescence, menstrual blood loss, pregnancy (2nd/3rd trimesters) |
| Chronic blood loss | Trauma, surgery, delivery, heavy menstrual bleeding |
| Chronic disease | Kidney disease, heart failure, inflammatory bowel disease, gastritis, peptic ulcer, intestinal cancer and benign tumours |
| Drug-related | Glucocorticoids, salicylates, non-steroidal anti-inflammatory drugs, proton-pump inhibitors, H2-receptor antagonists, drug-induced haemolytic anaemia |
| Genetic | Iron-refractory iron-deficiency anaemia, thalassaemia and sickle cell anaemia |
| Ferumoxytol | Iron Carboxymaltose | Iron Isomaltoside 1000 | Low Molecular Weight Iron Dextran | Iron Sucrose | Iron Gluconate | |
|---|---|---|---|---|---|---|
| Brand name | Feraheme® | Ferinject® | Monofer® | Cosmofer® | Venofer® | Ferlixit® |
| Maximum single dose | 510 mg | 1000 mg | 20 mg/kg | 20 mg/kg | 200 mg | 125 mg |
| Minimum administration time (minutes) | 15 | 15 | 15 | 60 | 30 | 30–60 |
| Replacement dose possible in a single infusion | No | Yes | Yes | Yes | No | No |
| Ferumoxytol | Iron Carboxymaltose | Iron Isomaltoside 1000 | Low Molecular Weight Iron Dextran | Iron Sucrose | Iron Gluconate | |
|---|---|---|---|---|---|---|
| Molecular weight (Da) | 185,000 | 150,000 | 150,000 | 103,000 | 43,000 | 37,500 |
| Carbohydrate ligand | Polyglucose sorbitol carboxymethylether | Carboxymaltose | Isomaltoside | Dextran polysaccharide | Sucrose | Gluconate, loosely associated sucrose |
| Relative stability of iron carbohydrate complex | High | High | High | High | Medium | Low |
| Reactivity with transferrin | Low | Low | Low | Low | Medium | High |
| Relative labile iron release | Low | Low | Low | Medium | High | High |
| Plasma half-life (hrs) | ~15 | 7–12 | 20 | 5–20 | 6 | ~1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.; Pereira, D.I.A.; Chappell, H.F.; Drakesmith, H. Intravenous Irons: From Basic Science to Clinical Practice. Pharmaceuticals 2018, 11, 82. https://doi.org/10.3390/ph11030082
Bhandari S, Pereira DIA, Chappell HF, Drakesmith H. Intravenous Irons: From Basic Science to Clinical Practice. Pharmaceuticals. 2018; 11(3):82. https://doi.org/10.3390/ph11030082
Chicago/Turabian StyleBhandari, Sunil, Dora I. A. Pereira, Helen F. Chappell, and Hal Drakesmith. 2018. "Intravenous Irons: From Basic Science to Clinical Practice" Pharmaceuticals 11, no. 3: 82. https://doi.org/10.3390/ph11030082
APA StyleBhandari, S., Pereira, D. I. A., Chappell, H. F., & Drakesmith, H. (2018). Intravenous Irons: From Basic Science to Clinical Practice. Pharmaceuticals, 11(3), 82. https://doi.org/10.3390/ph11030082





