Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice
Abstract
:1. Introduction
2. Results
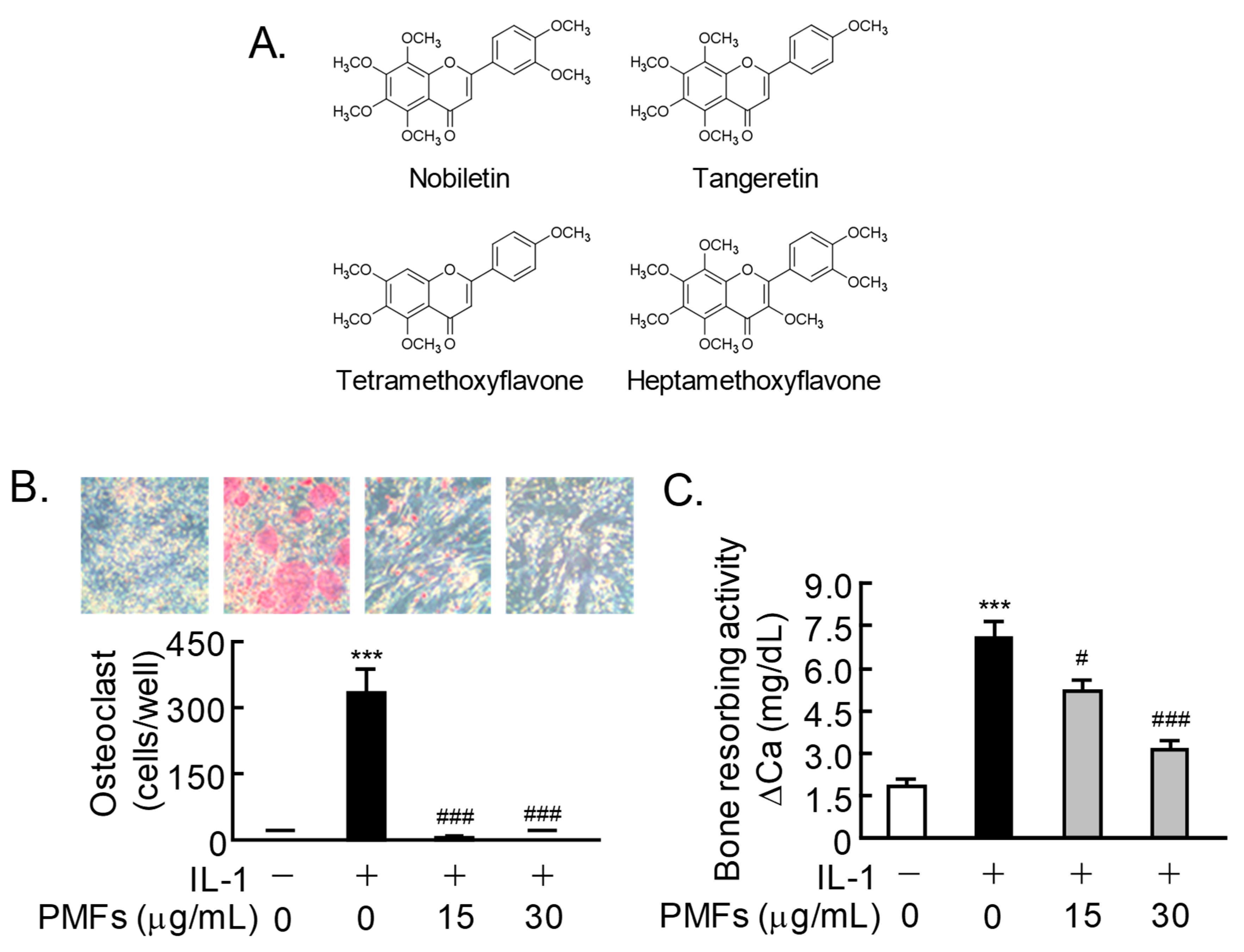
2.1. Effects of PMFs on IL-1-Induced Osteoclast Differentiation and Bone Resorption
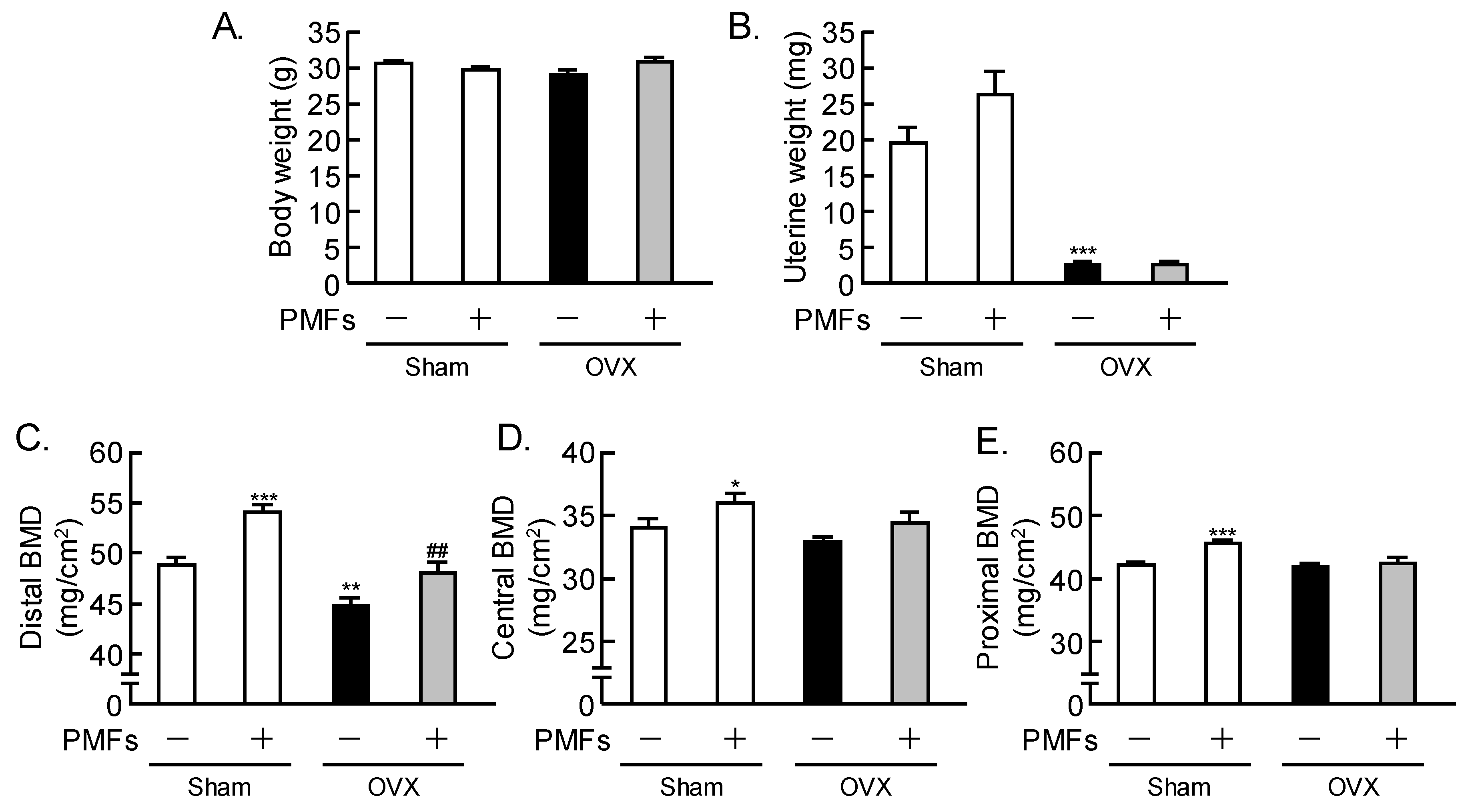
2.2. Effects of PMFs on Bone Mass in OVX Mice, an In Vivo Model of Osteoporosis
2.3. An Analysis of the Femoral Trabecular Bone Using μCT
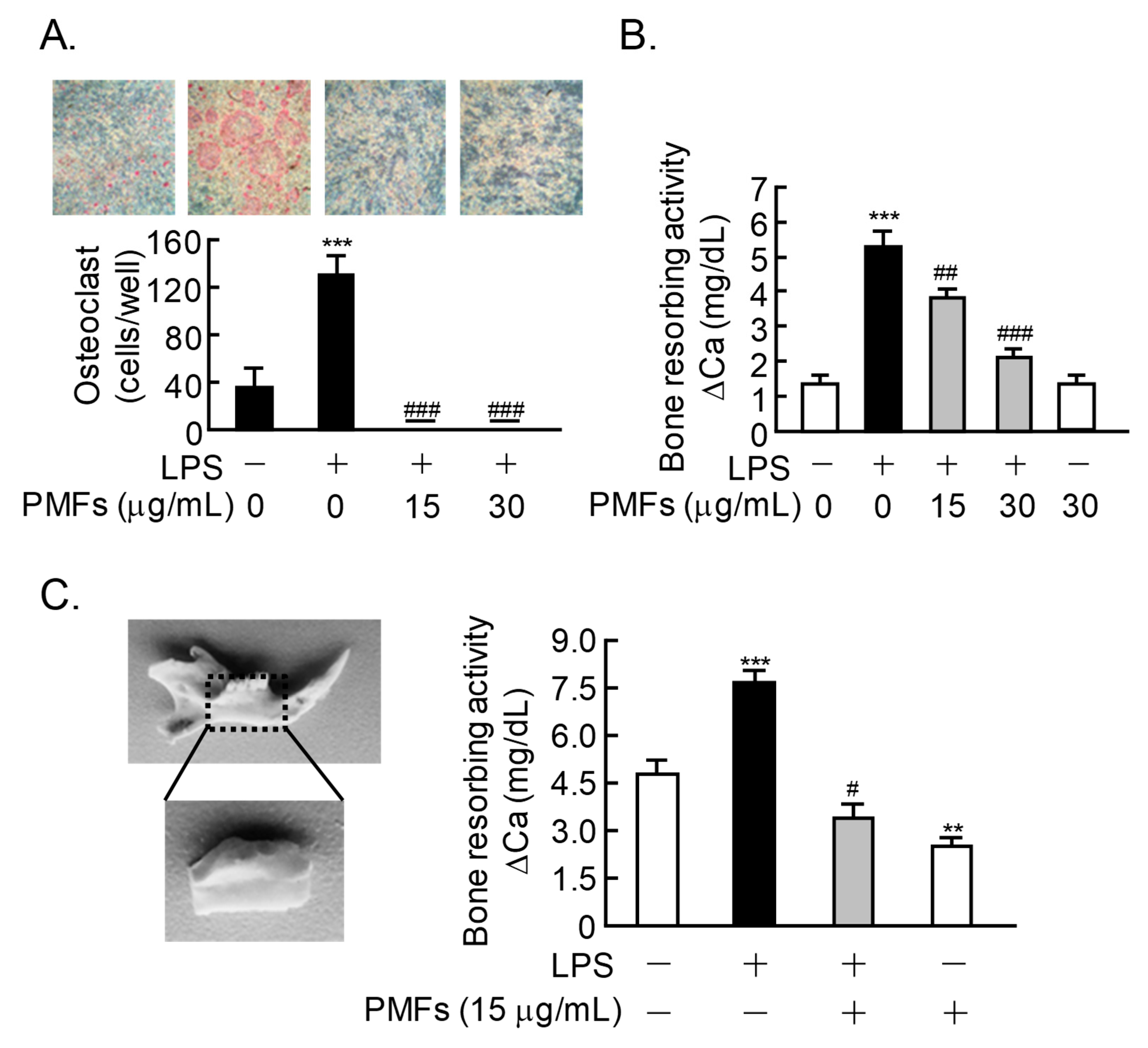
2.4. Effects of PMFs on LPS-Induced Osteoclast Formation and ex vivo Alveolar Bone Resorption
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Isolation of Primary Mouse Osteoblastic Cells
4.3. Co-Cultures of Mouse Bone Marrow Cells and Osteoblasts
4.4. Organ Cultures of Mouse Calvariae
4.5. Oral Administration of PMFs in OVX Mice
4.6. Organ Cultures of Mouse Mandibular Alveolar Bone
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lacey, D.; Timms, E.; Tan, H.; Kelley, M.; Dunstan, C.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Maeno, M.; Suzuki, N.; Fujisaki, K.; Tanaka, H.; Ogiso, B.; Ito, K. IL-1 alpha stimulates the formation of osteoclast-like cells by increasing M-CSF and PGE2 production and decreasing OPG production by osteoblasts. Life Sci. 2005, 77, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Udagawa, N.; Sato, N.; Takami, M.; Itoh, K.; Woo, J.-T.; Takahashi, N.; Nagai, K. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J. Immunol. 2004, 172, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Kobayashi, M.; Takita, M.; Matsumoto, C.; Miyaura, C.; Inada, M. Hyaluronan inhibits bone resorption by suppressing prostaglandin E synthesis in osteoblasts treated with interleukin-1. Biochem. Biophys. Res. Commun. 2009, 381, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Matsumoto, C.; Uematsu, S.; Akira, S.; Miyaura, C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol. 2006, 177, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, T.; Miyaura, C.; Inada, M.; Maruyama, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology 2000, 141, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Inada, M.; Suzawa, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J. Biol. Chem. 2000, 275, 19819–19823. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Kusano, K.; Masuzawa, T.; Chaki, O.; Onoe, Y.; Aoyagi, M.; Sasaki, T.; Tamura, T.; Koishihara, Y.; Ohsugi, Y. Endogenous bone-resorbing factors in estrogen deficiency: Cooperative effects of IL-1 and IL-6. J. Bone Miner. Res. 1995, 10, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [PubMed]
- Ichimaru, R.; Tominari, T.; Yoshinouchi, S.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Numabe, Y.; Murphy, G.; Nagase, H.; Miyaura, C.; et al. Raloxifene reduces the risk of local alveolar bone destruction in a mouse model of periodontitis combined with systemic postmenopausal osteoporosis. Arch. Oral Biol. 2017, 85, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Juluri, R.; Prashanth, E.; Gopalakrishnan, D.; Kathariya, R.; Devanoorkar, A.; Viswanathan, V.; Romanos, G.E. Association of Postmenopausal Osteoporosis and Periodontal Disease: A Double-Blind Case-Control Study. J. Int. Oral Health 2015, 7, 119–123. [Google Scholar] [PubMed]
- Miyata, Y.; Tanaka, H.; Shimada, A.; Sato, T.; Ito, A.; Yamanouchi, T.; Kosano, H. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sci. 2011, 88, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Weng, M.-S.; Lin, J.-K. Tangeretin suppresses IL-1β-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. Biochem. Pharmacol. 2007, 73, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, S.; Sung, J.; Yang, J.; Choi, Y.; Jeong, H.; Lee, J. Nobiletin Attenuates the Inflammatory Response Through Heme Oxygenase-1 Induction in the Crosstalk Between Adipocytes and Macrophages. J. Med. Food 2017, 20, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Li, S.; Chai, C.-Y.; Lo, C.-Y.; Dushenkov, S.; Ho, C.-T.; Pan, M.-H.; Wang, Y.-J. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3′,4′-didemethylnobiletin, derived from nobiletin. Carcinogenesis 2008, 29, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Guan, X.; Zhou, L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Boil. Ther. 2008, 7, 966–973. [Google Scholar] [CrossRef]
- Li, S.; Sang, S.; Pan, M.-H.; Lai, C.-S.; Lo, C.-Y.; Yang, C.; Ho, C.-T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-D.; Liao, Y.-C.; Shih, Y.-W.; Tsai, L.-Y. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine 2013, 20, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.-C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Miyoshi, K.; Tsumura, Y.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus polymethoxylated flavone, attenuates inflammation in the mouse hippocampus. Brain Sci. 2015, 5, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone ameliorates depressive-like behavior and hippocampal neurochemical changes in chronic unpredictable mild stressed mice by regulating the brain-derived neurotrophic factor: Requirement for ERK activation. Int. J. Mol. Sci. 2017, 18, 2133. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Tominari, T.; Matsumoto, C.; Hirata, M.; Takita, M.; Inada, M.; Miyaura, C. Nobiletin, a polymethoxy flavonoid, suppresses bone resorption by inhibiting NFκB-dependent prostaglandin E synthesis in osteoblasts and prevents bone loss due to estrogen deficiency. J. Pharmacol. Sci. 2011, 115, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Inoue, H.; Tominari, T.; Watanabe, K.; Hirata, M.; Miyaura, C.; Inada, M. Heptamethoxyflavone, a citrus flavonoid, suppresses inflammatory osteoclastogenesis and alveolar bone resorption. Biosci. Biotechnol. Biochem. 2015, 79, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, N.; Fujii, T.; Hashizume, R.; Masaki, H. A polymethoxyflavone mixture, extracted from orange peels, suppresses the UVB-induced expression of MMP-1. Exp. Dermatol. 2016, 25, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, N.; Hashizume, R.; Masaki, H. A polymethoxyflavone mixture extracted from orange peels, mainly containing nobiletin, 3,3′,4′,5,6,7,8-heptamethoxyflavone and tangeretin, suppresses melanogenesis through the acidification of cell organelles, including melanosomes. J. Dermatol. Sci. 2017, 88, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, N.; Fujii, T.; Masaki, H.; Okubo, T.; Shimada, K.; Hashizume, R. Orange peel extract, containing high levels of polymethoxyflavonoid, suppressed UVB-induced COX-2 expression and PGE2 production in HaCaT cells through PPAR-γ activation. Exp. Dermatol. 2014, 23, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Ikuina, T.; Hikima, T.; Tokiwano, T.; Yoshizawa, Y. Relationship between structure and antiproliferative activity of polymethoxyflavones towards HL60 cells. Anticancer Res. 2012, 32, 5239–5244. [Google Scholar] [PubMed]
- Murakami, A.; Song, M.; Katsumata, S.-I.; Uehara, M.; Suzuki, K.; Ohigashi, H. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: Possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 2007, 30, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Hwang, J.-H.; Ko, H.-C.; Park, J.-G.; Kim, S.-J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yang, B.; Zhao, H.; Xu, B.; Jiao, W.; Wang, Q.; Wang, Z.; Kuang, H. Tangeretin exerts anti-neuroinflammatory effects via NF-κB modulation in lipopolysaccharide-stimulated microglial cells. Int. Immunopharmacol. 2014, 19, 275–282. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, S.; Tominari, T.; Matsumoto, C.; Yoshinouchi, S.; Ichimaru, R.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Miyaura, C.; Inada, M. Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice. Pharmaceuticals 2018, 11, 7. https://doi.org/10.3390/ph11010007
Matsumoto S, Tominari T, Matsumoto C, Yoshinouchi S, Ichimaru R, Watanabe K, Hirata M, Grundler FMW, Miyaura C, Inada M. Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice. Pharmaceuticals. 2018; 11(1):7. https://doi.org/10.3390/ph11010007
Chicago/Turabian StyleMatsumoto, Shigeru, Tsukasa Tominari, Chiho Matsumoto, Shosei Yoshinouchi, Ryota Ichimaru, Kenta Watanabe, Michiko Hirata, Florian M. W. Grundler, Chisato Miyaura, and Masaki Inada. 2018. "Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice" Pharmaceuticals 11, no. 1: 7. https://doi.org/10.3390/ph11010007
APA StyleMatsumoto, S., Tominari, T., Matsumoto, C., Yoshinouchi, S., Ichimaru, R., Watanabe, K., Hirata, M., Grundler, F. M. W., Miyaura, C., & Inada, M. (2018). Effects of Polymethoxyflavonoids on Bone Loss Induced by Estrogen Deficiency and by LPS-Dependent Inflammation in Mice. Pharmaceuticals, 11(1), 7. https://doi.org/10.3390/ph11010007




