Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage
Abstract
1. Introduction
2. Materials & Methods
2.1. Bacterial Strains
2.2. Bacteriophage and Genome Analysis
2.3. Purification of Lys-phiSA012 Recombinant Protein
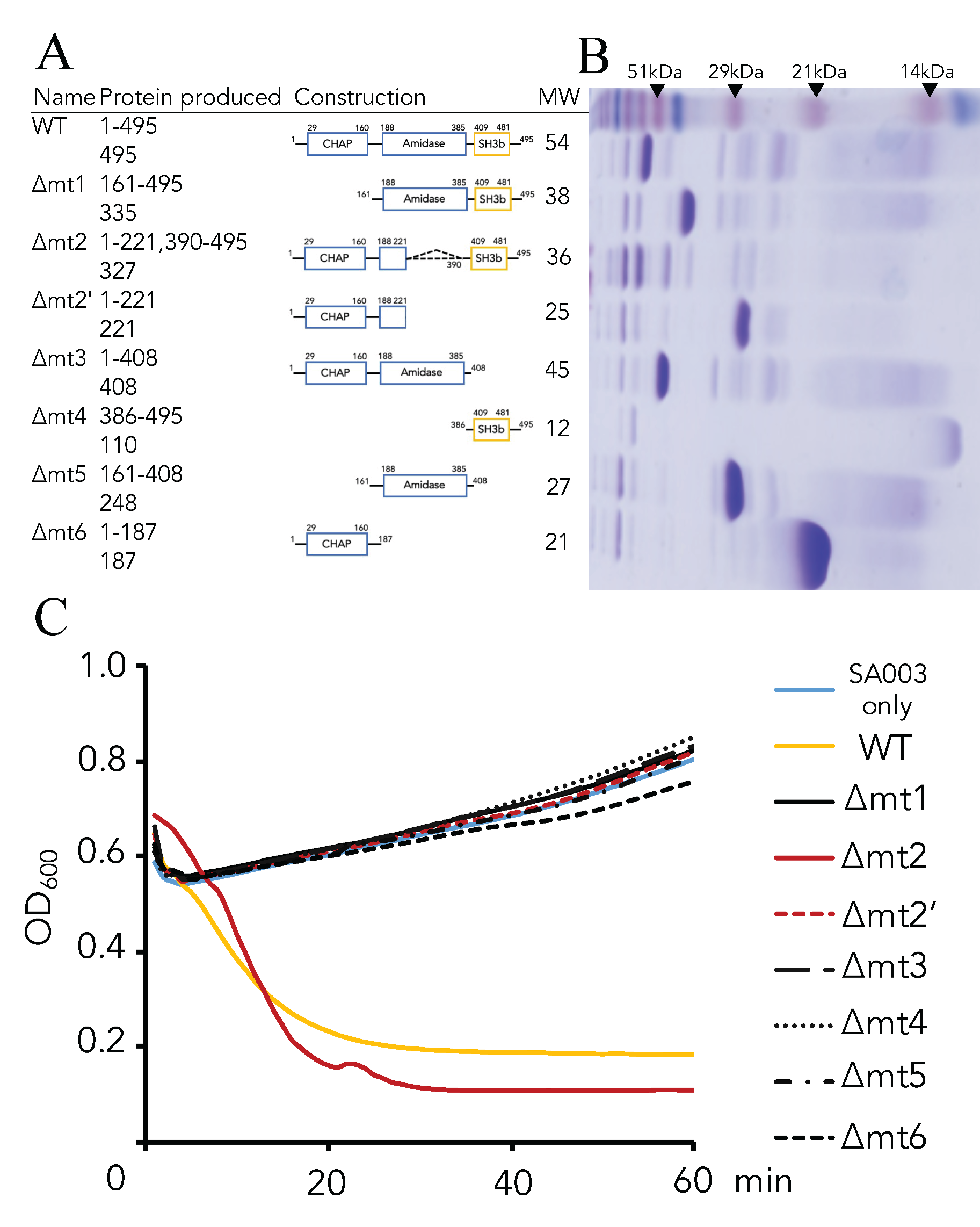
2.4. Generation of Domain(s) Deletion Mutants
2.5. Turbidity Reduction Assays
2.6. MIC and MBC Assays
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
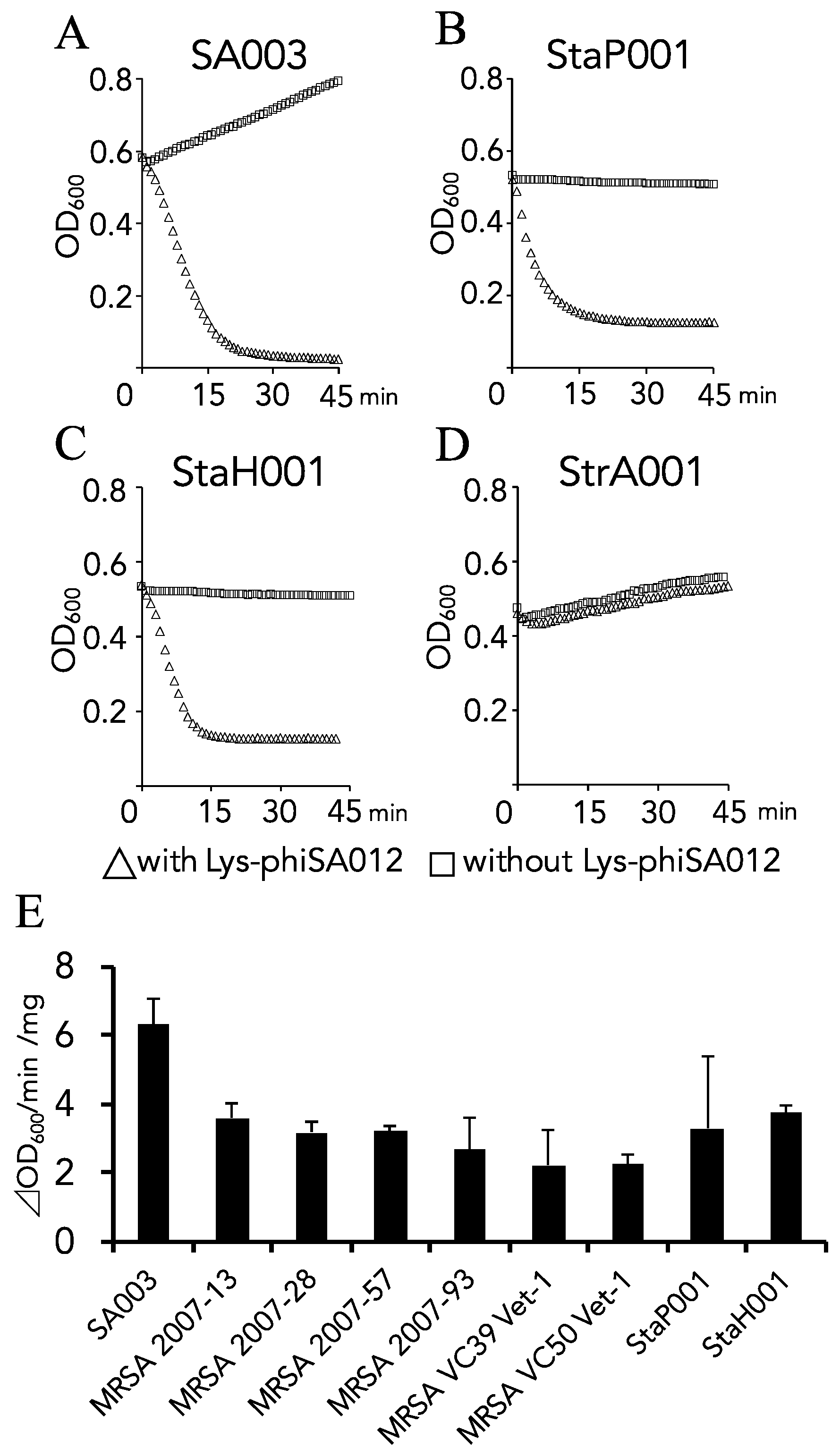
3.1. Lytic Activity and the Antimicrobial Spectrum of Lys-phiSA012.
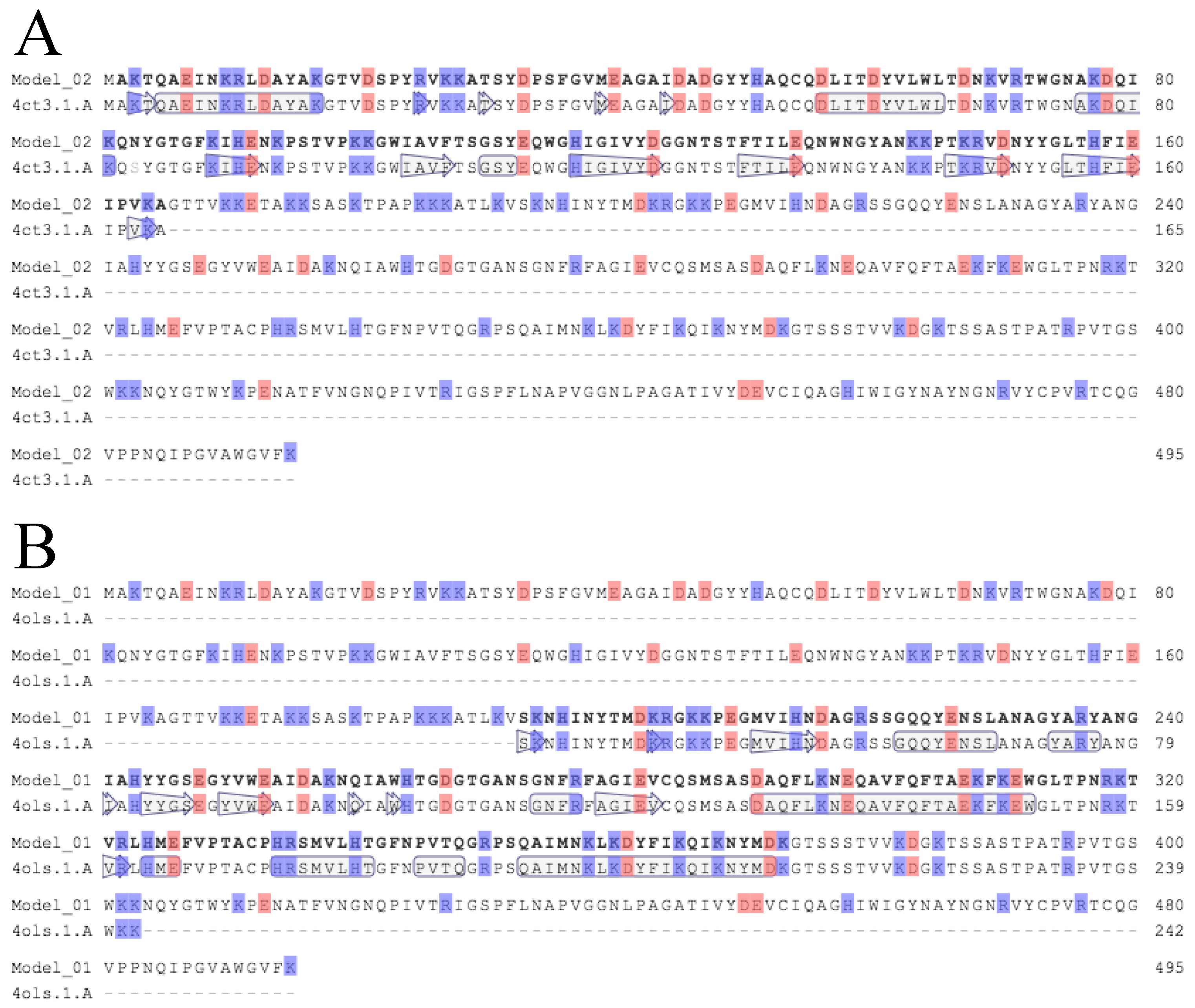
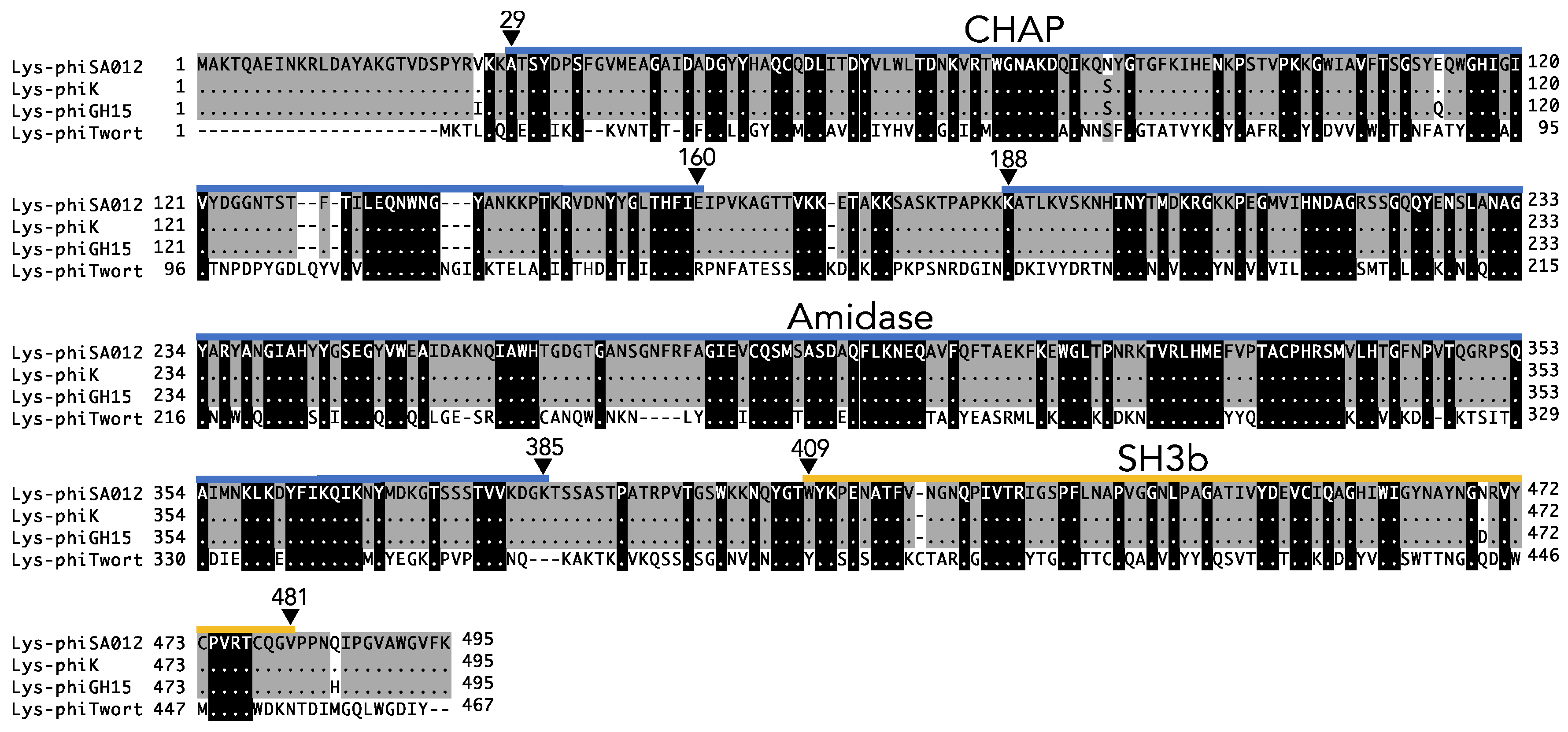
3.2. Critical Region of the Lys-phiSA012 Protein Required for Lytic Activity
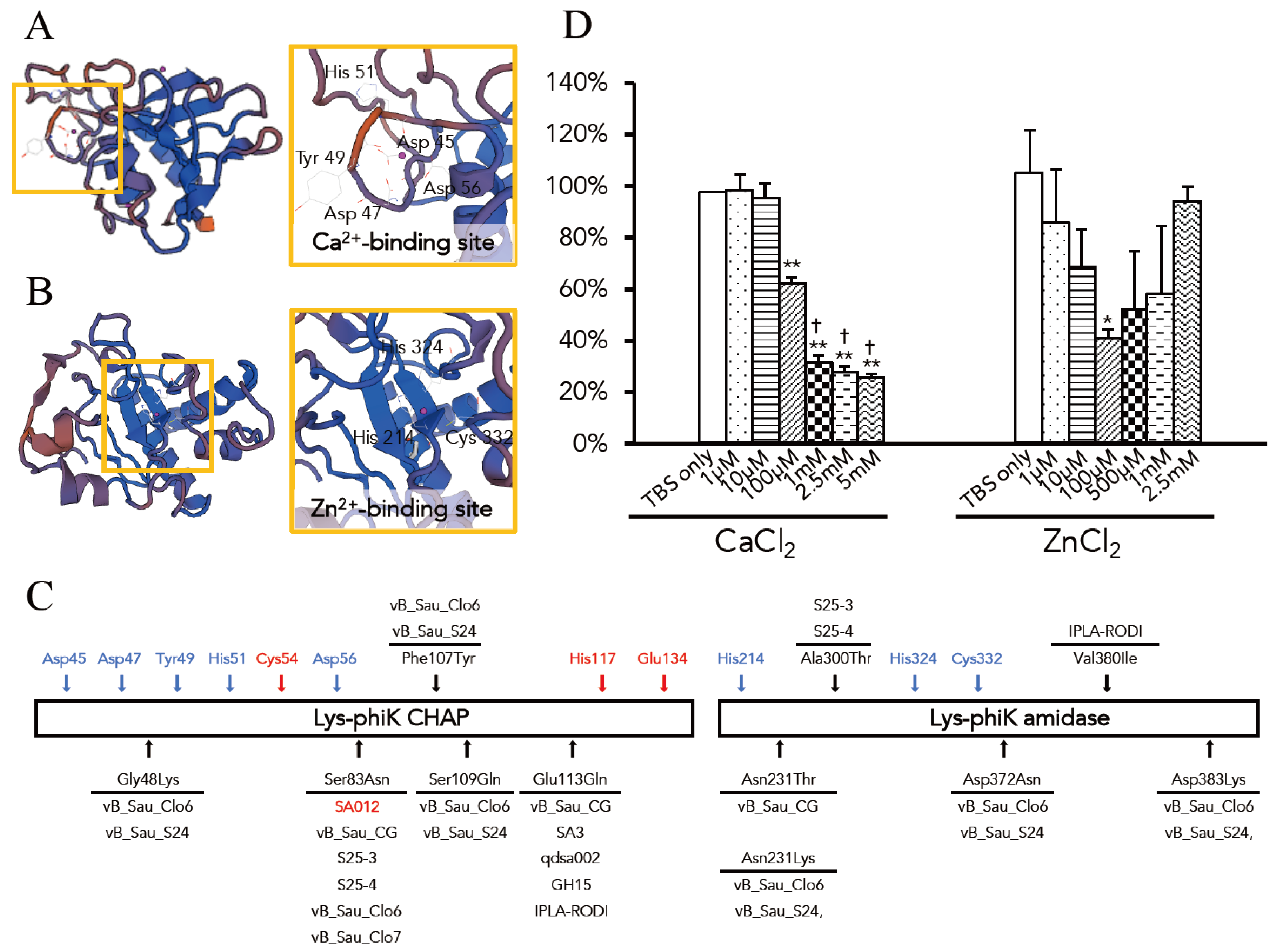
3.3. Optimal Ca2+ and Zn2+ Concentration for the Lytic Activity of Lys-phiSA012
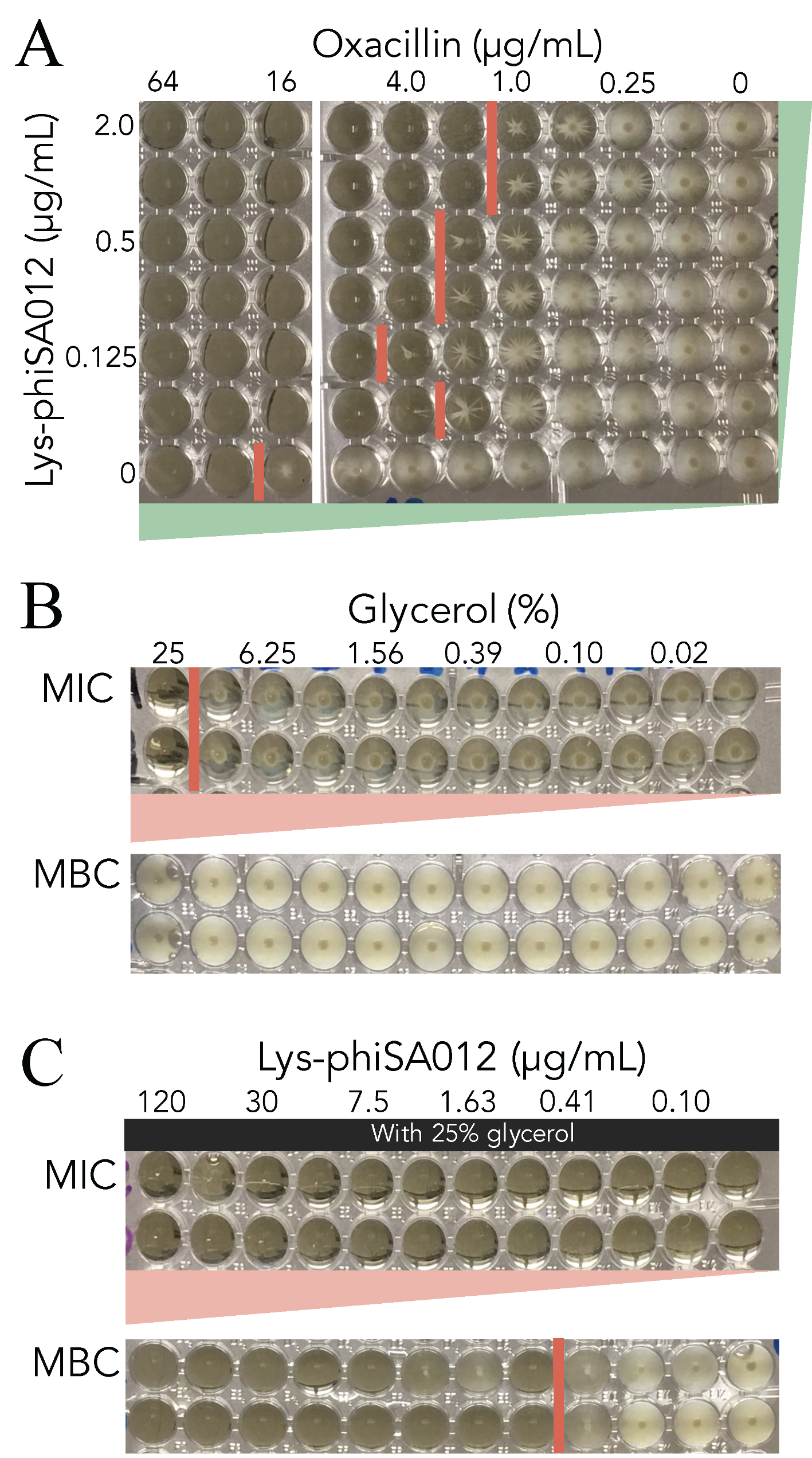
3.4. MIC Assays of Oxacillin w/wo Lys-phiSA012
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

References
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Clinical impact of antimicrobial resistance in European hospitals: Excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 2011, 55, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.; Davey, P.G.; Grundmann, H.; Burden Study Group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Kojima, A.; Harada, K.; Ishihara, K.; Takahashi, T.; Tamura, Y. Correlation between the usage volume of veterinary therapeutic antimicrobials and resistance in Escherichia coli isolated from the feces of food-producing animals in japan. Jpn. J. Infect. Dis. 2005, 58, 369–372. [Google Scholar] [PubMed]
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhang, X.L.; Li, W.; Lu, X.F.; Liu, B.; Wang, J. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess 2013, 185, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Gebreyes, W.A.; Abley, M.J.; Harper, A.L.; Forshey, B.M.; Male, M.J.; Martin, H.W.; Molla, B.Z.; Sreevatsan, S.; Thakur, S.; et al. Methicillin-resistant Staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS ONE 2013, 8, e63704. [Google Scholar] [CrossRef] [PubMed]
- Neyra, R.C.; Frisancho, J.A.; Rinsky, J.L.; Resnick, C.; Carroll, K.C.; Rule, A.M.; Ross, T.; You, Y.; Price, L.B.; Silbergeld, E.K. Multidrug-resistant and methicillin-resistant Staphylococcus aureus (MRSA) in hog slaughter and processing plant workers and their community in North Carolina (USA). Environ. Health Perspect. 2014, 122, 471–477. [Google Scholar] [PubMed]
- Khanna, T.; Friendship, R.; Dewey, C.; Weese, J.S. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 2008, 128, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C. Livestock-associated Staphylococcus aureus: The United States experience. PLoS Pathog. 2015, 11, e1004564. [Google Scholar] [CrossRef] [PubMed]
- Geenen, P.L.; Graat, E.A.; Haenen, A.; Hengeveld, P.D.; Van Hoek, A.H.; Huijsdens, X.W.; Kappert, C.C.; Lammers, G.A.; Van Duijkeren, E.; Van De Giessen, A.W. Prevalence of livestock-associated MRSA on Dutch broiler farms and in people living and/or working on these farms. Epidemiol. Infect. 2013, 141, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, A.; Castro, B.; Rodriguez-Alvarez, C.; Gonzalez, J.C.; Sierra, A.; Montesinos, M.I.; Abreu, R.; Arias, A. Prevalence and characteristics of methicillin-resistant Staphylococcus aureus in pigs and pig workers in Tenerife, Spain. Foodborne Pathog. Dis. 2012, 9, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Denis, O.; Suetens, C.; Hallin, M.; Catry, B.; Ramboer, I.; Dispas, M.; Willems, G.; Gordts, B.; Butaye, P.; Struelens, M.J. Methicillin-resistant Staphylococcus aureus st398 in swine farm personnel, Belgium. Emerg. Infect. Dis. 2009, 15, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Aubry-Damon, H.; Grenet, K.; Sall-Ndiaye, P.; Che, D.; Cordeiro, E.; Bougnoux, M.E.; Rigaud, E.; Le Strat, Y.; Lemanissier, V.; Armand-Lefevre, L.; et al. Antimicrobial resistance in commensal flora of pig farmers. Emerg. Infect. Dis. 2004, 10, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Streicher, K.L. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neoplasia 2002, 7, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yamane, I. Epidemiological survey and economical evaluation of bovine mastitis in tie-stall dairy farms. J. Jpn. Vet. Med. Assoc. 2006, 59, 674–678. [Google Scholar] [CrossRef][Green Version]
- Costa, E.O.; Benites, N.R.; Guerra, J.L.; Melville, P.A. Antimicrobial susceptibility of Staphylococcus spp. isolated from mammary parenchymas of slaughtered dairy cows. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Aires-de-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 22 February 2018).
- Haddad Kashani, H.; Schmelcher, M.; Sabzalipoor, H.; Seyed Hosseini, E.; Moniri, R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dabrowska, I.; Gregorczyk, K.P.; Wyzewski, Z. Phage therapy in bacterial infections treatment: One hundred years after the discovery of bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Iwano, H.; Hiyashimizu, Y.; Matsubara, K.; Higuchi, H.; Nagahata, H.; Niwa, H.; Katayama, Y.; Kinoshita, Y.; Hagiwara, K.; et al. Phage therapy is effective in a mouse model of bacterial equine keratitis. Appl. Environ. Microbiol. 2016, 82, 5332–5339. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Iwano, H.; Higuchi, H.; Yokota, H.; Usui, M.; Iwasaki, T.; Tamura, Y. Bacteriophage can lyse antibiotic-resistant Pseudomonas aeruginosa isolated from canine diseases. J. Vet. Med. Sci. 2016, 78, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Synnott, A.J.; Kuang, Y.; Kurimoto, M.; Yamamichi, K.; Iwano, H.; Tanji, Y. Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities. Appl. Environ. Microbiol. 2009, 75, 4483–4490. [Google Scholar] [CrossRef] [PubMed]
- Iwano, H.; Inoue, Y.; Takasago, T.; Kobayashi, H.; Furusawa, T.; Taniguchi, K.; Fujiki, J.; Yokota, H.; Usui, M.; Tanji, Y.; et al. Bacteriophage ФSA012 has a broad host range against Staphylococcus aureus and effective lytic capacity in a mouse mastitis model. Biology 2018, 8, 7. [Google Scholar]
- Meaden, S.; Koskella, B. Exploring the risks of phage application in the environment. Front. Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A novel antimicrobial endolysin, lyspa26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage lytic enzymes: Novel anti-infectives. Trends Microbiol. 2005, 13, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A bacteriolytic agent that detects and kills bacillus anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Pastagia, M.; Euler, C.; Chahales, P.; Fuentes-Duculan, J.; Krueger, J.G.; Fischetti, V.A. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob. Agents Chemother. 2011, 55, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rubio, L.; Martinez, B.; Rodriguez, A.; Donovan, D.M.; Gotz, F.; Garcia, P. The phage lytic proteins from the Staphylococcus aureus bacteriophage vB_SauS-phiiPLA88 display multiple active catalytic domains and do not trigger staphylococcal resistance. PLoS ONE 2013, 8, e64671. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Garcia, E.; Moscoso, M. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob. Agents Chemother. 2011, 55, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage lysin cf-301, a potent antistaphylococcal biofilm agent. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The recombinant phage lysin lysk has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xu, W.; Lei, L.; Huang, J.; Feng, X.; Sun, C.; Du, C.; Zuo, J.; Li, Y.; Du, T.; et al. Lysgh15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 2011, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Gaeng, S.; Wendlinger, G.; Maier, S.K.; Scherer, S. The two-component lysis system of Staphylococcus aureus bacteriophage twort: A large TTG-start holin and an associated amidase endolysin. FEMS Microbiol. Lett. 1998, 162, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Li, X.; Hu, L.; Cheng, M.; Xia, F.; Gong, P.; Wang, B.; Ge, J.; Zhang, H.; et al. Lysgh15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci. Rep. 2016, 6, 29344. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Feng, Y.; Feng, X.; Sun, C.; Lei, L.; Ding, W.; Niu, F.; Jiao, L.; Yang, M.; Li, Y.; et al. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-Hand-Like” calcium-binding phage lysin. PLoS Pathog. 2014, 10, e1004109. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Dong, S.; Baker, J.R.; Foster-Frey, J.; Pritchard, D.G.; Donovan, D.M. Lysk chap endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 2009, 294, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Gaitero, M.; Keary, R.; Garcia-Doval, C.; Coffey, A.; van Raaij, M.J. Crystal structure of the lytic chap(k) domain of the endolysin Lysk from Staphylococcus aureus bacteriophage k. Virol. J. 2014, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Shimokubo, N.; Sakagami, A.; Ueno, H.; Muramatsu, Y.; Kadosawa, T.; Yanagisawa, C.; Hanaki, H.; Nakajima, C.; Suzuki, Y.; et al. Occurrence and molecular characteristics of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius in an academic veterinary hospital. Appl. Environ. Microbiol. 2010, 76, 5165–5174. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Saito, M.; Shimokubo, N.; Muramatsu, Y.; Maetani, S.; Tamura, Y. Methicillin-resistant Staphylococcus aureus carriage among veterinary staff and dogs in private veterinary clinics in Hokkaido, Japan. Microbiol. Immunol. 2014, 58, 149–154. [Google Scholar] [CrossRef] [PubMed]
- clinical and Laboratory standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-First Inf. Suppl. 2011, 31, 68–76. [Google Scholar]
- Becker, S.C.; Swift, S.; Korobova, O.; Schischkova, N.; Kopylov, P.; Donovan, D.M.; Abaev, I. Lytic activity of the staphylolytic Twort phage endolysin CHAP domain is enhanced by the SH3b cell wall binding domain. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Yun, J.; Lim, J.A.; Shin, H.; Heu, S.; Ryu, S. Characterization of lysb4, an endolysin from the Bacillus cereus-infecting bacteriophage b4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Flamm, R.K.; Jones, R.N. Antimicrobial activity of daptomycin tested against Gram-positive pathogens collected in Europe, Latin America, and selected countries in the Asia-Pacific region (2011). Diagn. Microbiol. Infect. Dis. 2013, 75, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The swiss-model workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.; Arnold, K.; Kunzli, M.; Bordoli, L.; Schwede, T. The swiss-model repository and associated resources. Nucleic Acids Res. 2009, 37, D387–D392. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and SWISS-PDBVIEWER: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. Swiss-Model: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, V.; Peressutti Bacci, N.; Boncompain, C.A.; Amadio, A.F.; Carrasco, S.; Suarez, C.A.; Morbidoni, H.R. Broad-range lytic bacteriophages that kill Staphylococcus aureus local field strains. PLoS ONE 2017, 12, e0181671. [Google Scholar] [CrossRef] [PubMed]
- Takemura-Uchiyama, I.; Uchiyama, J.; Kato, S.; Inoue, T.; Ujihara, T.; Ohara, N.; Daibata, M.; Matsuzaki, S. Evaluating efficacy of bacteriophage therapy against Staphylococcus aureus infections using a silkworm larval infection model. FEMS Microbiol. Lett. 2013, 347, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T. Combined use of bacteriophage k and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.; Vandenheuvel, D.; Martinez, B.; Rodriguez, A.; Lavigne, R.; Garcia, P. Two phages, phiiPLA-RODI and phiiPLA-C1C, lyse mono- and dual-species staphylococcal biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Dreher-Lesnick, S.M.; Schreier, J.E.; Stibitz, S. Development of phage lysin Lysa2 for use in improved purity assays for live biotherapeutic products. Viruses 2015, 7, 6675–6688. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Cooney, J.C.; Ross, R.P.; Sleator, R.D.; McAuliffe, O.; O’Mahony, J.; Coffey, A. In silico modeling of the staphylococcal bacteriophage-derived peptidase chap(k). Bacteriophage 2011, 1, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.Y.; Donovan, D.M.; Becker, S.C.; Lebedev, D.N.; Priyma, A.D.; Koudriachova, H.V.; Kabanov, A.V.; Klyachko, N.L. Physicochemical characterization of the staphylolytic lysk enzyme in complexes with polycationic polymers as a potent antimicrobial. Biochimie 2013, 95, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Zou, D.; Huang, S.; Lei, H.; Yang, Z.; Su, Y.; He, X.; Zhao, Q.; Wang, Y.; Liu, W.; Huang, L. Sensitive and rapid detection of the plasmid-encoded colistin-resistance gene mcr-1 in enterobacteriaceae isolates by loop-mediated isothermal amplification. Front. Microbiol. 2017, 8, 2356. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.; Euler, C.; Collin, M.; Chahales, P.; Gorelick, K.J.; Fischetti, V.A. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ФMR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Foster-Frey, J.; Donovan, D.M. The phage k lytic enzyme lysk and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 2008, 287, 185–191. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Meaney, W.; Fitzgerald, G.F.; Elbreki, M.F.; Coffey, A. Potential of the polyvalent anti-staphylococcus bacteriophage k for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 2005, 71, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Pantucek, R.; Rosypalova, A.; Doskar, J.; Kailerova, J.; Ruzickova, V.; Borecka, P.; Snopkova, S.; Horvath, R.; Gotz, F.; Rosypal, S. The polyvalent staphylococcal phage phi 812: Its host-range mutants and related phages. Virology 1998, 246, 241–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmelcher, M.; Shabarova, T.; Eugster, M.R.; Eichenseher, F.; Tchang, V.S.; Banz, M.; Loessner, M.J. Rapid multiplex detection and differentiation of listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl. Environ. Microbiol. 2010, 76, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M.; Lardeo, M.; Foster-Frey, J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 2006, 265, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Schneewind, O. Target cell specificity of a bacteriocin molecule: A c-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996, 15, 4789–4797. [Google Scholar] [PubMed]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a bacteriophage lytic enzyme plygbs significantly increases its antibacterial activity against group b streptococci. Appl. Microbiol. Biotechnol. 2007, 74, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Extracellular and intracellular regulation of calcium homeostasis. Sci. World J. 2001, 1, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Jung, G.M.; Son, J.S.; Yoon, S.J.; Choi, Y.J.; Kang, S.H. Comparison of the antibacterial properties of phage endolysins sal-1 and lysk. Antimicrob. Agents Chemother. 2011, 55, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Z.; Fujiwara, T.; Komatsuzawa, H.; Sugai, M.; Sakon, J. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 2006, 281, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Loffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014, 304, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered endolysin-based “artilysins” to combat multidrug-resistant Gram-negative pathogens. Am. Soc. Microbiol. 2014, 5, e01379-14. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rubio, L.; Chang, W.L.; Gutierrez, D.; Lavigne, R.; Martinez, B.; Rodriguez, A.; Govers, S.K.; Aertsen, A.; Hirl, C.; Biebl, M.; et al. ‘Artilysation’ of endolysin lambdasa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci. Rep. 2016, 6, 35382. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strains | Name | Refarences and Remarks | ||||
|---|---|---|---|---|---|---|
| Staphylococcus aureus | SA003 | Synnott, A.J. et al. [28], Iwano, H. et al. [29] | ||||
| Staphylococcus pseudointermedius | StaP001 | Field isolates identified by their 16s ribosomal RNA sequences. | ||||
| Staphylococcus haemolyticus | StaH001 | |||||
| Streptcoccus agalactiae | StrA001 | |||||
| Scc mec | MLST | spa type | Antimicrobial-resistance pattern | Refarences | ||
| MRSA | MRSA 2007-13 | II | NT | t002 | MPIPC, GM, KM, EM, OTC, ERFX | Ishihara, K. et al. [47] |
| MRSA 2007-28 | II | NT | t1265 | MPIPC, KM, EM, | ||
| MRSA 2007-57 | IV | NT | t008 | MPIPC, GM, KM, EM | ||
| MRSA 2007-93 | II | NT | t062 | MPIPC, KM, EM, OTC, CP, ERFX | ||
| MRSA VC39 Vet-1 | IV | ST380 | t021 | MPIPC, SM, KM, GM, EM | Ishihara, K. et al. | |
| MRSA VC50 Vet-1 | IV | ST30 | t1852 | MPIPC, KM, GM, EM, CPFX | [48] | |
| Plasmids | Protein Produced (Amino Acids) | Forward Primes | Reverse Primers | Recipient Vectors |
|---|---|---|---|---|
| pGEX-Lys012WT | 1-495 | Lys012-1Fw | Lys012-495Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt1 | 161-495 | Lys012-161Fw | Lys012-495Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt2 | 1-221, 390-495 | 1-221; Lys012-1Fw | 1-221; Lys012-Δmt2Rv | pGEX-6P-2 |
| 390-495; Lys012-Δmt2Fw | 390-495; Lys012-495Rv | |||
| Overwrap; Lys012-1Fw | Overwrap; Lys012-495Rv | |||
| pGEX-Lys012Δmt2′ | 1-221 | Lys012-1Fw | Lys012-221Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt3 | 1-408 | Lys012-1Fw | Lys012-408Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt4 | 386-495 | Lys012-386Fw | Lys012-495Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt5 | 161-408 | Lys012-161Fw | Lys012-408Rv | pGEX-6P-2 |
| pGEX-Lys012Δmt6 | 1-187 | Lys012-1Fw | Lys012-187Rv | pGEX-6P-2 |
| Primers | Sequences | |||
| Lys012-1Fw | 5′-TCCCCAGGAATTCCCATGGCTAAGACTCAAGCAGA-3′ | |||
| Lys012-161Fw | 5′-TCCCCAGGAATTCCCATGATACCTGTAAAAGCAGGAA-3′ | |||
| Lys012-386Fw | 5′-TCCCCAGGAATTCCCATGACAAGTAGCGCA-3′ | |||
| Lys012-187Rv | 5′-CGCTCCAGTCGACCCCTATTTCTTTTTAGGTGCAG-3′ | |||
| Lys012-221Rv | 5′-CGCTCGAGTCGACCCCTATGAAGAACGACCTGC-3′ | |||
| Lys012-408Rv | 5′-CGCTAGTCGACCCCTAAGTTCCGTACTGGTTC-3′ | |||
| Lys012-495Rv | 5′-CGCTCGAGTCGACCCCTACTTGAATACTCCCCAGG-3′ | |||
| Lys012-Δmt2Fw | 5′-CACAACGATGCAGGTCGTTCTTCAAGTACACCGGCAACTAGACCAGTTAC-3′ | |||
| Lys012-Δmt2Rv | 5′-GTAACTGGTCTAGTTGCCGGTGTACTTGAAGAACGACCTGCATCGTTGTG-3′ | |||
| Homologous Protein | Source Organism | Residues | Protein ID in PDBe | Seq. Identity | Ligands | |
|---|---|---|---|---|---|---|
| Lys-phiSA012 CHAP | ORF30/31 | Staphylococcus virus K | 1–165 | 4ct3.1 | 99.39% | Ca2+ |
| CHAP domain | (Gene name: PhageK_071) | Cl− | ||||
| Hg2+ | ||||||
| Lys-phiSA012 AMID | Endolysin | Staphylococcus phage GH15 | 165–403 | 4ols | 100% | Zn2+ |
| N-acetylmuramoyl-l-alanine amidase | (Gene name: GH15_071) | Mg2+ | ||||
| Fe3+ |
| Source of Endolysin | Query Cover (Nucleotide) | Identity (Nucleotide) | Accession | Resideu at Position (Amino Asid) | Identity (Amino Acid) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 48 | 83 | 107 | 109 | 113 | 165 | 231 | 300 | 372 | 380 | 383 | 406 | 414 | 425 | 437 | 452 | 453 | 470 | 484 | 485 | 486 | 493 | |||||
| Bacteriophage K | 100% | 100% | AY176327.1 | V | G | S | F | S | E | A | N | A | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 100.00% |
| Bacteriophage JD007 | 100% | 99% | JX878671.1 | V | G | S | F | S | E | A | N | A | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 100.00% |
| Bacteriophage SA012 | 100% | 99% | AB903967.1 | V | G | N | F | S | E | A | N | A | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 99.80% |
| Bacteriophage vB_Sau_CG | 100% | 99% | KY794641.1 | V | G | N | F | S | Q | A | T | A | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 99.39% |
| Bacteriophage S25-3 | 100% | 99% | AB853330.1 | V | G | N | F | S | E | E | N | T | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 99.39% |
| Bacteriophage S25-4 | 100% | 99% | AB853331.1 | V | G | N | F | S | E | E | N | T | D | V | D | Y | N | V | V | V | C | N | N | Q | I | V | 99.39% |
| Bacteriophage SA3 | 100% | 96% | MF001365.1 | I | G | S | F | S | Q | A | N | A | D | V | D | Y | N | V | V | V | C | N | N | H | I | V | 99.39% |
| Bacteriophage qdsa002 | 100% | 96% | KY779849.1 | I | G | S | F | S | Q | A | N | A | D | V | D | Y | N | V | V | V | C | N | N | H | I | V | 99.39% |
| Bacteriophage GH15 | 100% | 96% | JQ686190.1 | I | G | S | F | S | Q | A | N | A | D | V | D | Y | N | V | V | V | C | D | N | H | I | V | 99.19% |
| Bacteriophage vB_Sau_Clo6 | 100% | 94% | KY794642.1 | V | K | N | Y | E | E | A | K | A | N | V | K | Y | N | V | V | I | C | D | S | Y | T | I | 97.98% |
| Bacteriophage vB_Sau_S24 | 100% | 94% | KY794643.1 | V | K | N | Y | E | E | A | K | A | N | V | K | Y | N | V | V | V | A | N | N | Q | V | T | 97.37% |
| Bacteriophage MCE-2014 | 99% | 99% | KJ888149.1 | V | G | S | F | S | E | A | N | A | D | V | D | Y | N | V | V | V | C | N | S | H | V | V | 99.39% |
| Bacteriophage IPLA-RODI | 99% | 95% | KP027446.1 | I | G | S | F | S | Q | A | N | A | D | I | D | F | S | I | I | V | C | N | N | H | I | V | 98.38% |
| CHAP | Amidase | SH3b | |||||||||||||||||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiki, J.; Nakamura, T.; Furusawa, T.; Ohno, H.; Takahashi, H.; Kitana, J.; Usui, M.; Higuchi, H.; Tanji, Y.; Tamura, Y.; et al. Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage. Pharmaceuticals 2018, 11, 25. https://doi.org/10.3390/ph11010025
Fujiki J, Nakamura T, Furusawa T, Ohno H, Takahashi H, Kitana J, Usui M, Higuchi H, Tanji Y, Tamura Y, et al. Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage. Pharmaceuticals. 2018; 11(1):25. https://doi.org/10.3390/ph11010025
Chicago/Turabian StyleFujiki, Jumpei, Tomohiro Nakamura, Takaaki Furusawa, Hazuki Ohno, Hiromichi Takahashi, Junya Kitana, Masaru Usui, Hidetoshi Higuchi, Yasunori Tanji, Yutaka Tamura, and et al. 2018. "Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage" Pharmaceuticals 11, no. 1: 25. https://doi.org/10.3390/ph11010025
APA StyleFujiki, J., Nakamura, T., Furusawa, T., Ohno, H., Takahashi, H., Kitana, J., Usui, M., Higuchi, H., Tanji, Y., Tamura, Y., & Iwano, H. (2018). Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage. Pharmaceuticals, 11(1), 25. https://doi.org/10.3390/ph11010025





