Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties
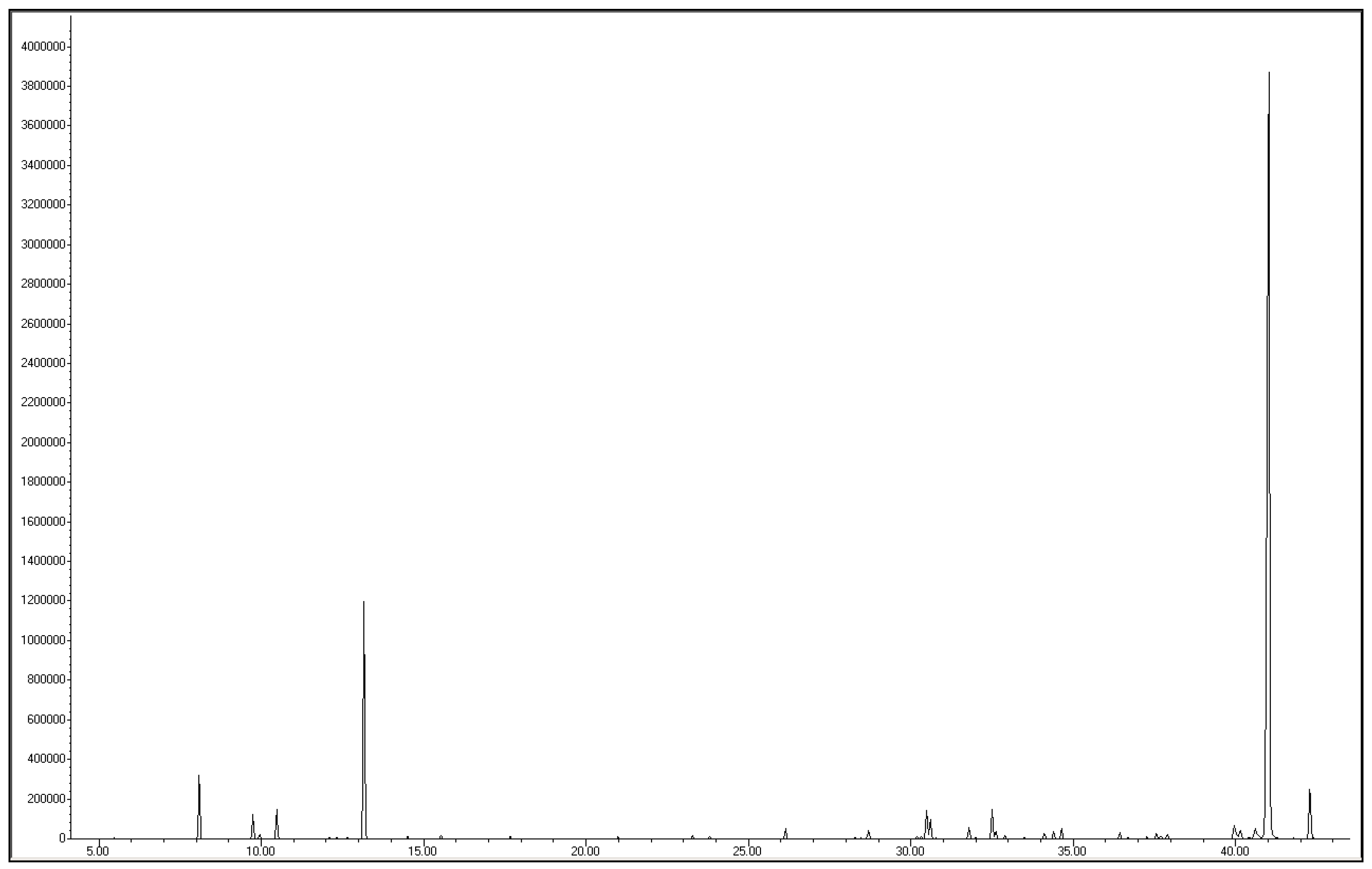
2.2. Chemical Composition
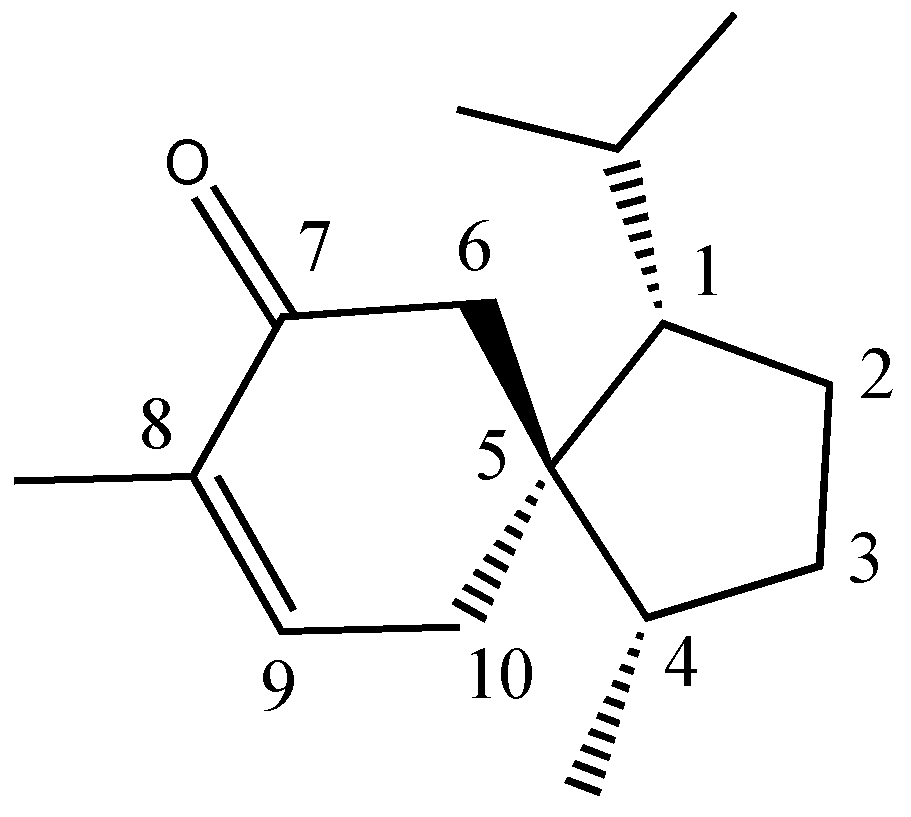
2.3. Isolation and Characterization of Acorenone B
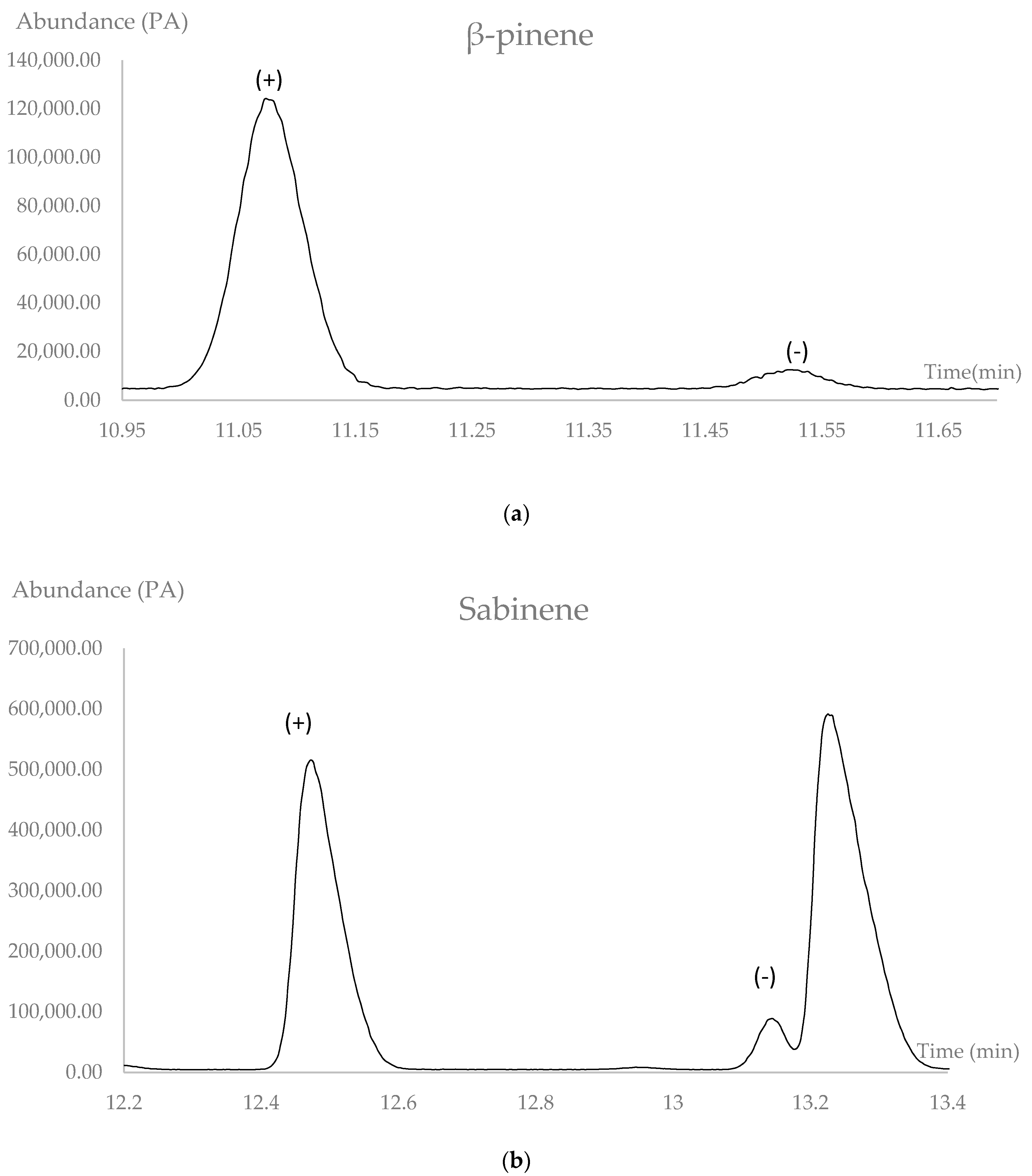
2.4. Enantiomeric Analysis
2.5. Antimicrobial Activity
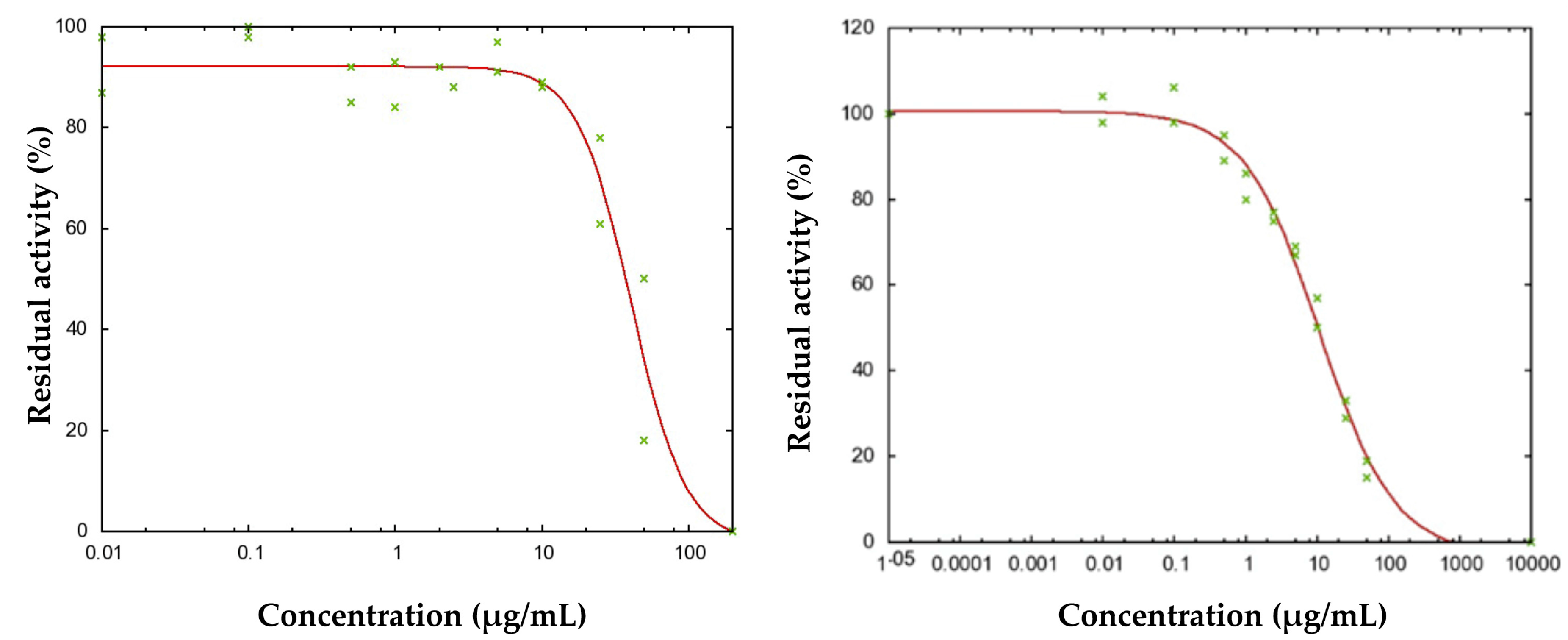
2.6. Cholinesterase Inhibition Test
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Essential Oil
3.3. Physical Analysis
3.4. Gas Chromatography Coupled to Mass-Spectrometry (GC-MS)
3.5. Gas Chromatography Coupled to Flame Ionization Detector (GC-FID)
3.6. Enantioselective GC Analysis
3.7. Isolation and Identification of Acorenone B
3.8. Antimicrobial Activity
3.9. Cholinesterase Inhibition Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; Obied, H.K. Biophenols of mints: Antioxidant, acetylcholinesterase, butyrylcholinesterase and histone deacetylase inhibition activities targeting Alzheimer’s disease treatment. J. Funct. Foods 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Fadaeinasab, M.; Hadi, A.H.A.; Kia, Y.; Basiri, A.; Murugaiyah, V. Cholinesterase enzymes inhibitors from the leaves of Rauvolfia reflexa and their molecular docking study. Molecules 2013, 18, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Zachow, L.L.; Ávila, J.M.; Saldanha, G.A.; Mostardeiro, M.A.; da Silva, U.F.; Morel, A.F.; Dalcol, I.I. Chemical composition and evaluation of prolyl oligopeptidase and acetylcholinesterase inhibitory activities of Leonurus Sibiricus L. from Brazil. Nat. Prod. Res. 2017, 31, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Chuiko, G.; Podgornaya, V.; Zhelnin, Y. Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: Cross-species and cross-family differences. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 55–61. [Google Scholar] [CrossRef]
- Manalo, R.V.; Silvestre, M.A.; Barbosa, A.L.A.; Medina, P.M. Coconut (Cocos nucifera) Ethanolic Leaf Extract Reduces Amyloid-β (1–42) Aggregation and Paralysis Prevalence in Transgenic Caenorhabditis elegans Independently of Free Radical Scavenging and Acetylcholinesterase Inhibition. Biomedicines 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, A.; Akıncıoğlu, H.; Gülçin, İ.; Durdagi, S.; Supuran, C.T.; Göksu, S. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg. Med. Chem. 2015, 23, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, L.; Osorio, J. Variación de la actividad de la enzima Butirilcolinesterasa en usuarias de anticonceptivos hormonales. In Anales de la Facultad de Medicina; Universidad Nacional Mayor de San Marcos: Lima, Peru, 2000. [Google Scholar]
- Çokuğraş, A.N. Butyrylcholinesterase: structure and physiological importance. Turk. J. Biochem. 2003, 28, 54–61. [Google Scholar]
- Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic Essential Oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) Growing in Central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef] [PubMed]
- Lamamra, M.; Laouer, H.; Amira, S.; Orhan, I.E.; Senol, F.S.; Demirci, B.; Akkal, S. Chemical Composition and Cholinesterase Inhibitory Activity of Different Parts of Daucus aristidis Coss. Essential Oils from Two Locations in Algeria. Rec. Natl. Prod. 2017, 11, 147. [Google Scholar]
- Baldemir, A.; Topçu, H.; Paksoy, M.Y.; Motalebipour, E.Z.; Kafkas, S. First microsatellite markers for Scaligeria lazica Boiss.(Apiaceae) by next-generation sequencing: Population structure and genetic diversity analysis. Biotechnol. Biotechnol. Equip. 2017, 31, 535–543. [Google Scholar] [CrossRef]
- Izco, J.; Pulgar, Í.; Aguirre, Z.; Santin, F. Estudio florístico de los páramos de pajonal meridionales de Ecuador. Rev. Peru. Biol. 2007, 14, 237–246. [Google Scholar] [CrossRef]
- Abad, X. Determinación de la Actividad Antimicrobiana de los Extractos Totales de Cuatro Especies Vegetales de las Provincias de Loja y Zamora Chinchipe: Piper ecuadorense (Matico), Lepechinia mutica Benth (Turuyante), Fuschia ayavacensis (Pena-Pena), Niphogeton dissecta (Culantrillo del cerro), Empleando los Métodos de Difusión en Placa, Concentración Mínima Inhibitoria e Inhibición del Crecimiento Radial; Departamento de Química, Universidad Técnica Particular de Loja: Loja, Ecuador, 2009; p. 88. [Google Scholar]
- Rajabi, Z.; Ebrahimi, M.; Farajpour, M.; Mirza, M.; Ramshini, H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crops Prod. 2014, 61, 233–239. [Google Scholar] [CrossRef]
- Benyelles, B.; Allali, H.; Dib, M.E.A.; Djabou, N.; Paolini, J.; Costa, J. Chemical Composition Variability of Essential Oils of Daucus gracilis Steinh. from Algeria. Chem. Biodivers. 2017. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [PubMed]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography–mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Triki, M.A.; Rebai, A. Chemical compositions, antibacterial and antioxidant activities of essential oil and various extracts of Geranium sanguineum L. flowers. Arch. Appl. Sci. Res. 2011, 3, 135–144. [Google Scholar]
- Skaltsa, H.D.; Mavrommati, A.; Constantinidis, T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae). Phytochemistry 2001, 57, 235–244. [Google Scholar] [CrossRef]
- Zalkow, L.H.; Baxter, J.T.; McClure, R.J.; Gordon, M.M. A Phytochemical Investigation of Bothriochloa intermedia. J. Natl. Prod. 1980, 43, 598–608. [Google Scholar] [CrossRef]
- Yousefbeyk, F.; Golfakhrabadi, F.; Amouei, A.; Ghasemi, S.; Amin, M.; Gohari, A.; Samadi, N.; Amini, M.; Amin, G. Phytochemical Investigation and Antifungal Activity of Daucus littoralis Smith sub sp. hyrcanicus Rech. f. Res. J. Phytochem. 2015, 9, 33–44. [Google Scholar]
- Kubeczka, K.-H.; Bohn, I.; Schultze, W.; Formaček, V. The Composition of the Essential Oils of Chaerophyllum hirsutum L. J. Essent. Oil Res. 1989, 1, 249–259. [Google Scholar] [CrossRef]
- Lawrence, B.M.; Morton, J.K. Acorenone-B in Angelica lucida oil. Phytochemistry 1974, 13, 528. [Google Scholar] [CrossRef]
- Kaul, V.K.; Vats, S. Essential oil composition of Bothriochloa pertusa and phyletic relationship in aromatic grasses. Biochem. Syst. Ecol. 1998, 26, 347–356. [Google Scholar] [CrossRef]
- Lin, J.; Dou, J.; Xu, J.; Aisa, H.A. Chemical composition, antimicrobial and antitumor activities of the essential oils and crude extracts of Euphorbia macrorrhiza. Molecules 2012, 17, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, A. Chemical constituents, antimicrobial and antioxidant activity of the hexane extract from root and seed of Levisticum persicum Freyn and Bornm. J. Med. Plants Res. 2011, 5, 5127–5131. [Google Scholar]
- Yousefbeyk, F.; Gohari, A.R.; Sourmaghi, M.H.S.; Amini, M.; Jamalifar, H.; Amin, M.; Golfakhrabadi, F.; Ramezani, N.; Amin, G. Chemical Composition and Antimicrobial Activity of Essential Oils from Different Parts of Daucus littoralis Smith subsp. hyrcanicus Rech. f. J. Essent. Oil Bear. Plants 2014, 17, 570–576. [Google Scholar] [CrossRef]
- Bahl, J.; Padalia, R.C.; Verma, R.S.; Bansal, R.P. Essential Oil Composition of Bothriochloa bladhii (Retz.) ST Blake: An Introduction from Tropical Region of Western Ghats of India. J. Essent. Oil Bear. Plants 2014, 17, 136–141. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; García, M.Á.; Marina, M.L. Characteristics and enantiomeric analysis of chiral pyrethroids. J. Chromatogr. A 2010, 1217, 968–989. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.R.D.; Lopes, P.M.; Azevedo, M.M.B.D.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of a-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative Anti-Infectious Bronchitis Virus (IBV) Activity of (−)-Pinene: Effect on Nucleocapsid (N) Protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias Instituto Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Kosasa, T.; Kuriya, Y.; Yamanishi, Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Bernardo-Gil, M.G.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Zeb, A.; Hameed, A.; Khan, L.; Khan, I.; Dalvandi, K.; Iqbal Choudhary, M.; Basha, F.Z. Quinoxaline derivatives: Novel and selective butyrylcholinesterase inhibitors. Med. Chem. 2014, 10, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Bloniecki, V.; Aarsland, D.; Blennow, K.; Cummings, J.; Falahati, F.; Winblad, B.; Freund-Levi, Y. Effects of Risperidone and Galantamine Treatment on Alzheimer’s Disease Biomarker Levels in Cerebrospinal Fluid. J. Alzheimer's Dis. 2017, 57, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Vita Finzi, P.V.; Vidari, G. Phytochemical and ethnomedicinal study of Huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. J. Ethnopharmacol. 2016, 193, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.K.; Van Rijn, R.M.; Verpoorte, R. Qualitative determination of false positive effects in the acetylcholinesterase assays using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
| Component | LRI a | LRI lit b | % c | δ | Literature for LRI |
|---|---|---|---|---|---|
| α-Pinene | 930 | 932 | 3.94 | 1.79 | [18] |
| Sabinene | 969 | 969 | 1.41 | 0.28 | [18] |
| β-Pinene | 974 | 974 | 0.30 | 0.09 | [18] |
| β-Myrcene | 987 | 988 | 2.14 | 0.37 | [18] |
| p-Cymene | 1022 | 1020 | trace | -- | [18] |
| Limonene | 1027 | 1024 | 0.13 | 0.03 | [18] |
| (Z)-β-Ocimene | 1034 | 1032 | 0.10 | 0.04 | [18] |
| (E)-β-Ocimene | 1045 | 1044 | 29.64 | 1.63 | [18] |
| γ-Terpinolene | 1055 | 1056 | 0.29 | 0.30 | [19] |
| Unidentified | 1072 | -- | trace | -- | -- |
| α-Pinene oxide | 1093 | 1099 | trace | -- | [18] |
| Unidentified | 1155 | -- | trace | -- | -- |
| (E,E) 2,6-Dimethyl-3,5,7-octatriene-2-ol | 1205 | 1207 | trace | -- | [20] |
| Citronellol | 1224 | 1223 | 0.16 | 0.07 | [18] |
| Unidentified | 1256 | -- | 0.15 | 0.01 | -- |
| Geranial | 1264 | 1264 | 0.07 | 0.01 | [18] |
| Methyl geranate | 1318 | 1322 | 0.51 | 0.04 | [18] |
| Unidentified | 1334 | -- | 0.06 | 0.01 | -- |
| α-Copaene | 1371 | 1374 | trace | -- | [18] |
| β-Funebrene | 1411 | 1413 | 0.17 | 0.02 | [18] |
| (E)-Caryophyllene | 1414 | 1417 | 0.24 | 0.02 | [18] |
| β-Cedrene | 1417 | 1419 | 1.23 | 0.13 | [18] |
| cis-Thujopsene | 1420 | 1429 | 0.97 | 0.10 | [18] |
| (E)-β-Farnesene | 1450 | 1454 | 1.13 | 0.13 | [18] |
| allo-aromadendrene | 1454 | 1458 | trace | -- | [18] |
| α-Himachalene | 1457 | 1449 | trace | -- | [18] |
| cis-Cadina-1(6),4-diene | 1466 | 1461 | 1.70 | 0.17 | [18] |
| γ-Muurolene | 1469 | 1478 | 0.36 | 0.04 | [18] |
| Germacrene-D | 1475 | 1484 | 0.38 | 0.05 | [18] |
| ar-Curcumene | 1477 | 1479 | 0.15 | 0.01 | [18] |
| α-Zingiberene | 1491 | 1493 | 0.34 | 0.02 | [18] |
| (E,E)-α-Farnesene | 1501 | 1505 | trace | -- | [18] |
| Unidentified | 1506 | -- | 0.23 | 0.02 | -- |
| δ-Cadinene | 1513 | 1522 | 0.36 | 0.03 | [18] |
| β-Sesquiphellandrene | 1519 | 1521 | 0.89 | 0.08 | [18] |
| (E)-Nerolidol | 1557 | 1561 | 0.08 | 0.01 | [18] |
| Unidentified | 1565 | -- | 0.42 | 0.07 | -- |
| Spathulenol | 1569 | 1577 | 0.13 | 0.01 | [18] |
| Unidentified | 1581 | -- | 0.40 | 0.03 | -- |
| Unidentified | 1585 | -- | 0.15 | 0.01 | -- |
| Geranyl isovalerate | 1594 | 1606 | 0.57 | 0.05 | [18] |
| Cedrol | 1600 | 1600 | 0.33 | 0.03 | [18] |
| Unidentified | 1609 | -- | 0.07 | 0.01 | -- |
| α-Cadinol | 1645 | 1652 | 0.08 | 0.01 | [18] |
| Unidentified | 1655 | -- | 1.20 | 0.05 | -- |
| Acorenone | 1666 | 1655 | 0.11 | 0.01 | [21] |
| Acorenone B | 1683 | 1675 | 41.01 | 3.35 | [22] |
| (3E)-Butylidene phthalide | 1718 | 1717 | 5.54 | 0.93 | [18] |
| Unidentified | 1777 | -- | 0.11 | 0.04 | -- |
| Sandaracopimaradiene | 1943 | 1942 | trace | -- | [23] |
| Biformene | 1983 | 1990 | trace | -- | [18] |
| Unidentified | 2008 | -- | trace | -- | -- |
| (E,E)-Geranyl linalool | 2016 | 2026 | trace | -- | [18] |
| Unidentified | 2047 | -- | trace | -- | -- |
| Unidentified | 2052 | -- | 0.64 | 0.19 | -- |
| Monoterpene hydrocarbons | - | - | 37.97 | ||
| Oxygenated monoterpene | - | - | 0.25 | ||
| Sesquiterpene hydrocarbons | - | - | 9.71 | ||
| Oxygenated sesquiterpene | - | - | 42.31 | ||
| Others | - | - | 6.22 | ||
| Total amount of compounds | - | - | 96.46% |
| Components | RT a (min) | LRI b | Enantiomeric Distribution (%) | e.e. (%) |
|---|---|---|---|---|
| (+)-β-pinene | 11.08 | 957 | 86.9 | 73.8 |
| (−)-β-pinene | 11.52 | 965 | 13.1 | |
| (+)-Sabinene | 12.47 | 983 | 80.9 | 61.8 |
| (−)-Sabinene | 13.15 | 997 | 19.1 |
| Microorganism | E.O, (Niphogeton dissecta) mg/mL | Acorenone B (mg/mL) |
|---|---|---|
| Candida albicans | NA | NA |
| Enterococcus faecalis | 10 | NA |
| Escherichia coli | NA | NA |
| Micrococcus luteus | NA | NA |
| Staphylococcus aureus | 5 | NA |
| Compound | AChE (IC50) μg/mL | BChE (IC50) μg/mL |
|---|---|---|
| Niphogeton dissecta essential oil | NA | 11.5 |
| Acorenone B | 40.8 | 10.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calva, J.; Bec, N.; Gilardoni, G.; Larroque, C.; Cartuche, L.; Bicchi, C.; Montesinos, J.V. Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr. Pharmaceuticals 2017, 10, 84. https://doi.org/10.3390/ph10040084
Calva J, Bec N, Gilardoni G, Larroque C, Cartuche L, Bicchi C, Montesinos JV. Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr. Pharmaceuticals. 2017; 10(4):84. https://doi.org/10.3390/ph10040084
Chicago/Turabian StyleCalva, James, Nicole Bec, Gianluca Gilardoni, Christian Larroque, Luis Cartuche, Carlo Bicchi, and José Vinicio Montesinos. 2017. "Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr" Pharmaceuticals 10, no. 4: 84. https://doi.org/10.3390/ph10040084
APA StyleCalva, J., Bec, N., Gilardoni, G., Larroque, C., Cartuche, L., Bicchi, C., & Montesinos, J. V. (2017). Acorenone B: AChE and BChE Inhibitor as a Major Compound of the Essential Oil Distilled from the Ecuadorian Species Niphogeton dissecta (Benth.) J.F. Macbr. Pharmaceuticals, 10(4), 84. https://doi.org/10.3390/ph10040084








