Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and In Vivo Characterization
Abstract
1. Introduction
2. Results and Discussion
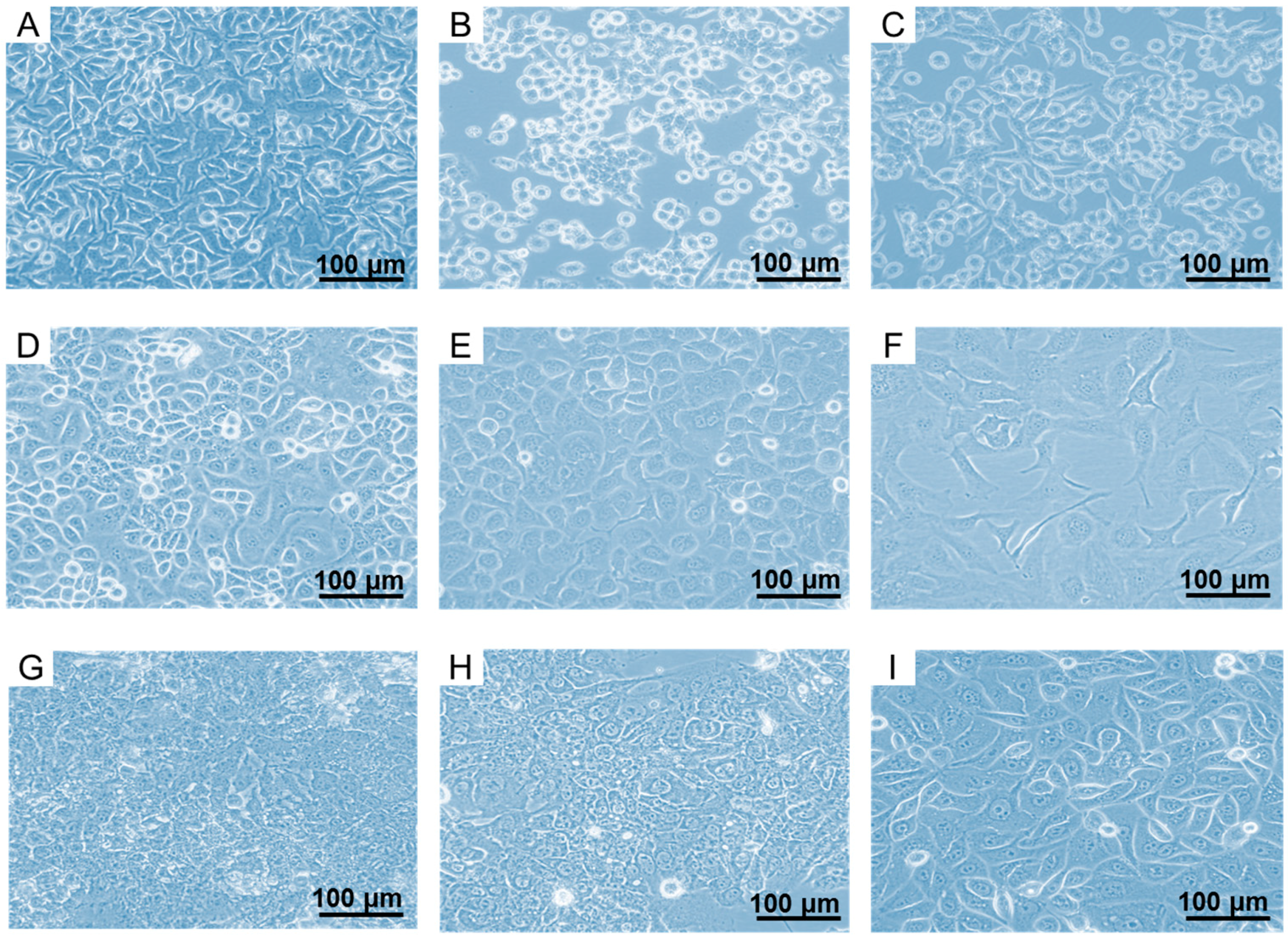
2.1. In Vitro Culturing of FR-Expressing Cancer Cell Lines
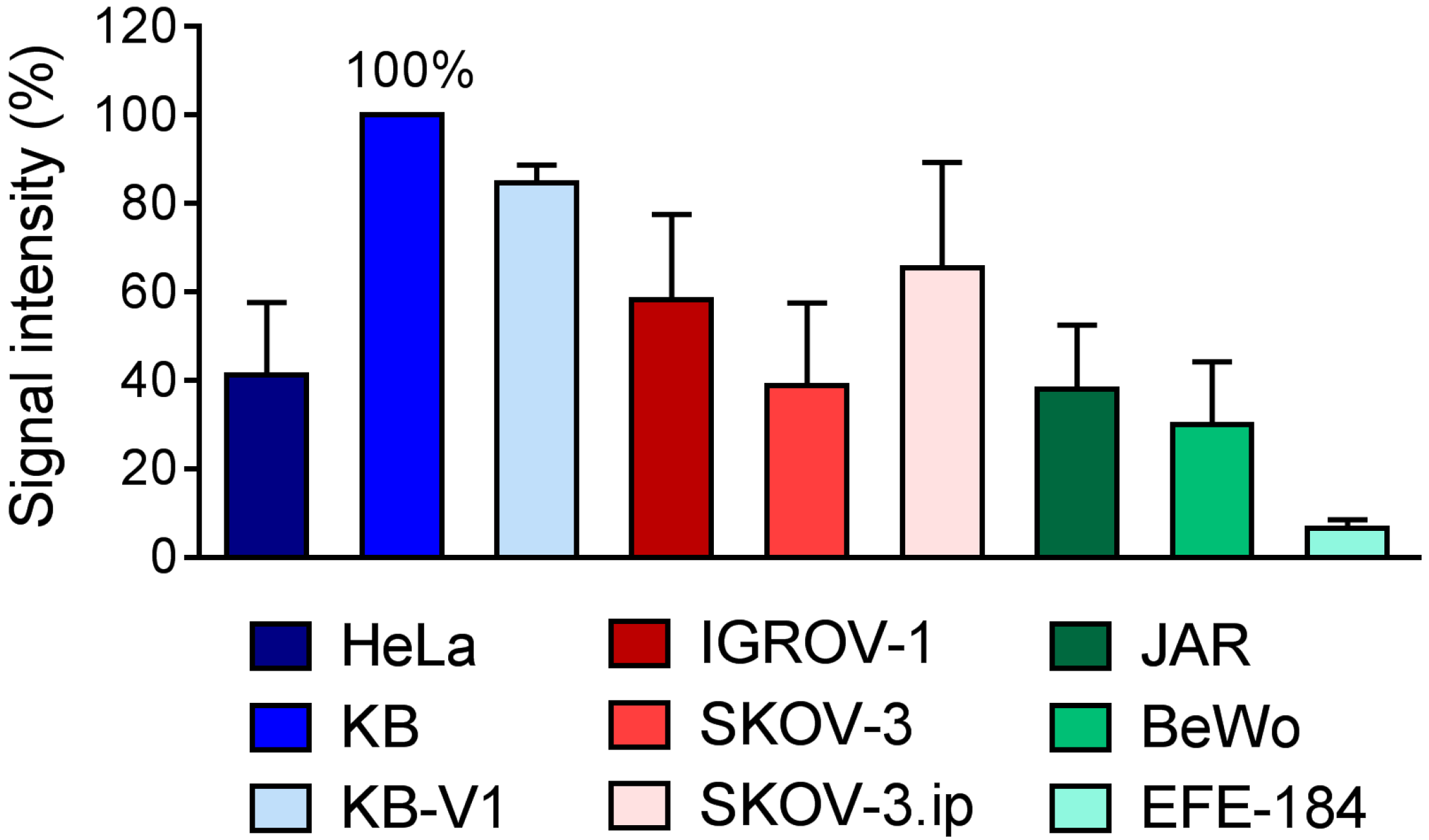
2.2. Determination of FR-Expression Levels of Cells Cultured In Vitro
2.3. Tumor Cell Characterization beyond FR-Expression
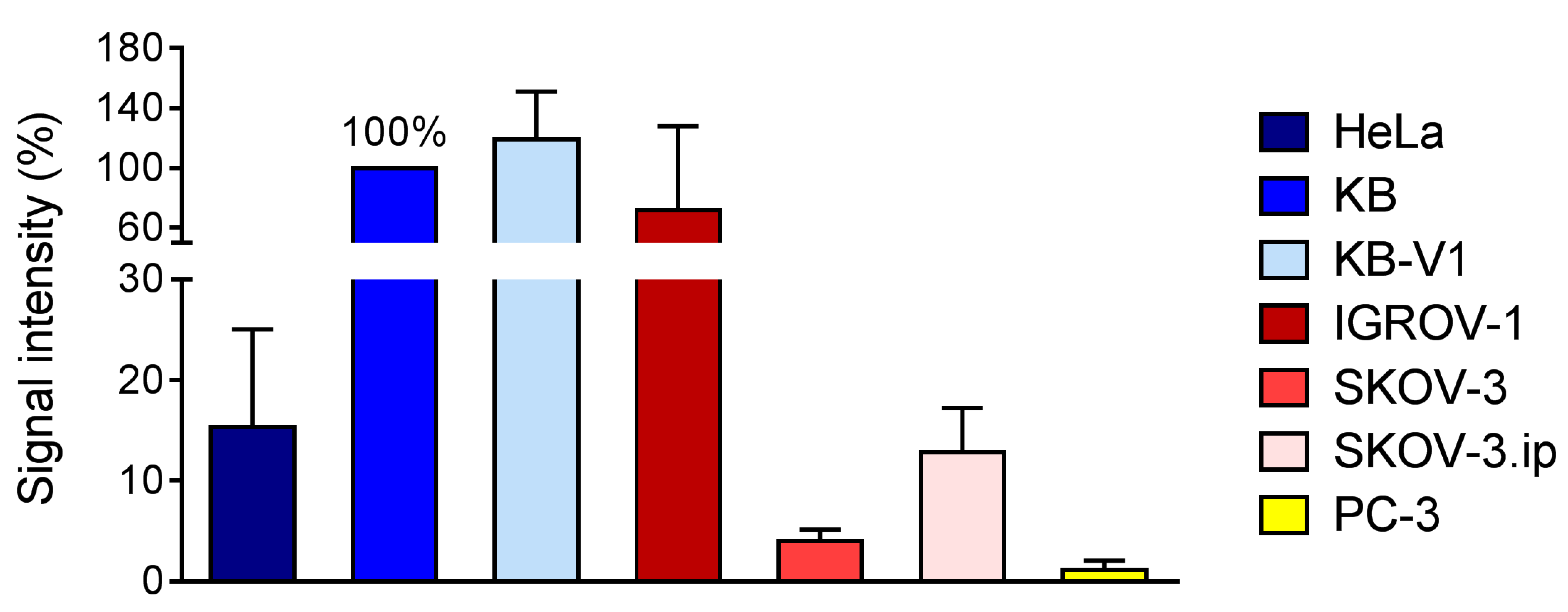
2.3.1. Expression of L1-Cell Adhesion Molecule
2.3.2. Expression of Human Epidermal Growth Factor Receptor-2
2.3.3. Sensitivity towards Chemotherapeutics
2.4. Gynecologic Tumor Xenograft Mouse Models
2.5. FR-Expression Levels in Tumor Xenografts
2.5.1. Determination of FR-Expression Using Autoradiography
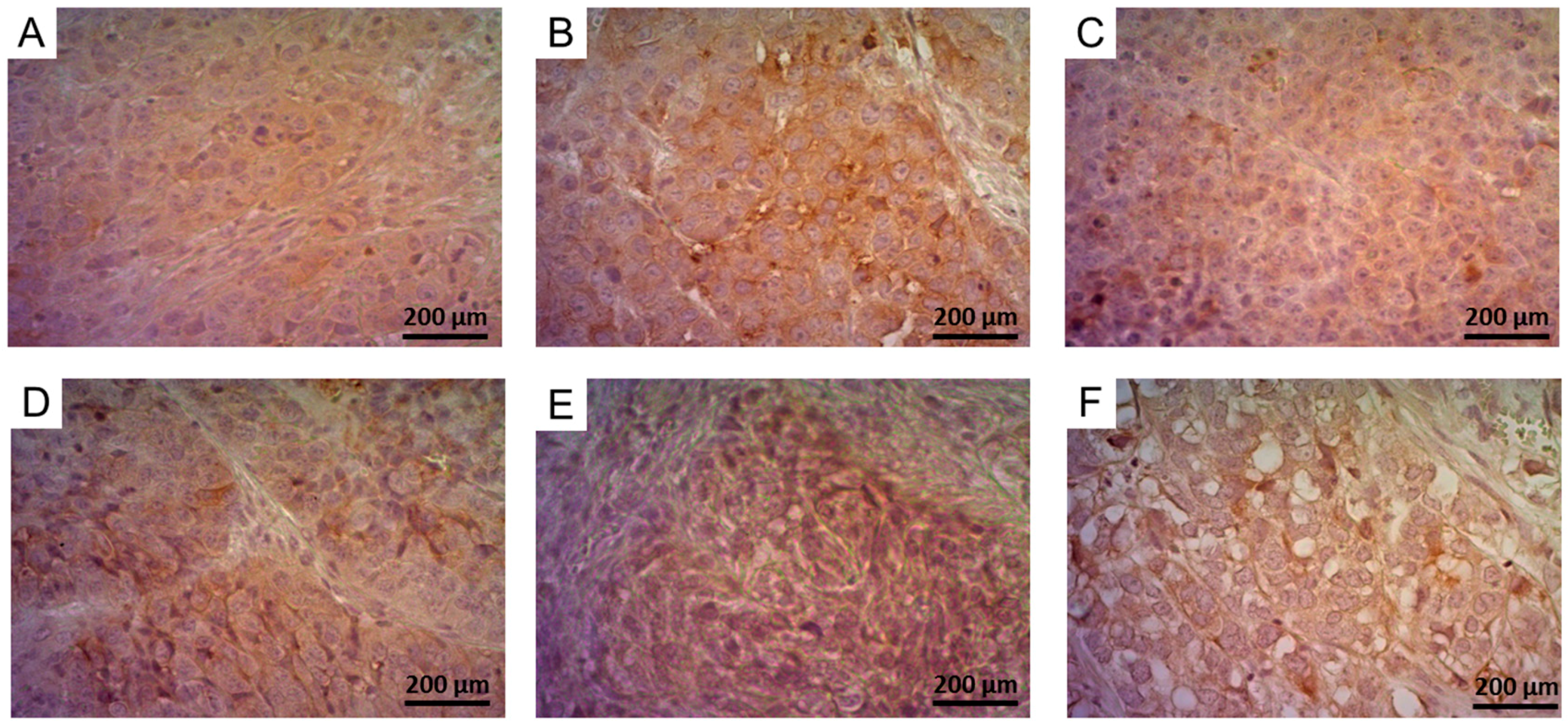
2.5.2. Determination of FR-Expression Using Immunohistochemistry
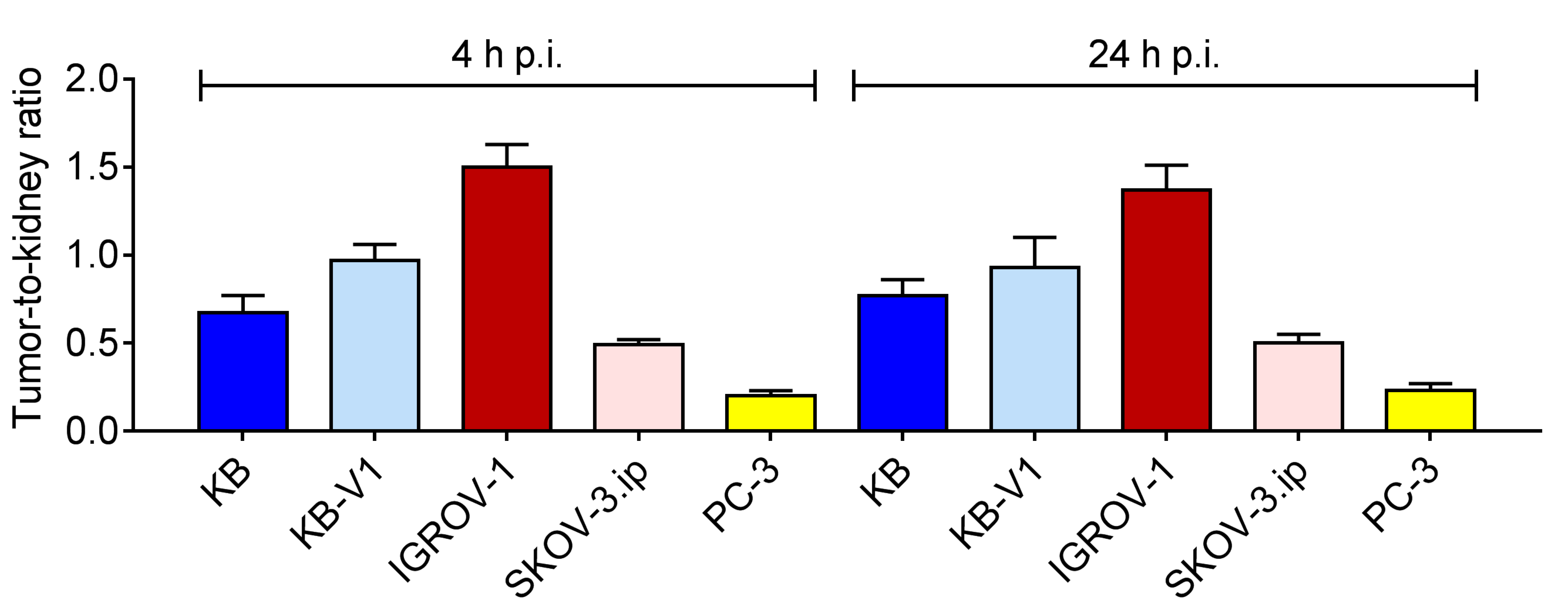
2.6. Biodistribution Experiments
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Preparation of 177Lu-Folate
4.3. Cell Lines and Cell Culture
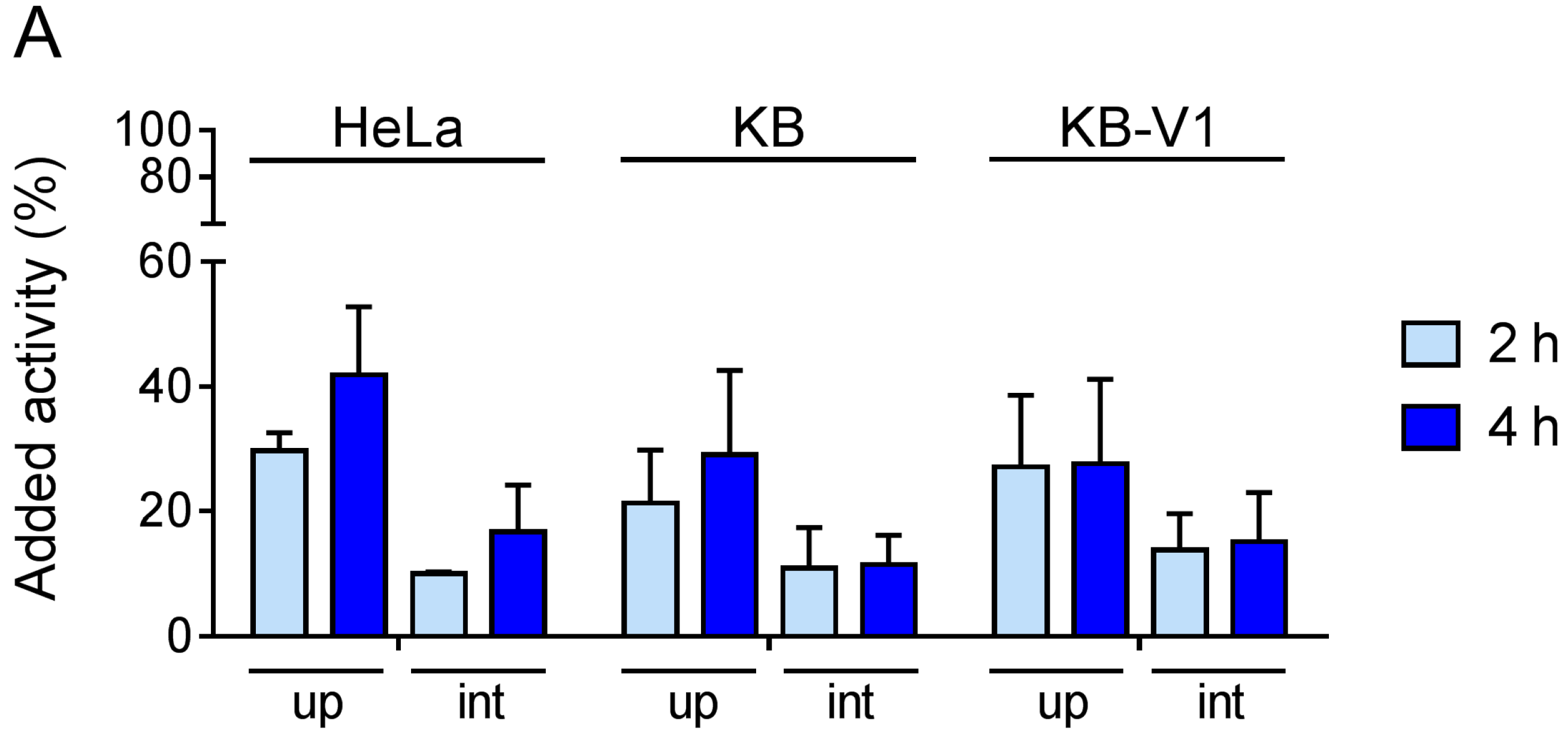
4.4. Cell Internalization Experiments
4.5. Western Blot
4.6. In Vitro Autoradiography
4.7. Immunohistochemistry
4.8. Animal Experiments
4.9. Biodistribution Experiments
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garin-Chesa, P.; Campbell, I.; Saigo, P.E.; Lewis, J.L.; Old, L.J.; Rettig, W.J. Trophoblast and ovarian cancer antigen LK26—Sensitivity and specificity in immunopathology and molecular identification as a folate-binding protein. Am. J. Pathol. 1993, 142, 557–567. [Google Scholar] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Hansen, S.I.; Hoiermadsen, M.; Bostad, L. A high-affinity folate binding-protein in proximal tubule cells of human kidney. Kidney Int. 1992, 41, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Birn, H.; Spiegelstein, O.; Christensen, E.I.; Finnell, R.H. Renal tubular reabsorption of folate mediated by folate binding protein 1. J. Am. Soc. Nephrol. 2005, 16, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. ESMO 2015, 26, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Paulos, C.M.; Reddy, J.A.; Leamon, C.P.; Turk, M.J.; Low, P.S. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004, 66, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, J.; Lantrip, D.A.; Waters, D.J.; Mathias, C.J.; Green, M.A.; Fuchs, P.L.; Low, P.S. Design and synthesis of [111In]DTPA-folate for use as a tumor-targeted radiopharmaceutical. Bioconjugate Chem. 1997, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.A.; Dehdashti, F.; Mutch, D.G.; Podoloff, D.A.; Wendt, R.; Sutton, G.P.; Burt, R.W.; Ellis, P.R.; Mathias, C.J.; Green, M.A.; et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: Initial clinical results. J. Nucl. Med. 2003, 44, 700–707. [Google Scholar] [PubMed]
- Reddy, J.A.; Xu, L.C.; Parker, N.; Vetzel, M.; Leamon, C.P. Preclinical evaluation of 99mTc-EC20 for imaging folate receptor-positive tumors. J. Nucl. Med. 2004, 45, 857–866. [Google Scholar] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Vlahov, I.R.; Westrick, E.; Parker, N.; Nicoson, J.S.; Vetzel, M. Comparative preclinical activity of the folate-targeted vinca alkaloid conjugates EC140 and EC145. Int. J. Cancer 2007, 121, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Vlahov, I.R.; Vetzel, M.; Parker, N.; Nicoson, J.S.; Xu, L.C.; Westrick, E. Synthesis and biological evaluation of EC72: A new folate-targeted chemotherapeutic. Bioconjug. Chem. 2005, 16, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.A.; Westrick, E.; Santhapuram, H.K.; Howard, S.J.; Miller, M.L.; Vetzel, M.; Vlahov, I.; Chari, R.V.; Goldmacher, V.S.; Leamon, C.P. Folate receptor-specific antitumor activity of EC131, a folate-maytansinoid conjugate. Cancer Res. 2007, 67, 6376–6382. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.A.; Dorton, R.; Westrick, E.; Dawson, A.; Smith, T.; Xu, L.C.; Vetzel, M.; Kleindl, P.; Vlahov, I.R.; Leamon, C.P. Preclinical evaluation of EC145, a folate-vinca alkaloid conjugate. Cancer Res. 2007, 67, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Vetzel, M.; Dorton, R.; Westrick, E.; Parker, N.; Wang, Y.; Vlahov, I. Folate targeting enables durable and specific antitumor responses from a therapeutically null tubulysin b analogue. Cancer Res. 2008, 68, 9839–9844. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Reber, J.; Brandt, S.; Bernhardt, P.; Groehn, V.; Schibli, R.; Müller, C. Folate receptor-targeted radionuclide therapy: Preclinical investigation of anti-tumor effects and potential radionephropathy. Nucl. Med. Biol. 2015, 42, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.T.; Joyrich, R.N.; Naumann, R.W.; Shah, N.P.; Maurer, A.H.; Strauss, H.W.; Uszler, J.M.; Symanowski, J.T.; Ellis, P.R.; Harb, W.A. Phase ii study of treatment of advanced ovarian cancer with folate-receptor-targeted therapeutic (vintafolide) and companion SPECT-based imaging agent (99mTc-etarfolatide). Ann. Oncol. ESMO 2014, 25, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.J.; Shetty, A.; Lu, Y.; Ellis, R.; Low, P.S. A phase i study of folate immune therapy (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J. Immunother. 2013, 36, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Vlahov, I.R.; Westrick, E.; Dawson, A.; Dorton, R.; Vetzel, M.; Santhapuram, H.K.; Wang, Y. Preclinical antitumor activity of a novel folate-targeted dual drug conjugate. Mol. Pharm. 2007, 4, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Klein, P.J.; Vlahov, I.R.; Dorton, R.; Bloomfield, A.; Nelson, M.; Westrick, E.; Parker, N.; Bruna, K.; et al. Reducing undesirable hepatic clearance of a tumor-targeted vinca alkaloid via novel saccharopeptidic modifications. J. Pharmacol. Exp. Ther. 2011, 336, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, M.; Thakur, A.; Rinaldi, F. Degradation of bms-753493, a novel epothilone folate conjugate anticancer agent. Drug Dev. Ind. Pharm. 2013, 39, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Huang, L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J. Biol. Chem. 1996, 271, 8481–8487. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, S.R.; Zhou, R.; Kung, H.F.; Chen, I.W. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Acad. Radiol. 2004, 11, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schubiger, P.A.; Schibli, R. In vitro and in vivo targeting of different folate receptor-positive cancer cell lines with a novel 99mTc-radiofolate tracer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Maitani, Y. Folate-linked nanoparticle-mediated suicide gene therapy in human prostate cancer and nasopharyngeal cancer with herpes simplex virus thymidine kinase. Cancer Gene Ther. 2005, 12, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.R. Human cancer cell lines: Fact and fantasy. Nat. Rev. Mol. Cell Biol. 2000, 1, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zeng, X.; Wang, Z.; Chen, Q. Cell line cross-contamination: KB is not an oral squamous cell carcinoma cell line. Eur. J. Oral Sci. 2009, 117, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Mornet, E.; Carmoy, N.; Laine, C.; Lemiegre, L.; Le Gall, T.; Laurent, I.; Marianowski, R.; Ferec, C.; Lehn, P.; Benvegnu, T.; et al. Folate-equipped nanolipoplexes mediated efficient gene transfer into human epithelial cells. Int. J. Mol. Sci. 2013, 14, 1477–1501. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Mathias, C.J.; Wang, S.; Waters, D.J.; Turek, J.J.; Low, P.S.; Green, M.A. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J. Nucl. Med. 1998, 39, 1579–1585. [Google Scholar] [PubMed]
- Mathias, C.J.; Hubers, D.; Low, P.S.; Green, M.A. Synthesis of [99mTc]DTPA-folate and its evaluation as a folate-receptor-targeted radiopharmaceutical. Bioconjug. Chem. 2000, 11, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Gey, G.O.; Coffman, W.D.; Kubicek, M.T. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952, 264–265. [Google Scholar]
- Shen, D.W.; Cardarelli, C.; Hwang, J.; Cornwell, M.; Richert, N.; Ishii, S.; Pastan, I.; Gottesman, M.M. Multiple drug-resistant human kb carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J. Biol. Chem. 1986, 261, 7762–7770. [Google Scholar] [PubMed]
- Endicott, J.A.; Ling, V. The biochemistry of p-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 1989, 58, 137–171. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.T. P-glycoproteins and multidrug resistance. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Moodie, F.M.; Satsangi, J. Multidrug resistance 1 gene (p-glycoprotein 170): An important determinant in gastrointestinal disease? Gut 2003, 52, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Benard, J.; Da Silva, J.; De Blois, M.C.; Boyer, P.; Duvillard, P.; Chiric, E.; Riou, G. Characterization of a human ovarian adenocarcinoma line, igrov1, in tissue culture and in nude mice. Cancer Res. 1985, 45, 4970–4979. [Google Scholar] [PubMed]
- Schultz, R.M.; Andis, S.L.; Shackelford, K.A.; Gates, S.B.; Ratnam, M.; Mendelsohn, L.G.; Shih, C.; Grindey, G.B. Role of membrane-associated folate binding protein in the cytotoxicity of antifolates in KB, IGROV1, and L1210A cells. Oncol. Res. 1995, 7, 97–102. [Google Scholar] [PubMed]
- Hua, W.; Christianson, T.; Rougeot, C.; Rochefort, H.; Clinton, G.M. Skov3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J. Steroid Biochem. Mol. Biol. 1995, 55, 279–289. [Google Scholar] [CrossRef]
- Yu, D.; Wolf, J.K.; Scanlon, M.; Price, J.E.; Hung, M.C. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by e1a. Cancer Res. 1993, 53, 891–898. [Google Scholar] [PubMed]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Doucette, M.M.; Stevens, V.L. Folate receptor function is regulated in response to different cellular growth rates in cultured mammalian cells. J. Nutr. 2001, 131, 2819–2825. [Google Scholar] [PubMed]
- Tran, T.; Shatnawi, A.; Zheng, X.; Kelley, K.M.; Ratnam, M. Enhancement of folate receptor alpha expression in tumor cells through the glucocorticoid receptor: A promising means to improved tumor detection and targeting. Cancer Res. 2005, 65, 4431–4441. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Hasui, S.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Iseki, K. The mechanism of carrier-mediated transport of folates in bewo cells: The involvement of heme carrier protein 1 in placental folate transport. Biosci. Biotechnol. Biochem. 2008, 72, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, A.; Rachmilewitz, J.; Eldar-Geva, T.; Salant, T.; Schneider, T.; de Groot, N. Differentiation of choriocarcinoma cell line (JAR). Cancer Res. 1992, 52, 3713–3717. [Google Scholar] [PubMed]
- Serrano, M.A.; Macias, R.I.; Briz, O.; Monte, M.J.; Blazquez, A.G.; Williamson, C.; Kubitz, R.; Marin, J.J. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, JEG-3 and JAR of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta 2007, 28, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, A.; Klimek, J.; Masaoka, M.; Kaminski, M.; Kedzior, J.; Majczak, A.; Niemczyk, E.; Wozniak, M.; Trzonkowski, P.; Wakabayashi, T. Partial characterization of human choriocarcinoma cell line jar cells in regard to oxidative stress. Acta Biochim. Pol. 2004, 51, 1023–1038. [Google Scholar] [PubMed]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benešová, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Müller, C. Preclinical comparison of albumin-binding radiofolates: Impact of linker entities on the in vitro and in vivo properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, E.; Keating, J.J.; Kularatne, S.A.; Jiang, J.; Judy, R.; Predina, J.; Nie, S.; Low, P.; Singhal, S. Comparison of folate receptor targeted optical contrast agents for intraoperative molecular imaging. Int. J. Mol. Imaging 2015, 2015, 469047. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ahn, R.; Van den Bossche, J.; Thompson, D.H.; O’Halloran, T.V. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol. Cancer Ther. 2009, 8, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Tischler, V.; Pfeifer, M.; Hausladen, S.; Schirmer, U.; Bonde, A.K.; Kristiansen, G.; Sos, M.L.; Weder, W.; Moch, H.; Altevogt, P.; et al. L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol. Cancer 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Zeng, Z.L.; Yang, J.; Ren, C.; Wang, D.S.; Wu, W.J.; Xu, R.H. L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J. Hematol. Oncol. 2013, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Arlt, M.J.; Novak-Hofer, I.; Gast, D.; Gschwend, V.; Moldenhauer, G.; Grunberg, J.; Honer, M.; Schubiger, P.A.; Altevogt, P.; Kruger, A. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006, 66, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, S.; Bianchi, M.; Colombo, N.; Fasani, R.; Goisis, G.; Casadio, C.; Viale, G.; Liu, J.; Herlyn, M.; Godwin, A.K.; et al. The differential role of L1 in ovarian carcinoma and normal ovarian surface epithelium. Cancer Res. 2008, 68, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Gast, D.; Riedle, S.; Riedle, S.; Schabath, H.; Schlich, S.; Schneider, A.; Issa, Y.; Stoeck, A.; Fogel, M.; Joumaa, S.; et al. L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int. J. Cancer 2005, 115, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, I.C.; Stelloo, E.; Nout, R.A.; Nijman, H.W.; Edmondson, R.J.; Church, D.N.; MacKay, H.J.; Leary, A.; Powell, M.E.; Mileshkin, L.; et al. Prognostic significance of L1cam expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol. 2016, 29, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An enitec collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Magalhaes, M.C.; Jelovac, D.; Connolly, R.M.; Wolff, A.C. Treatment of HER2-positive breast cancer. Breast 2014, 23, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Tuefferd, M.; Couturier, J.; Penault-Llorca, F.; Vincent-Salomon, A.; Broet, P.; Guastalla, J.P.; Allouache, D.; Combe, M.; Weber, B.; Pujade-Lauraine, E.; et al. HER2 status in ovarian carcinomas: A multicenter gineco study of 320 patients. PLoS ONE 2007, 2, e1138. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the gynecologic oncology group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Wenzel, C.; Steger, G.G. Trastuzumab in the management of early and advanced stage breast cancer. Biologics 2007, 1, 19–31. [Google Scholar] [PubMed]
- Chavez-Blanco, A.; Perez-Sanchez, V.; Gonzalez-Fierro, A.; Vela-Chavez, T.; Candelaria, M.; Cetina, L.; Vidal, S.; Duenas-Gonzalez, A. HER2 expression in cervical cancer as a potential therapeutic target. BMC Cancer 2004, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, M.E.; Ricci, A.; Paris, L.; Surrentino, E.; Liliac, L.; Bagnoli, M.; Canevari, S.; Mezzanzanica, D.; Podo, F.; Iorio, E.; et al. Monitoring response to cytostatic cisplatin in a HER2(+) ovary cancer model by mri and in vitro and in vivo mr spectroscopy. Br. J. Cancer 2014, 110, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wilken, J.A.; Webster, K.T.; Maihle, N.J. Trastuzumab sensitizes ovarian cancer cells to egfr-targeted therapeutics. J. Ovarian Res. 2010, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.K.; Dunk, C.E.; Amsalem, H.; Maxwell, C.; Keating, S.; Lye, S.J. HER1 signaling mediates extravillous trophoblast differentiation in humans. Biol. Reprod. 2010, 83, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J. Nat. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, A.W.; Mornex, F.; Partensky, C.; Descos, L.; Case, L.D.; Melin, S.A.; Levine, E.A.; Mishra, G.; Limentani, S.A.; Kachnic, L.A.; et al. Adjuvant gemcitabine and concurrent radiation for patients with resected pancreatic cancer: A phase II study. Br. J. Cancer 2006, 95, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bischof, M.; Huber, P.; Stoffregen, C.; Wannenmacher, M.; Weber, K.J. Radiosensitization by pemetrexed of human colon carcinoma cells in different cell cycle phases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 289–292. [Google Scholar] [CrossRef]
- Wang, W.B.; Yang, Y.; Zhao, Y.P.; Zhang, T.P.; Liao, Q.; Shu, H. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J. Gastroenterol. 2014, 20, 15682–15690. [Google Scholar] [CrossRef] [PubMed]
- Bergman, A.M.; Pinedo, H.M.; Talianidis, I.; Veerman, G.; Loves, W.J.; van der Wilt, C.L.; Peters, G.J. Increased sensitivity to gemcitabine of p-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br. J. Cancer 2003, 88, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, C.; Tonini, G.; Pisano, C.; Di Napoli, M.; Cecere, S.C.; Tambaro, R.; Facchini, G.; Pignata, S. Ovarian cancer standard of care: Are there real alternatives? Chin. J. Cancer 2015, 34, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: The icon3 randomised trial. Lancet 2002, 360, 505–515.
- Umsumarng, S.; Pintha, K.; Pitchakarn, P.; Sastraruji, K.; Sastraruji, T.; Ung, A.T.; Jatisatienr, A.; Pyne, S.G.; Limtrakul, P. Inhibition of p-glycoprotein mediated multidrug resistance by stemofoline derivatives. Chem. Pharm. Bull. (Tokyo) 2013, 61, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Ngo, H.; Martin, M.C.; Wolf, J.K. An evaluation of cytotoxicity of the taxane and platinum agents combination treatment in a panel of human ovarian carcinoma cell lines. Gynecol. Oncol. 2005, 98, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schibli, R.; Forrer, F.; Krenning, E.P.; de Jong, M. Dose-dependent effects of (anti)folate preinjection on 99mTc-radiofolate uptake in tumors and kidneys. Nucl. Med. Biol. 2007, 34, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Vlahov, I.R.; Santhapuram, H.K.; Leamon, C.P.; Schibli, R. Tumor targeting using 67Ga-DOTA-Bz-folate—Investigations of methods to improve the tissue distribution of radiofolates. Nucl. Med. Biol. 2011, 38, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Reber, J.; Haller, S.; Leamon, C.P.; Müller, C. 177Lu-EC0800 combined with the antifolate pemetrexed: Preclinical pilot study of folate receptor targeted radionuclide tumor therapy. Mol. Cancer Ther. 2013, 12, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schibli, R.; Krenning, E.P.; de Jong, M. Pemetrexed improves tumor selectivity of 111In-DTPA-folate in mice with folate receptor-positive ovarian cancer. J. Nucl. Med. 2008, 49, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Grunberg, J.; Knogler, K.; Waibel, R.; Novak-Hofer, I. High-yield production of recombinant antibody fragments in HEK-293 cells using sodium butyrate. Biotechniques 2003, 34, 968–972. [Google Scholar] [PubMed]

| Cancer Type | Cell Line | 5-FU IC50 (μM) | GEM IC50 (nM) | PMX IC50 (nM) | CIS IC50 (μM) | DOX IC50 (nM) | PCX IC50 (nM) |
|---|---|---|---|---|---|---|---|
| Cervical | HeLa | 16.2 | 56.4 | 8.9 ** | 2.8 | 36.2 | 9.5 |
| KB | 28.6 * | 120 | 4.5 ** | 1.9 | 15.4 | 2.7 | |
| KB-V1 | 9.1 | 12.8 | 5.3 | 0.3 | 211 | 597 | |
| Ovarian | IGROV-1 | 2.0 | 11.8 | 41.8 | 0.8 | 23.9 | 5.0 |
| SKOV-3 | 8.0 ** | 20.8 | 14.9 * | 5.6 | 96.7 ** | 4.0 | |
| SKOV-3.ip | 3.1 | 45.0 * | 6.4 * | 1.9 | 449 ** | 9.6 | |
| Choriocarcinoma | JAR | 8.2 | 30.6 | 33.8 | 0.9 | 9.8 | 3.1 |
| BeWo | 4.2 | 1.2 | 6.4 | 0.4 | 2.2 | 1.6 | |
| Endometrial | EFE-184 | 9.7 * | 15.8 | n.d. | 1.6 | 390 | 90.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwowska, K.; Schmid, R.M.; Cohrs, S.; Schibli, R.; Müller, C. Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and In Vivo Characterization. Pharmaceuticals 2017, 10, 72. https://doi.org/10.3390/ph10030072
Siwowska K, Schmid RM, Cohrs S, Schibli R, Müller C. Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and In Vivo Characterization. Pharmaceuticals. 2017; 10(3):72. https://doi.org/10.3390/ph10030072
Chicago/Turabian StyleSiwowska, Klaudia, Raffaella M. Schmid, Susan Cohrs, Roger Schibli, and Cristina Müller. 2017. "Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and In Vivo Characterization" Pharmaceuticals 10, no. 3: 72. https://doi.org/10.3390/ph10030072
APA StyleSiwowska, K., Schmid, R. M., Cohrs, S., Schibli, R., & Müller, C. (2017). Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and In Vivo Characterization. Pharmaceuticals, 10(3), 72. https://doi.org/10.3390/ph10030072







