Assessment of Bone Metastases in Patients with Prostate Cancer—A Comparison between 99mTc-Bone-Scintigraphy and [68Ga]Ga-PSMA PET/CT
Abstract
:1. Introduction
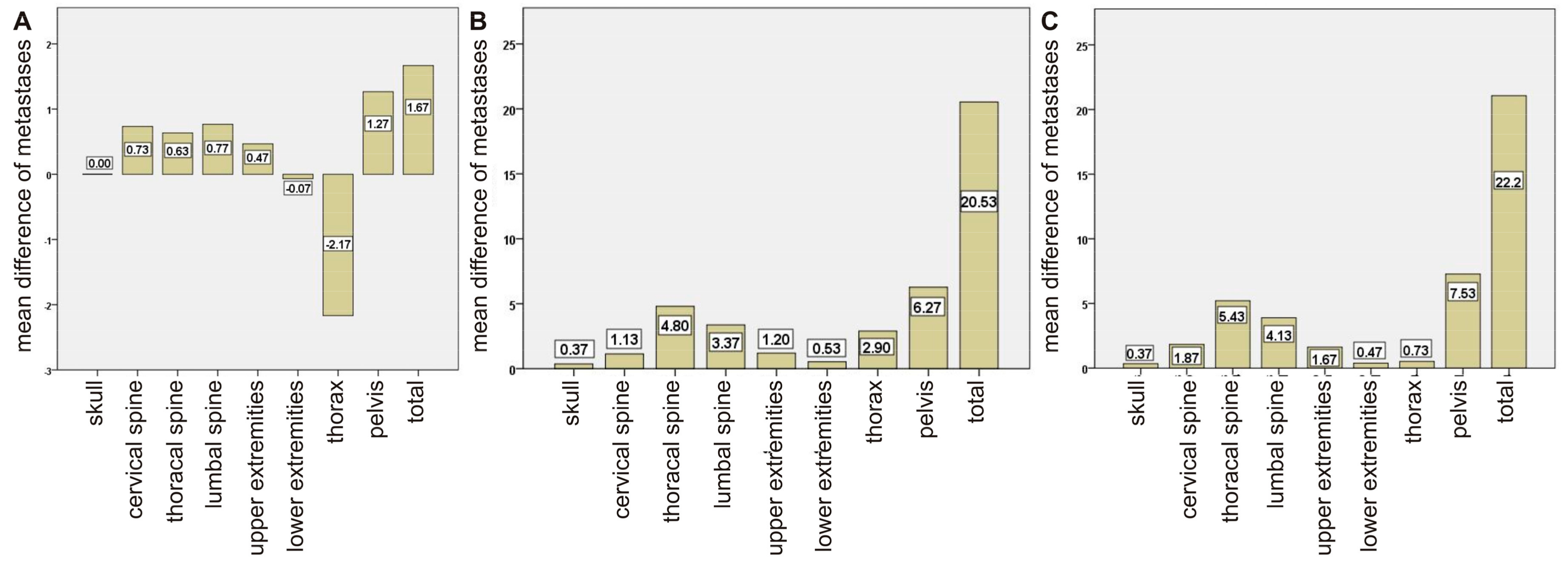
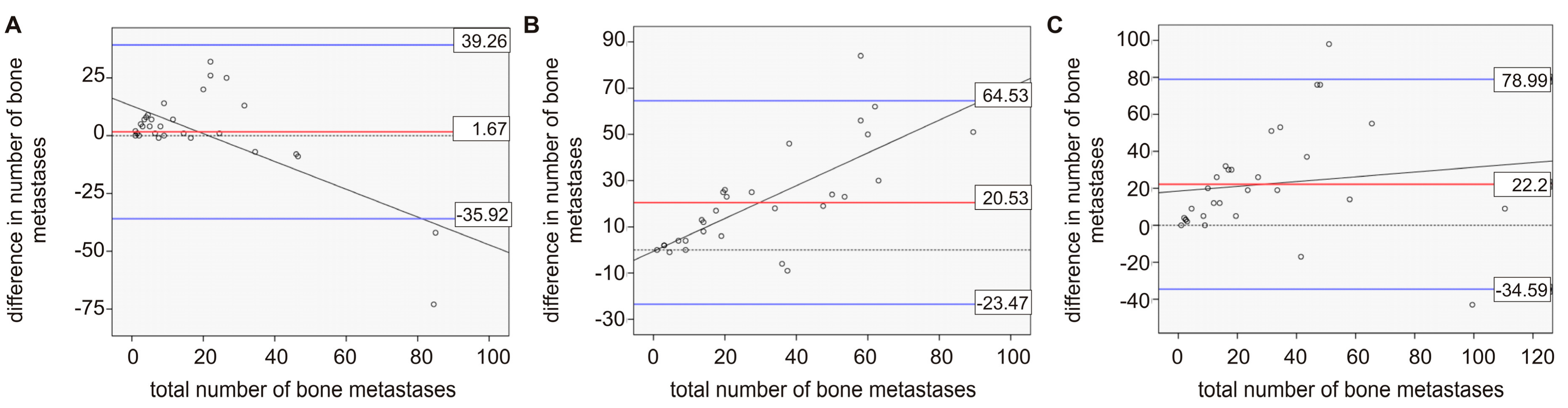
2. Results
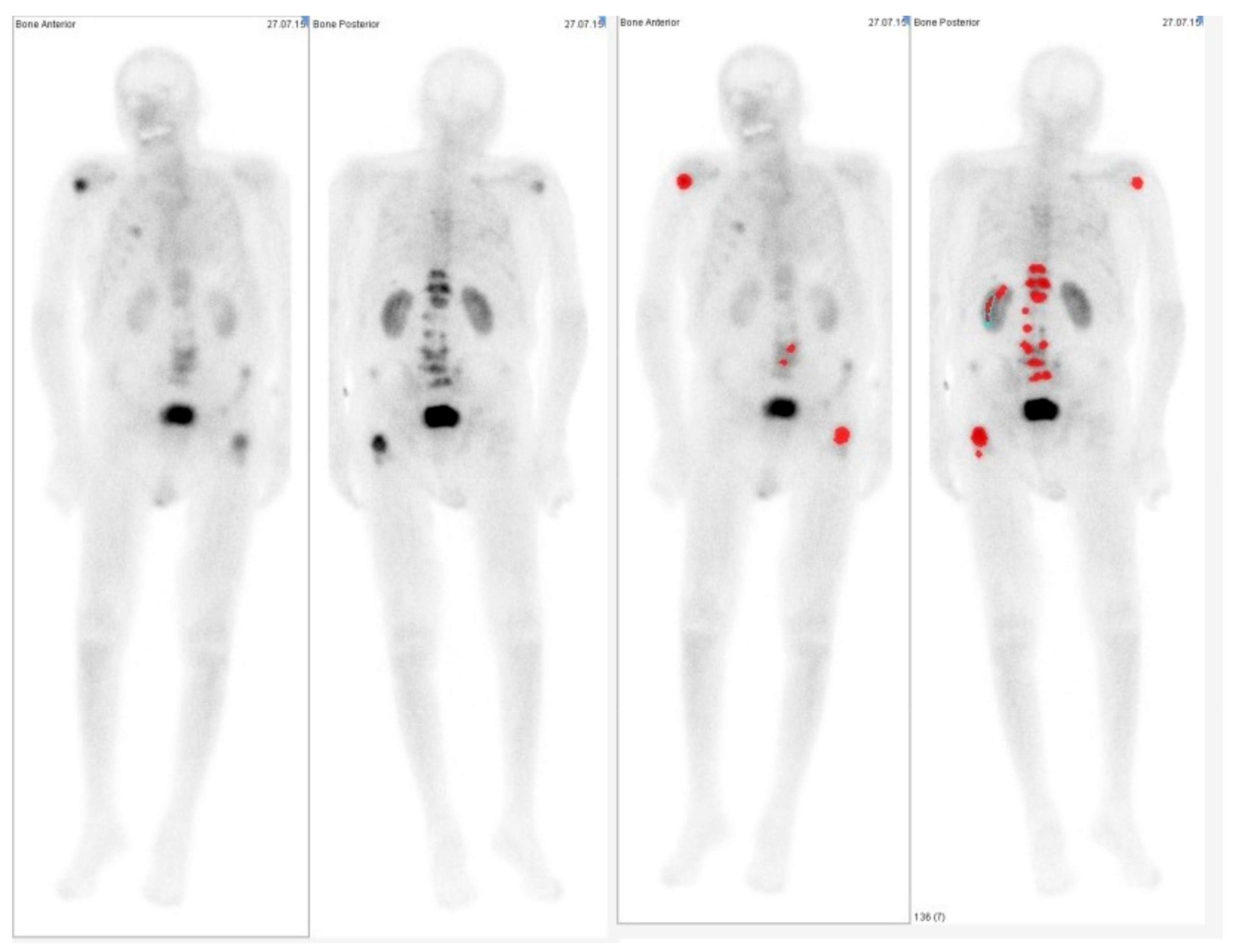
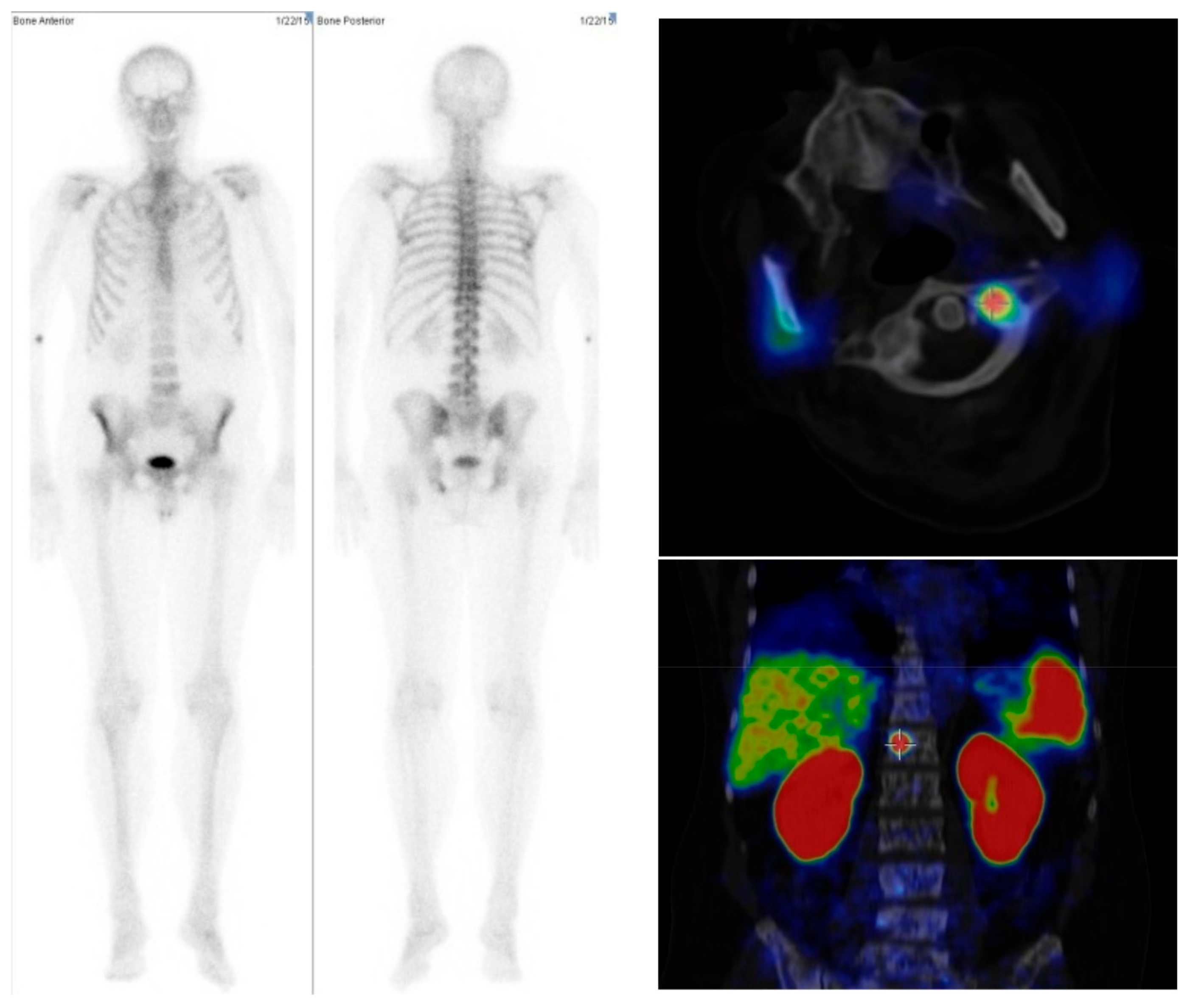
2.1. Comparison between Bone Scan and PET/CT
2.2. Comparison of Imaging and Serum Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patient Population and Treatment
4.2. Imaging
4.3. Data Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Prostate Cancer; Site NCIW, Ed.; Surveillance, Epidemiology, and End Results Program: Rockville, MD, USA, 2015. [Google Scholar]
- Jacobs, S.C. Spread of prostatic cancer to bone. Urology 1983, 21, 337–344. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Imbriaco, M.; Larson, S.M.; Yeung, H.W.; Mawlawi, O.R.; Erdi, Y.; Venkatraman, E.S.; Scher, H.I. A new parameter for measuring metastatic bone involvement by prostate cancer: The bone scan index. Clin. Cancer Res. 1998, 4, 1765–1772. [Google Scholar] [PubMed]
- Sadik, M.; Hamadeh, I.; Nordblom, P.; Suurkula, M.; Hoglund, P.; Ohlsson, M.; Edenbrandt, L. Computer-assisted interpretation of planar whole-body bone scans. J. Nucl. Med. 2008, 49, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Ulmert, D.; Kaboteh, R.; Fox, J.J.; Savage, C.; Evans, M.J.; Lilja, H.; Abrahamsson, P.A.; Björk, T.; Gerdtsson, A.; Bjartell, A.; et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the bone scan index. Eur. Urol. 2012, 62, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Morris, M.J.; Kaboteh, R.; Bath, L.; Sadik, M.; Gjertsson, P.; Lomsky, M.; Edenbrandt, L.; Minarik, D.; Bjartell, A.; et al. Analytic validation of the automated bone scan index as an imaging biomarker to standardize quantitative changes in bone scans of patients with metastatic prostate cancer. J. Nucl. Med. 2016, 57, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M. EXINI quantitative bone scan index: Expanded utility for the planar radionuclide bone scan. J. Nucl. Med. 2016, 57, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, A.G.; Burger, I.A.; Sala, E.; Hricak, H.; Weber, W.A.; Vargas, H.A. Molecular imaging of prostate cancer. Radiographics 2016, 36, 142–159. [Google Scholar] [CrossRef]
- Jadvar, H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat. Rev. Urol. 2009, 6, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, G.S.; Schoder, H.; Ravizzini, G.C.; Gonen, M.; Fox, J.J.; Humm, J.; Morris, M.J.; Scher, H.I.; Larson, S.M. Prognostic value of baseline [18F]-fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin. Cancer Res. 2010, 16, 6093–6099. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A.; Mittra, E.; Yaghoubi, S.S.; Dick, D.W.; Quon, A.; Goris, M.L.; Gambhir, S.S. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: Results of the pilot-phase study. J. Nucl. Med. 2009, 50, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Loening, A.; Jamali, M.; Barkhodari, A.; Mosci, C.; Jackson, T.; Obara, P.; Taviani, V.; Gambhir, S.S.; Vasanawala, S.; et al. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J. Nucl. Med. 2016, 56, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Nekolla, S.G.; Maurer, T.; Weirich, G.; Wester, H.J.; Schwaiger, M. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom. Imaging 2015, 40, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of hybrid 68Ga-PSMA Ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.T.; Radtke, J.P.; Hadaschik, B.A.; Kopp-Schneider, A.; Eder, M.; Kopka, K.; Haberkorn, U.; Roethke, M.; Schlemmer, H.P.; Afshar-Oromieh, A. Comparison of hybrid 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Schreiter, V.; Gericke, M.; Heimann, U.; Steffen, I.; Stelter, L.; Maurer, M.H.; Hamm, B.; Brenner, W.; Theilig, D.; Kahn, J.; et al. Comparison of [68Ga]Ga-PSMA-HBED-CC PET versus Whole-Body Bone Scintigraphy for the Detection of Bone Metastases in Patients with Prostate Cancer. J. Nucl. Med. Radiat. Ther. 2016, 7, 302. [Google Scholar]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Bieth, M.; Kroenke, M.; Tauber, R.; Bahlbender, M.; Retz, M.; Nekolla, SG; Menze, B.; Maurer, T.; Eiber, M.; Schwaiger, M. Exploring new multimodal quantitative imaging indices for the assessment of osseous tumor burden in prostate cancer using 68Ga-PSMA-PET/CT. J. Nucl. Med 2017. [Google Scholar] [CrossRef] [PubMed]
- Sadik, M.; Suurkula, M.; Hoglund, P.; Jarund, A.; Edenbrandt, L. Quality of planar whole-body bone scan interpretations—A nationwide survey. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, L.; Balmus, C.; Ahmadzadehfar, H.; Essler, M.; Strunk, H.; Bundschuh, R.A. Assessment of Bone Metastases in Patients with Prostate Cancer—A Comparison between 99mTc-Bone-Scintigraphy and [68Ga]Ga-PSMA PET/CT. Pharmaceuticals 2017, 10, 68. https://doi.org/10.3390/ph10030068
Thomas L, Balmus C, Ahmadzadehfar H, Essler M, Strunk H, Bundschuh RA. Assessment of Bone Metastases in Patients with Prostate Cancer—A Comparison between 99mTc-Bone-Scintigraphy and [68Ga]Ga-PSMA PET/CT. Pharmaceuticals. 2017; 10(3):68. https://doi.org/10.3390/ph10030068
Chicago/Turabian StyleThomas, Lena, Caroline Balmus, Hojjat Ahmadzadehfar, Markus Essler, Holger Strunk, and Ralph A. Bundschuh. 2017. "Assessment of Bone Metastases in Patients with Prostate Cancer—A Comparison between 99mTc-Bone-Scintigraphy and [68Ga]Ga-PSMA PET/CT" Pharmaceuticals 10, no. 3: 68. https://doi.org/10.3390/ph10030068
APA StyleThomas, L., Balmus, C., Ahmadzadehfar, H., Essler, M., Strunk, H., & Bundschuh, R. A. (2017). Assessment of Bone Metastases in Patients with Prostate Cancer—A Comparison between 99mTc-Bone-Scintigraphy and [68Ga]Ga-PSMA PET/CT. Pharmaceuticals, 10(3), 68. https://doi.org/10.3390/ph10030068




