The Binding Effect of Proteins on Medications and Its Impact on Electrochemical Sensing: Antipsychotic Clozapine as a Case Study
Abstract
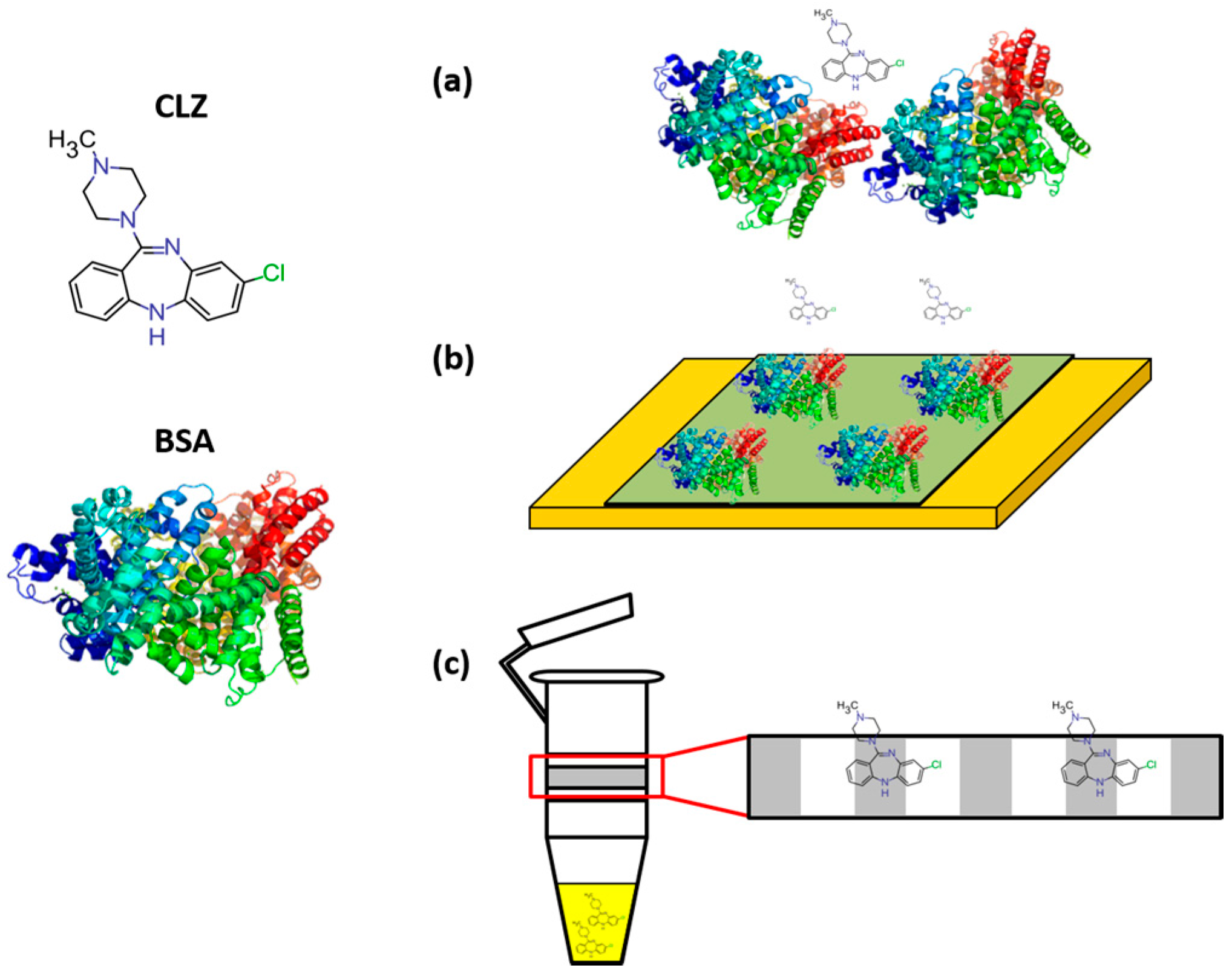
:1. Introduction
2. Results
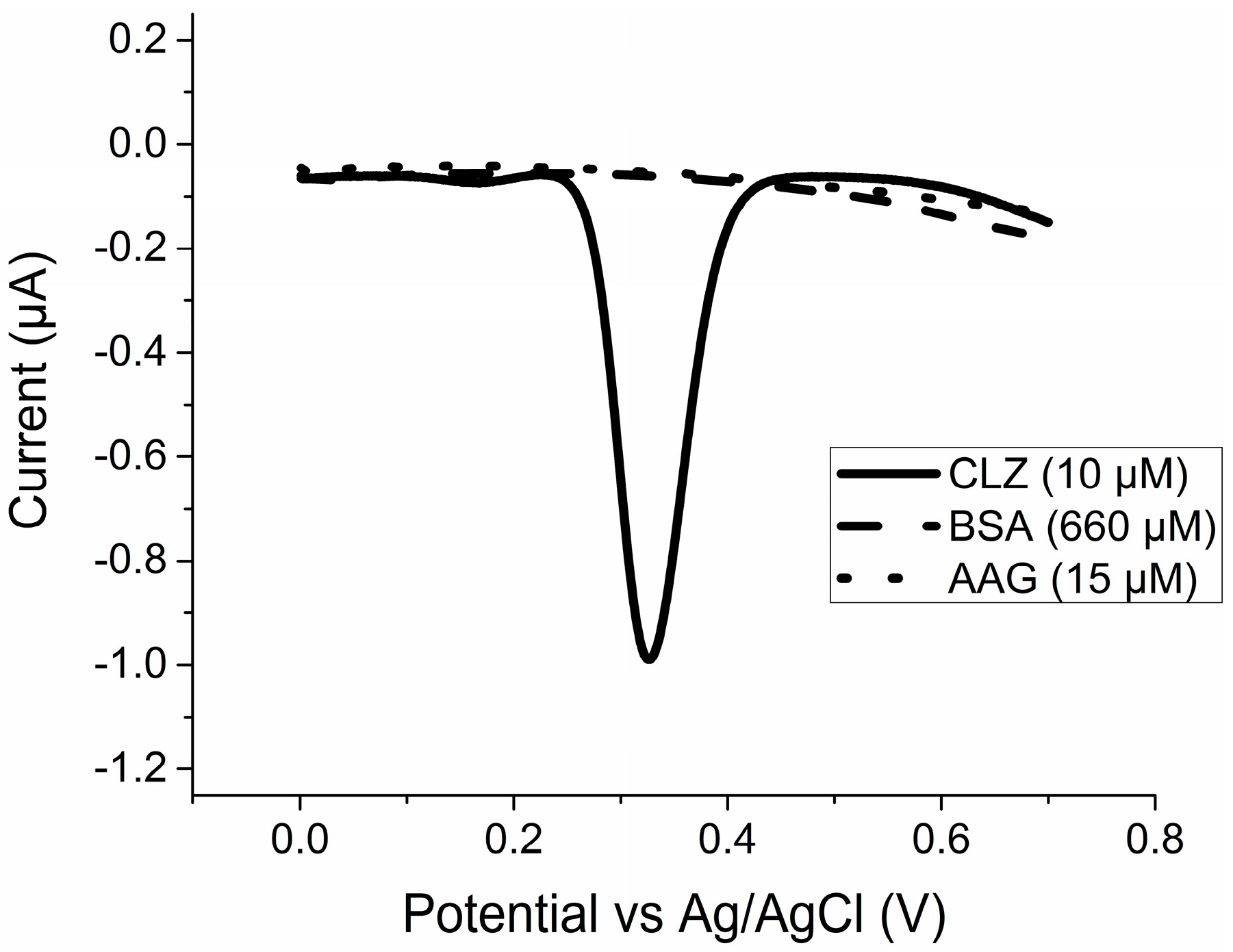
2.1. Pure Species Redox Activity
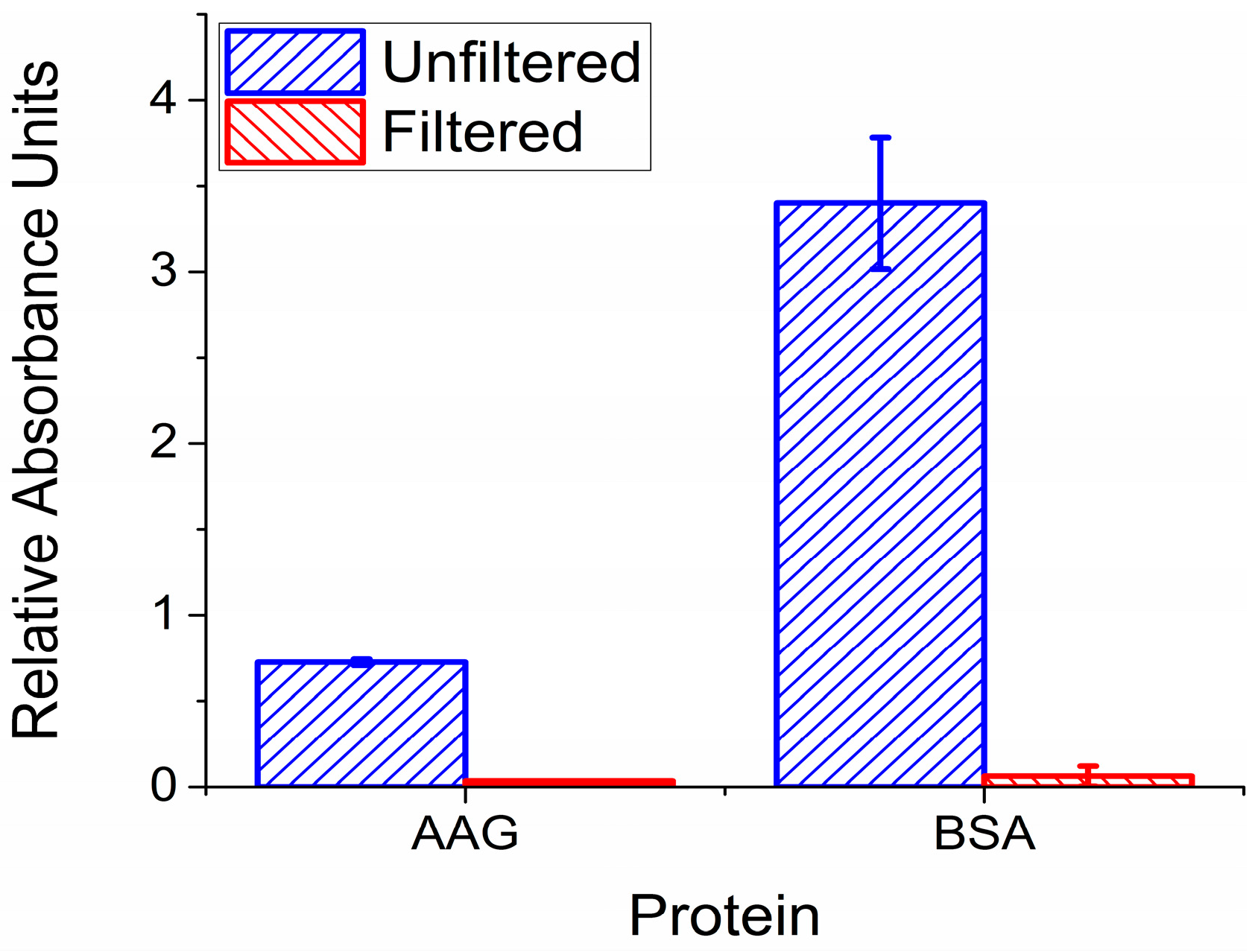
2.2. Validation of Filter Functionality
2.3. Quantification of Protein Fouling/Screening
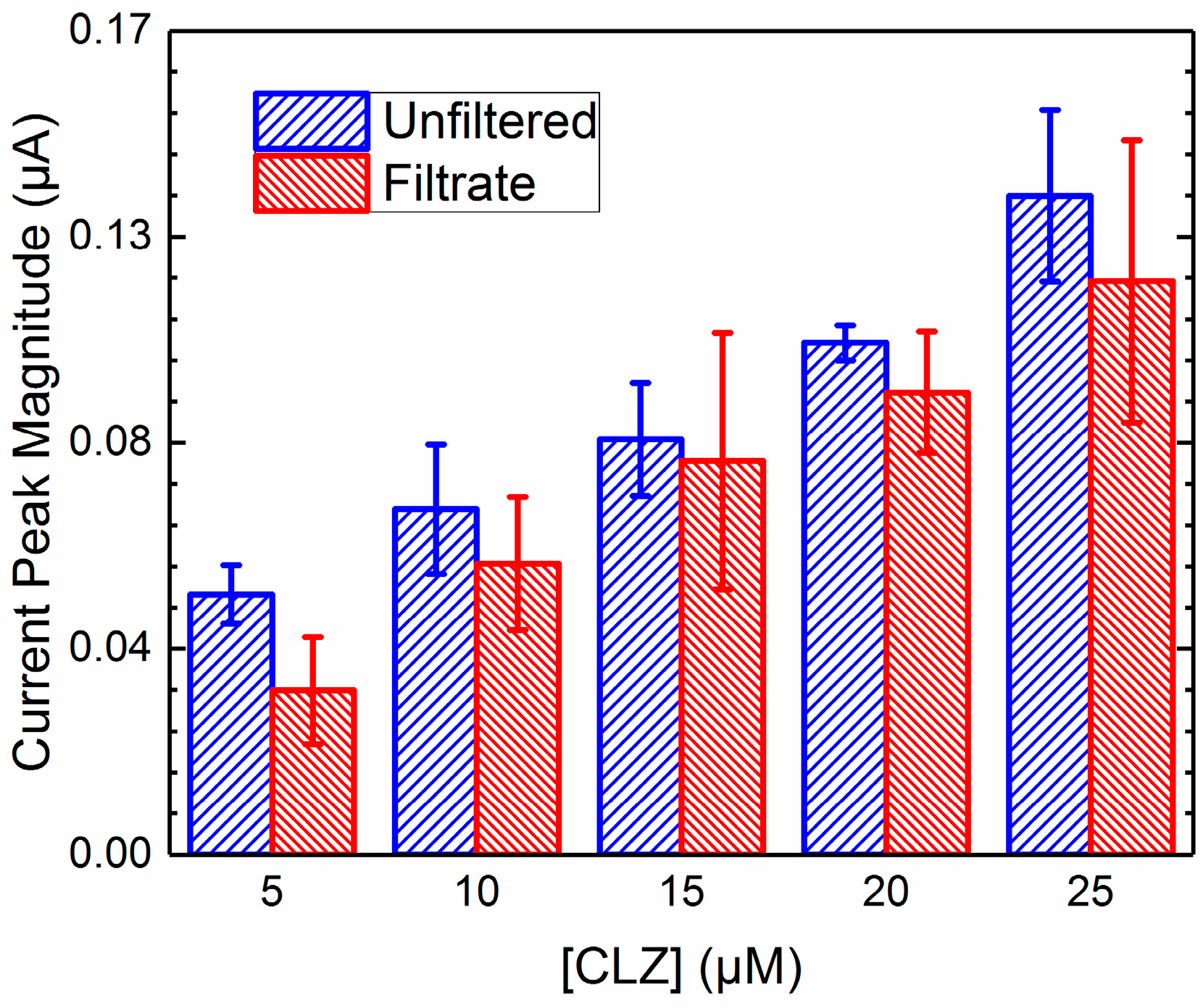
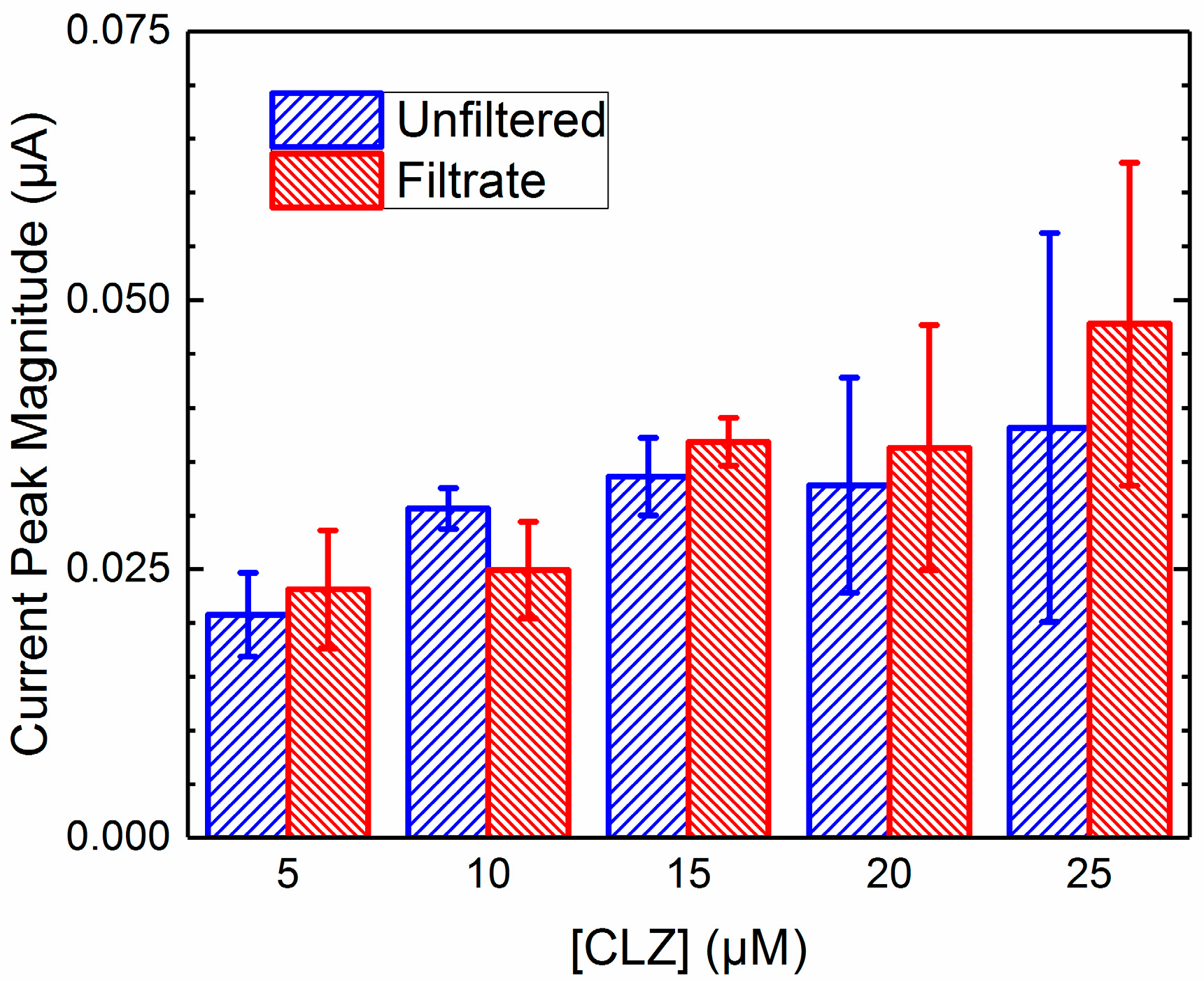
2.4. Unfiltered vs. Filtrate Comparison
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Free CLZ Isolation—Filtration
4.3. Ultraviolet Visible Spectroscopy
4.4. Percentage Protein Binding
4.5. Electrochemistry
4.6. Mass Spectrometry
4.7. Electrode Fouling/Screening
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nwobodo, N. Therapeutic drug monitoring in a developing nation: A clinical guide. JRSM Open 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xue, J.; Shao, J.; Jia, L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 2012, 17, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoav, H.; Winkler, T.E.; Kim, E.; Chocron, S.E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Redox cycling-based amplifying electrochemical sensor for in situ clozapine antipsychotic treatment monitoring. Electrochim. Acta 2014, 130, 497–503. [Google Scholar] [CrossRef]
- Farhadi, K.; Karimpour, A. Electrochemical Behavior and Determination of Clozapine on a Glassy Carbon Electrode Modified by Electrochemical Oxidation. Anal. Sci. 2007, 23, 479. [Google Scholar] [CrossRef] [PubMed]
- Chocron, S.E.; Weisberger, B.M.; Ben-Yoav, H.; Winkler, T.E.; Kim, E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Multidimensional Mapping Method Using an Arrayed Sensing System for Cross-Reactivity Screening. PLoS ONE 2015, 10, e0116310. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Chocron, S.E.; Ben-Yoav, H.; Winkler, T.E.; Liu, Y.; Glassman, M.; Wolfram, C.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Programmable “Semismart” Sensor: Relevance to Monitoring Antipsychotics. Adv. Funct. Mater. 2015, 25, 2156–2165. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Wang, B.; Yang, L.-P.; Liu, J.-P. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab. Rev. 2010, 42, 268–354. [Google Scholar] [CrossRef] [PubMed]
- Bagoji, A.M.; Gowda, J.I.; Gokavi, N.M.; Nandibewoor, S.T. Multi-spectroscopic and voltammetric evidences for binding, conformational changes of bovine serum albumin with thiamine. J. Biomol. Struct. Dyn. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-Q.; Jiang, F.-L.; Zhou, B.; Li, R.; Liu, Y. Interaction between a cationic porphyrin and bovine serum albumin studied by surface plasmon resonance, fluorescence spectroscopy and cyclic voltammetry. Photochem. Photobiol. Sci. 2011, 10, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Parakh, P.; Basu, D. Cannabis and psychosis: Have we found the missing links? Asian J. Psychiatry 2013, 6, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.W.; Kreyenbuhl, J.; Kelly, D.L.; Noel, J.M.; Boggs, D.L.; Fischer, B.A.; Himelhoch, S.; Fang, B.; Peterson, E.; Keller, W. The 2009 Schizophrenia PORT Psychopharmacological Treatment Recommendations and Summary Statements. Schizophr. Bull. 2010, 36, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G.; et al. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry 2011, 21, 195–235. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Bogers, J.; van Dijk, D.; Bakker, B.; Schulte, P. Beyond White Blood Cell Monitoring: Screening in the Initial Phase of Clozapine Therapy. J. Clin. Psyciatry 2012, 73, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.E.; Ben-Yoav, H.; Chocron, S.E.; Kim, E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Electrochemical Study of the Catechol-Modified Chitosan System for Clozapine Treatment Monitoring. Langmuir 2014, 30, 14686–14693. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoav, H.; Chocron, S.E.; Winkler, T.E.; Kim, E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. An Electrochemical Micro-System for Clozapine Antipsychotic Treatment Monitoring. Electrochim. Acta 2015, 163, 260–270. [Google Scholar] [CrossRef]
- Kim, E.; Winkler, T.E.; Kitchen, C.; Kang, M.; Banis, G.; Bentley, W.E.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Redox Probing for Chemical Information of Oxidative Stress. Anal. Chem. 2017, 89, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, E.; Winkler, T.E.; Banis, G.; Liu, Y.; Kitchen, C.A.; Kelly, D.L.; Ghodssi, R.; Payne, G.F. Reliable clinical serum analysis with reusable electrochemical sensor: Toward point-of-care measurement of the antipsychotic medication clozapine. Biosens. Bioelectron. 2017, 95, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. J. Diabetes Sci. Technol. 2010, 4, 1540–1562. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Albumin–drug interaction and its clinical implication. Serum Albumin 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Bailey, D.N.; Briggs, J.R. The Binding of Selected Therapeutic Drugs to Human Serum α-1 Acid Glycoprotein and to Human Serum Albumin In Vitro. Ther. Drug Monit. 2004, 26, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Scheife, R. Protein binding: What does it mean? DICP 1989, 23, 7–8. [Google Scholar] [CrossRef]
- Israili, Z.H.; Dayton, P.G. Human Alpha-1-Glycoprotein and its Interactions With Drugs. Drug Metab. Rev. 2001, 33, 161–235. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, R.; Yusufi, B.; Barnes, T. Comparability of whole-blood and plasma clozapine and norclozapine concentrations. Br. J. Clin. Pharmacol. 2003, 56, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, F. Protein binding study of clozapine by capillary electrophoresis in the frontal analysis mode. J. Pharm. Biomed. Anal. 2004, 35, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Rowell, F.J.; Hui, S.M.; Fairbairn, A.F.; Eccleston, D. Total and free serum haloperidol levels in schizophrenic patients and the effect of age thioridazine and fatty acid on haloperidol-serum protein binding in vitro. Br. J. Clin. Pharmacol. 1981, 11, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Vasavanonda, S.; Kumar, G.; Sham, H.L.; Johnson, M.; Grabowski, B.; Denissen, J.F.; Kohlbrenner, W.; Plattner, J.J.; Leonard, J.M.; et al. Human Serum Attenuates the Activity of Protease Inhibitors toward Wild-Type and Mutant Human Immunodeficiency Virus. Virology 1998, 250, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Maekawa, C.; Iwanaga, K.; Morimoto, K.; Kakemi, M. Bioavailability assessment of disopyramide using pharmacokinetic-pharmacodynamic (PK-PD) modeling in the rat. Biol. Pharm. Bull. 2000, 23, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.N.; White, R.F.; Barr, A.M.; Honer, W.G.; Procyshyn, R.M. Elevated clozapine plasma concentration secondary to a urinary tract infection: Proposed mechanisms. J. Psychiatry Neurosci. JPN 2016, 41, E67–E68. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.F. The Action of Potassium Cyanide and Potassium Ferricyanide on Certain Respiratory Pigments. J. Gen. Physiol. 1928, 11, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Anderle, G.; Mendelsohn, R. Thermal denaturation of globular proteins. Fourier transform-infrared studies of the amide III spectral region. Biophys. J. 1987, 52, 69–74. [Google Scholar] [CrossRef]
- Goto, Y.; Calciano, L.J.; Fink, A.L. Acid-induced folding of proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Markus, G.; McClintock, D.; Castellani, B. Tryptic Hydrolysis of Human Serum Albumin. J. Biol. Chem. 1967, 242, 4395–4401. [Google Scholar] [PubMed]
- Singh, S.M.; Cabello-Villegas, J.; Hutchings, R.L.; Mallela, K.M. Role of partial protein unfolding in alcohol-induced protein aggregation. Proteins 2010, 78, 2625–2637. [Google Scholar] [CrossRef] [PubMed]
- Lyman, W.J.; Rosenblatt, D.H.; Reehl, W.J. Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic Compounds; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- O’Neil, M.; Royal Society of Chemistry (Great Britain). The Merck Index, 15th ed.; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Schaber, G.; Stevens, I.; Gaertner, H.J.; Dietz, K.; Breyer-Pfaff, U. Pharmacokinetics of clozapine and its metabolites in psychiatric patients: Plasma protein binding and renal clearance. Br. J. Clin. Pharmacol. 1998, 46, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. CRC 2016, 46, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Martinez-Manez, R. The Supramolecular Chemistry of Organic-Inorganic Hybrid Materials, 1st ed.; Wiley: New York, NY, USA, 2010; ISBN 0-470-37621-X. [Google Scholar]
- Lee, K.-J.; Mower, R.; Hollenbeck, T.; Castelo, J.; Johnson, N.; Gordon, P.; Sinko, P.; Holme, K.; Lee, Y.-H. Modulation of Nonspecific Binding in Ultrafiltration Protein Binding Studies. Pharm. Res. 2003, 20, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
| [CLZ] (μM) | NSMB (%) |
|---|---|
| 5 | 18.7 ± 5.5 |
| 10 | 26.9 ± 7.1 |
| 15 | 27.9 ± 6.3 |
| 20 | 17.3 ± 11.2 |
| 25 | 30.2 ± 16.9 |
| [FoFi] (μM) | Signal Reduction Due to Screening (%) |
|---|---|
| 5 | 1.7 ± 6.0 |
| 10 | 25.7 ± 4.7 |
| 15 | 14.7 ± 3.0 |
| 20 | 16.0 ± 21.0 |
| 25 | 7.1 ± 2.2 |
| [CLZ] (μM) | Binding to AAG (%) | Binding to BSA (%) |
|---|---|---|
| 5 | 36.8 ± 7.1 | 73.2 ± 5.0 |
| 10 | 29.8 ± 13.1 | 67.0 ± 2.0 |
| 15 | 28.7 ± 9.7 | 69.4 ± 3.3 |
| 20 | 31.4 ± 2.4 | 76.7 ± 7.1 |
| 25 | 25.6 ± 9.7 | 77.1 ± 10.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banis, G.E.; Winkler, T.; Barton, P.; Chocron, S.E.; Kim, E.; Kelly, D.L.; Payne, G.F.; Ben-Yoav, H.; Ghodssi, R. The Binding Effect of Proteins on Medications and Its Impact on Electrochemical Sensing: Antipsychotic Clozapine as a Case Study. Pharmaceuticals 2017, 10, 69. https://doi.org/10.3390/ph10030069
Banis GE, Winkler T, Barton P, Chocron SE, Kim E, Kelly DL, Payne GF, Ben-Yoav H, Ghodssi R. The Binding Effect of Proteins on Medications and Its Impact on Electrochemical Sensing: Antipsychotic Clozapine as a Case Study. Pharmaceuticals. 2017; 10(3):69. https://doi.org/10.3390/ph10030069
Chicago/Turabian StyleBanis, George E., Thomas Winkler, Patricia Barton, Sheryl E. Chocron, Eunkyoung Kim, Deanna L. Kelly, Gregory F. Payne, Hadar Ben-Yoav, and Reza Ghodssi. 2017. "The Binding Effect of Proteins on Medications and Its Impact on Electrochemical Sensing: Antipsychotic Clozapine as a Case Study" Pharmaceuticals 10, no. 3: 69. https://doi.org/10.3390/ph10030069
APA StyleBanis, G. E., Winkler, T., Barton, P., Chocron, S. E., Kim, E., Kelly, D. L., Payne, G. F., Ben-Yoav, H., & Ghodssi, R. (2017). The Binding Effect of Proteins on Medications and Its Impact on Electrochemical Sensing: Antipsychotic Clozapine as a Case Study. Pharmaceuticals, 10(3), 69. https://doi.org/10.3390/ph10030069







