Development and Validation in Porcine and Human Models of a Bioimpedance Spectroscopy System for the Objective Assessment of Kidney Graft Viability
Abstract
1. Introduction
1.1. Hypothesis and Objectives
- Can a bioimpedance measurement device be developed and adapted to the surgical environment to provide real-time information on the ischemic status of the kidney organ during the transplant process?
- Can measurement schemes and new bioimpedance models be defined to improve the problem of repeatability of bioimpedance measurements in organs?
- The main objective is to present an intelligent bioimpedance spectroscopy device for assessing kidney viability during renal transplantation. To address this objective, the starting point is a multifrequency bioimpedance device patented by the Biomedical Engineering Group of the University of Seville [46], which has been validated and used in various clinical applications [47,48]. This work focuses on the research, development, and evaluation of instrumentation adapted to the renal transplant scenario and its approximation to the surgical environment, emphasizing the need for sterilization. Some preliminary and partial results on the researched aspects were presented in [49,50,51]. They are expanded on and discussed in this paper from a more integrated perspective. Other novelties of this work are related to the sensing stage and a modification of the four-electrode measurement scheme to provide greater accuracy and repeatability in bioimpedance estimations. On the other hand, the situation generated by the COVID-19 pandemic raised the need to adapt the device so that measurements could be performed autonomously by clinical staff without technical personnel intervention, leading to modifications in the user interface and data storage compared to the starting device [47].
- Another objective of the work is the proposal of a new Cole model, designed to address the problem of parameter repeatability in bioimpedance measurements. This model is distinguished by its ability to offer accurate and repeatable estimations in short-duration measurements, ranging from 1 to 5 min, and facilitate the analysis of long-term parametric variations, where samples are separated by intervals of 1 h or more. This duality allows for a more complete and reliable evaluation of bioimpedance dynamics at different time scales, overcoming the limitations of the conventional models.
1.2. Structure of the Work
2. Materials and Methods
2.1. User-Centered Design Methodology
- A series of improvements with the purpose of adapting its functionality for measurements in kidneys and in a surgical environment. The previous prototype required the assistance of specialized technical personnel to carry out the measurements, which limited its autonomy and hindered its implementation in the surgical environment, especially during the COVID-19 pandemic. The need to minimize the presence of additional personnel in the operating room, to reduce the risk of contagion, and ensure compliance with safety protocols made it essential to develop a system that would allow the clinical team to perform measurements autonomously and safely. The transition to autonomous use of the measurement device by the clinical team required modifications to both the hardware and the measurement protocols.
- The improvements provided by the process have resulted in an optimization of the current generation hardware and the input stage of the instrumentation amplifier.
- The measurement procedure has undergone changes due to optimizations made to the measurement scheme to improve repeatability, from localized measurements to longitudinal measurements.
- Processing algorithms for estimating bioimpedance parameters that synthesize the organ’s bioelectrical behavior have resulted in several models with the aim of minimizing fitting error and improving measurement repeatability.
- The need to sterilize the electrodes has led to a protocol for using the device that had to be approved by the Central Sterilization Unit of the Preventive Medicine and Public Health Service at the Virgen del Rocío University Hospital in Seville.
- The greatest variability in prototypes was established in the design of the probes, until a suitable solution was found for safe use in the operating room.
- A physical integration of the control device and the measurement device described in [48] was carried out. This integration resulted in a unique and compact housing, which houses both the data acquisition and processing components, as well as the user interface.
- A standardized and easy-to-follow measurement protocol was established to facilitate data management during transplantation and the recording of data of interest.
- User interface has been designed according to the needs of the clinical staff, allowing the visualization of results and the control of the device efficiently and safely. Usability and accessibility of the user interface have been established through different means (graphic, acoustic, and touch).
2.2. Technical and Clinical Requirements
- A compact and sterilizable design, indispensable for its use in the operating room.
- Immunity to electromagnetic interference, crucial for measurement stability.
- Portability, which facilitates its transport and handling.
- Measurement accuracy, to ensure the reliability of the results.
- Biocompatibility, which ensures the absence of adverse reactions in the patient.
- Cost minimization, in order to expand its accessibility.
2.3. Reference Pattern
2.4. Types of Measurements
- Bioimpedance measurements in pig kidneys: In order to evaluate the repeatability and reliability of bioimpedance measurements, an experimental study was conducted using kidneys obtained from pigs slaughtered in abattoirs intended for human consumption. The selection of this animal species was based on the marked anatomical similarity between porcine and human kidneys, validated by comparative measurements in human nephrectomy samples (see Section 3.2), which ensures the relevance of the results obtained for clinical application in humans. In addition, measurements in pig kidneys have allowed for the analysis of the evolution of ischemia and cell death in renal tissue, avoiding the use of human samples. The measurement protocol in pig kidneys is described below: Approximately 20 min after the animal’s sacrifice, the organ was extracted, initiating the sequence of measurements. To recreate the conditions prevalent in the surgical environment, porcine kidneys were immersed in an organ preservation solution (CELSIOR® from IGL, Lissieu, France), both at the beginning of the experiment and during intervals between measurements. The temperature of the organs was maintained in a range of 2 °C to 8 °C using ice in a refrigerated container, thus simulating the storage and preservation conditions common in an operating room. Immediately before each measurement, the organs were removed from the preservation solution, excess moisture was carefully removed using sterile gauze, and they were placed on an insulating surface. This procedure was carried out to ensure the accuracy of the measurements and avoid the influence of external factors, such as moisture or the conductivity of the support surface.
- Bioimpedance measurements in human kidneys: In order to validate the applicability of the device in measurements on human kidneys, a second evaluation was carried out using kidneys extracted from patients undergoing complete nephrectomy. This study was approved by the Ethics Committee of the Virgen del Rocío University Hospital of Seville, ensuring compliance with ethical and legal principles in research with human samples. In this case, only several measurements were considered at a specific point in time to analyze measurement repeatability and confirm the suitability of using pig kidneys as a model for the human kidney due to similarity in results.
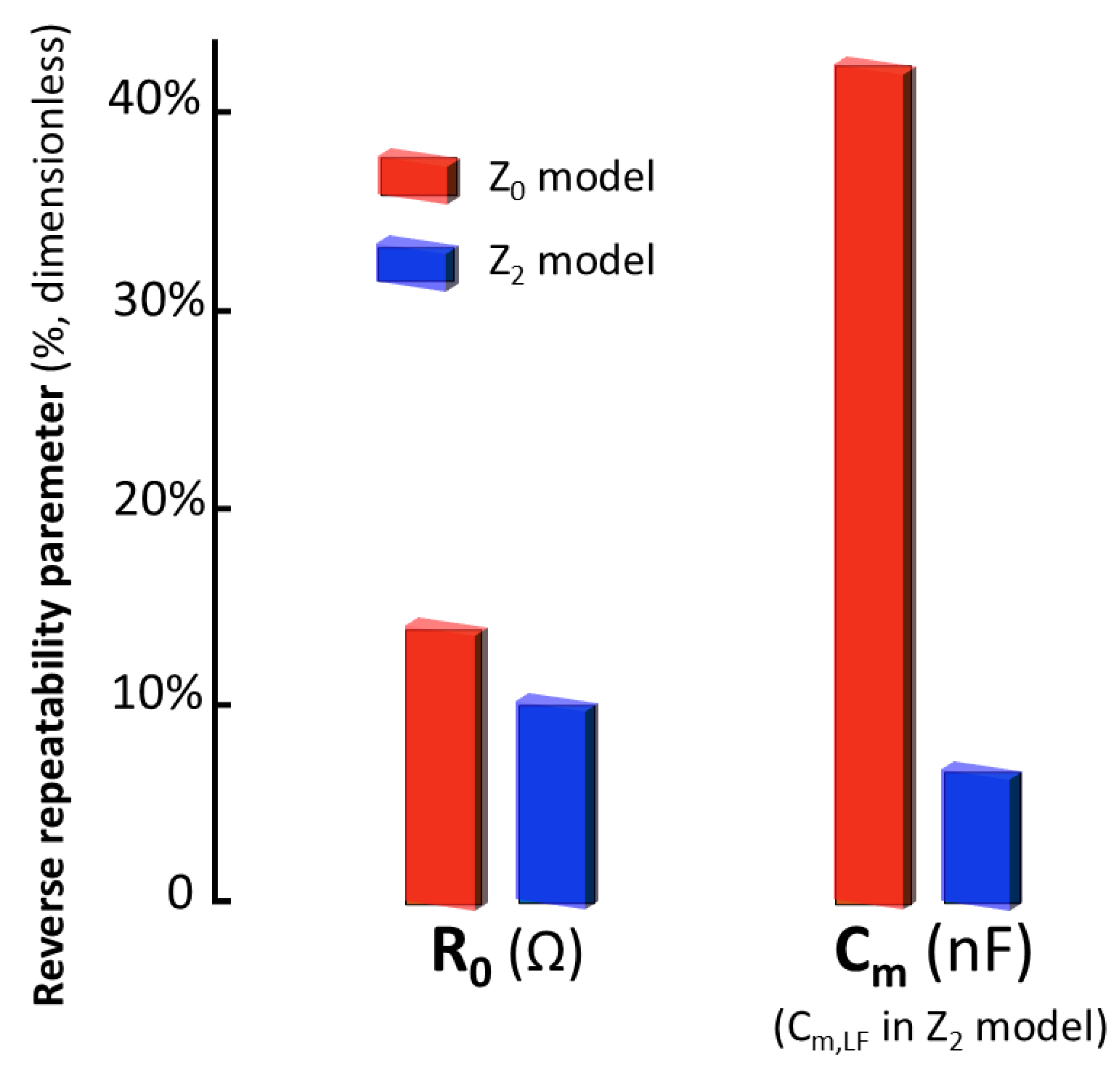
2.5. Study of Repeatability
- Short-term repeatability assessment: Consecutive measurements, comprised within a global time interval of less than 5 min, were analyzed. Between each measurement, the probe was removed from the kidney, allowing for the evaluation of the influence of slight variations in electrode position on the contact area. This scenario provided information on the repeatability of measurements under short-term stability conditions.
- Long-term evolution assessment: Measurements spaced over time were analyzed to record the evolution of parameters as the ischemia process progressed. This scenario allowed for the evaluation of the models’ ability to capture the dynamic changes in bioimpedance over an extended period.
2.6. Study of New Bioimpedance Models
- Cole model with parasitic capacitance (), which incorporates a parasitic shunt capacitance () with the kidney’s bioimpedance. This inclusion is justified by the need to model the capacitive effects that may arise due to the presence of conductive elements in the probes and electrodes used in the measurement, as well as the capacitive effects derived from the influence of external ground. Parasitic capacitance represents an additional impedance that can affect measurement accuracy, especially at high frequencies. By incorporating this capacitance into the model, the aim is to improve the representation of the total system impedance and, therefore, obtain more accurate estimates of kidney bioimpedance.
- Cole model with frequency-dependent membrane capacitance (): In this work, a new model has been proposed in which membrane capacitance increases linearly with frequency. This modification sought to improve the model’s ability to represent the complex interaction between electrical current and the cellular structures of renal tissue.
- (Resistance at zero frequency, in ohms): It represents the resistance that tissue offers to the passage of a direct electrical current. In the biological context, it is mainly associated with the resistance of the extracellular fluid.
- (Resistance at infinite frequency, in ohms): It represents the tissue’s resistance when the frequency tends to infinity. In this case, the electrical current passes through both the extracellular and intracellular fluids, so reflects the total resistance of the tissue.
- (Intracellular resistance, in ohms): It is calculated from and (), and represents the resistance offered by intracellular components to the passage of electrical current.
- (Extracellular resistance, in ohms): It corresponds to , and represents the resistance offered by the extracellular fluid to the passage of electrical current.
- f (Frequency, in Hz): It is the frequency of the electrical current used in the bioimpedance measurement. The tissue’s response varies depending on the frequency, which allows for obtaining information about its different components.
- (Membrane capacitance, in Farads): It represents the electrical capacitance of cell membranes. Its value is associated with the insulating behavior of cell membranes.
- (Membrane capacitance at low frequency, in Farads): It is the value of the membrane capacitance at low frequencies of the model proposed in this work.
- A (Modification factor of , in Farads per Hz): It is a parameter that models the dependence of membrane capacitance on frequency.
- (Phase delay, in seconds): It represents the delay in the phase of the electrical current due to the length of the cables and the characteristics of the hardware used in the measurement. This parameter is important to correct possible errors in bioimpedance measurements.
3. Results
3.1. Design and Development of the Bioimpedance Prototype
- Measurement probe: In order to guarantee the safety and sterility of the measurement environment, a configuration has been adopted that incorporates the electrodes into two ergonomic probes, designed to facilitate their handling by the operator. This arrangement allows for the individual extraction of the electrodes for sterilization, minimizing the risk of contamination and ensuring asepsis during the measurement procedure. The optimal design of the probe, both in its location and assembly, was the subject of an exhaustive analysis by the clinical and technical team. This collaborative process resulted in the identification of a solution that allows for obtaining accurate and reliable measurements, while facilitating the usability and handling of the probe by medical personnel during the kidney transplant procedure. During the design and optimization process of the probe, an exhaustive analysis of various factors that could influence the performance of the measurement system was carried out. The distance, shape, and dimensions of the probe and electrodes were thoroughly investigated, in order to determine the optimal configuration for obtaining accurate and reliable measurements. Likewise, mechanical and physical aspects of the probe, such as weight, measurement pressure, and grip area, were analyzed in depth, with the aim of ensuring its ergonomics and facilitating its handling during the surgical procedure. Figure 3 illustrates a selection of the probes evaluated during the design process, with two main types being distinguished:
- (a)
- Probes for localized measurements: These probes were designed to measure in a specific area of the kidney, with fixed distances in the separation between the electrodes. Probe 1 was a first approximation, but was discarded for having sharp elements that could damage the organ. Probes 2, 3, and 4 had a rounded shape at their ends, more suitable for their placement on the organ, with different configurations of distances between the electrodes, but these probes were also discarded for not being suitable for heat sterilization. Probe 5 implemented the most sensitive option of the previous probes, thanks to the greater distance between the electrodes intended for voltage measurement, and was specifically designed to be heat-sterilized by means of a housing composed of stainless steel and silicone suitable for withstanding high temperatures. Probe 6 was based on disposable or sterilizable commercial electrodes, specifically intraoperative subdural neurophysiology electrodes (MS04R-IP10X-0JH from Ad-Tech, Ashburn, VA, USA). In all the probes for localized measurements, current injection was carried out through the outer electrodes, while voltage measurement was established through the inner electrodes.
- (b)
- Probes for longitudinal measurements: Figure 3b shows an illustrative image of the electrode arrangement for longitudinal measurements in the kidney. The current injector electrodes are positioned at opposite ends of the organ, while the voltage measurement electrodes are located adjacent and close to the injector electrodes, also at the ends of the kidney. This configuration allows a uniform distribution of current through the renal tissue and the measurement of the impedance of the entire kidney, which is fundamental to obtain bioimpedance measurements representative of the physiological state of the organ. As will be seen in Section 3.3, the adopted measurement configuration demonstrated superiority in terms of repeatability compared to localized measurements in specific regions of the organ. This global measurement scheme minimizes the influence of local variations in the bioimpedance of the renal tissue, thus providing more consistent and reliable results. Different options were evaluated in which the probe incorporated a removable and sterilizable element, which could be incorporated into the probe’s handle by means of a connector. Probes 7 and 8 showed two solutions based on BNC connectors, which were discarded due to the small size of the electrode tips that could cause damage to the kidney. Probe 9 provided another option with a more rounded tip based on an electrical plug. Probe 10 demonstrated the finally adopted solution, based on commercial electrosurgery electrodes (F4068 from FIAB, Vicchio, Italy).
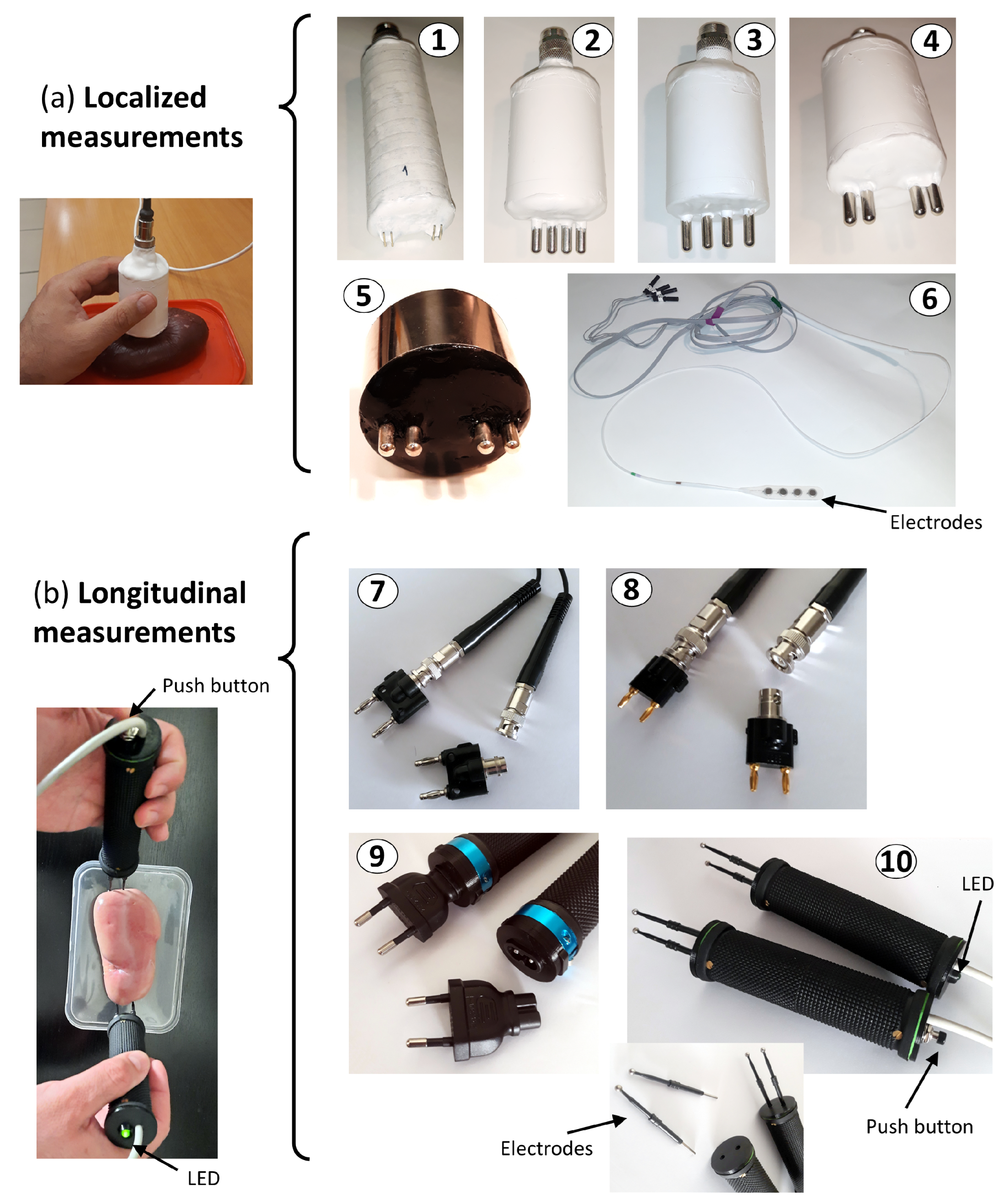
- Electrodes: A four-electrode configuration has been implemented instead of the standard two-electrode configuration, with the aim of removing the influence of the electrodes’ own impedance on the measurements. The impedance of the electrodes, which can vary significantly depending on their material and geometry, as well as the contact with the tissue, introduce sources of error in bioimpedance measurements. By using a four-electrode configuration, current injection is separated from voltage measurement, thus eliminating the influence of electrode impedance on tissue bioimpedance measurement. As previously mentioned in the description of the measurement probe, and as can also be seen in Figure 3, different electrodes were studied and analyzed. However, the exhaustive investigation of sterilization procedures led to the selection of a design alternative based on electrodes approved for sanitary use, with CE certification, and compatible with high-temperature autoclave sterilization. After a detailed evaluation of the different options, the FIAB F4068 commercial electrosurgery electrodes were chosen, and they were integrated into a particular solution of probes for longitudinal measurements. In this configuration, the electrodes can be easily inserted and removed individually thanks to a connector integrated inside the probe. These electrodes, characterized by their smoothed spherical shape with a diameter of 4 mm at the contact end with the renal tissue, minimize the risk of iatrogenic damage during the measurement process. The atraumatic morphology of these electrodes is crucial to ensure patient safety and renal graft integrity during bioimpedance evaluation. Figure 3b illustrates the details of probe 10 developed for its application in the surgical environment, highlighting the characteristics of the electrodes and their integration into the probe assembly. In addition, its design allows for high-temperature autoclave sterilization, which facilitates its reuse and reduces the risk of nosocomial infections.
- Measurement cable: The system design incorporates a cable that connects the probe to the measurement subsystem, with the purpose of optimizing the ergonomics of the procedure and facilitating the sterilization of the components. The measurement cable has a length of 180 cm to allow physical separation of the measurement area from the device. In addition, it is protected with electromagnetic shielding to mitigate the influence of electromagnetic noise in the clinical environment, where the presence of electronic equipment and interference sources is common. This separation between the probe and the main device allows for greater freedom of movement during measurement, which is especially useful in surgical environments where space and organization are critical. To ensure the asepsis of the measurement procedure, the use of a sterile and disposable cover has been implemented that covers both the measurement probes and the connection cable, with sufficient length to allow comfortable handling. An 8-pin connector with a threaded/bayonet locking mechanism is used to ensure a firm and stable connection, preventing accidental disconnections during the measurement procedure. The design of the connectors facilitates their installation and handling, which is crucial in surgical environments where speed and precision are essential. Of the 8 pins, 4 are used for bioimpedance measurements, and the rest are used for shielding and handling a push button and an indicator LED on the probes, as will be described later.
- Measurement subsystem: The measurement subsystem is responsible for the acquisition and processing of signals and the estimation of parameters that allow the evaluation of the electrical properties of renal tissue. This device is responsible for generating the electrical current, measuring the resulting voltage, and calculating the complex impedance of the tissue. The choice of the multifrequency spectroscopy technique, instead of the standard single-frequency method, is based on the need to increase the sensitivity and specificity of the measurement system by enabling the differentiation between intracellular and extracellular compartments. This selective analysis capability facilitates the implementation of a measurement normalization process, an essential aspect in the proposed system. Normalization allows correcting the variations inherent to the individual characteristics of the kidneys, thus ensuring that accurate and comparable measurements are obtained between different organs and patients. At each frequency, estimates of both the modulus and phase of the complex impedance occur. This wide frequency coverage allows for detailed characterization of the electrical properties of the renal tissue, providing valuable information about its composition and structure. Based on the system described in [47], the main elements of the measurement subsystem that have been modified with respect to the previous prototype are described below:
- (a)
- Regarding the device’s power supply, the previous prototype integrated the TPS65133 DC-DC converter from Texas Instruments (Dallas, TX, USA), designed to supply the ±5 V voltage levels required by the analog sensing stage [47]. However, this converter exhibited susceptibility to start-up failures in the case of the initial current demands exceeding a predefined threshold. The mitigation of this problem was achieved through the implementation of a soft start procedure, orchestrated through a manual activation sequence of three switches. In the current prototype, the incorporation of two load distribution switches (TPS22918DBVT from Texas Instruments, Dallas, TX, USA), controlled by software, has been chosen. This modification allows the device’s power-on management through a single button, thus simplifying autonomous operation, eliminating the possibility of operational errors derived from incorrect power-on sequences, and substantially improving the reliability and usability of the device.
- (b)
- The generation of sinusoidal signals, whose frequency is configurable, has been implemented using the Analog Devices AD9854 DDS (Wilmington, MA, USA) integrated circuit, which allows generating sinusoidal signals with high precision and stability over a wide frequency range.
- (c)
- The conversion of the sinusoidal voltage generated by the AD9854 DDS into a constant amplitude current (0.4 mA RMS) is performed by an improved Howland current pump, based on the previous design described in [47]. The amplitude of the current used is well below the international safety limits established for bioimpedance applications [52], which ensures the safety of the organ during the measurement procedure. Compared to the previous device [47], an improvement is proposed in the electrical current generation stage. This modification has the main objective of making the injected current independent of the load impedance value, which in this context corresponds to the bioimpedance of the kidney. The dependence of the injected current on the load impedance can introduce significant errors in bioimpedance measurements, since variations in the renal tissue impedance (due to factors such as hydration, temperature, or physiological state) would directly affect the magnitude of the current. By improving the stability of the constant current source, it is ensured that the injected current remains stable regardless of fluctuations in the kidney impedance. This results in more accurate and reliable measurements, which is fundamental for the objective evaluation of renal graft viability. The independence of the injected current from the load impedance also allows for greater repeatability of measurements, which is crucial for monitoring subtle changes in the bioelectrical properties of renal tissue during transplantation. Figure 4 illustrates the comparative design of the current source and the quantifiable improvements obtained. Unlike the preliminary prototype, where calibration compensated for load variations, the new current generation stage ensures more precise measurement and strict control of the electrical current.
- (d)
- An instrumentation amplifier (INA) based on operational amplifiers is used for voltage measurement. The gain of this amplifier has been adjusted to provide the largest dynamic range in the impedances obtained in a kidney, and ensuring to avoid signal saturation in all possible cases. A modification of the instrumentation amplifier design has been implemented, schematically represented in Figure 5b, which increases the input impedance by an order of magnitude (factor of 10). This optimization is crucial for the accuracy of the voltage measurement, since the high input impedance minimizes the influence of the electrode impedance on the obtained estimates (see Figure 5b). Reducing the effects of load impedance is essential to ensure the reliability of bioimpedance measurements, especially in biological tissues that exhibit variable intrinsic impedance. The implementation of this circuit modification also contributes to obtaining more accurate and representative measurements of the bioelectrical properties of renal tissue.
- (e)
- The measurement subsystem includes a first processing of the acquired signals to determine the modulus and phase of the bioimpedance at each of the frequencies, applying the quadrature measurement technique described in [47]. The processing results are sent through a formatted data structure to the control subsystem via a wired serial data interface.


- Control subsystem: The control architecture was implemented using an M5Stack module (M5Stack Technology, Shenzhen, China), which was physically integrated together with the measurement subsystem in the same housing and measurement device. This convergence of functionalities in a single compact unit eliminated the dependence on an external device, such as a smartphone [47] or laptop [48], thus optimizing the portability and operational efficiency of the system. Based on the previous system, the main elements of the control subsystem that have been modified with respect to the previous prototype are described below:
- (a)
- Data storage: The persistence of acquired data is guaranteed by their storage in files hosted on an SD memory card. This external storage strategy minimizes the vulnerability of information to overwrite events that could compromise the integrity of the device’s internal memory. The use of wireless communications is restricted exclusively to external debugging procedures. Patient data were anonymized to ensure information security and privacy.
- (b)
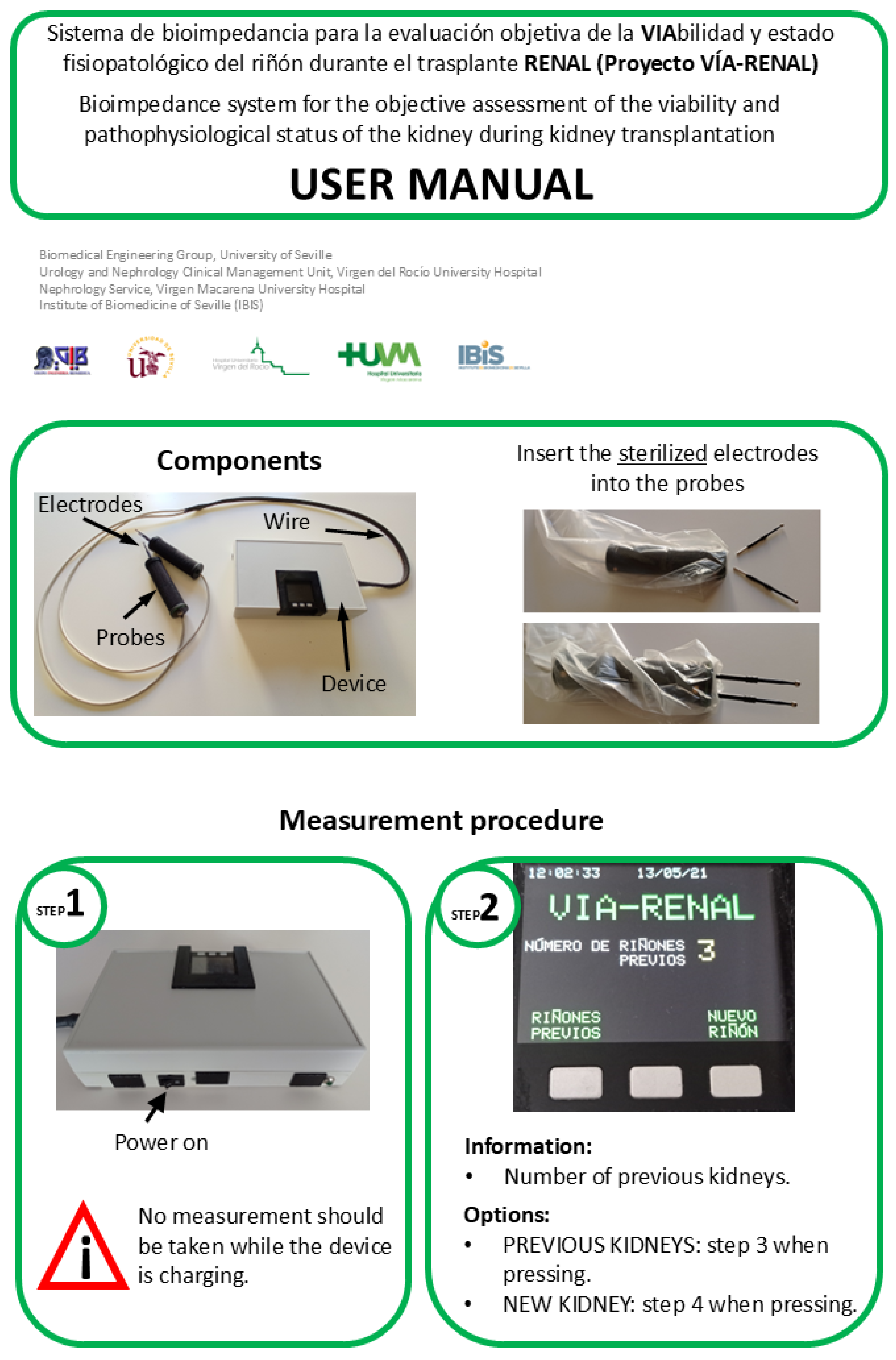
- Graphical user interface: The device’s human-machine interface is materialized through the LCD screen and three push buttons integrated into the M5Stack module, which allow direct interaction with the control software and navigation through specific menus. This graphical interface facilitates the execution of a standardized measurement protocol, designed in collaboration with the clinical team, and provides the following essential functionalities: (1) assignment of a unique numerical identifier to each renal graft; (2) specification of the graft’s laterality (right/left) for correct anatomical identification; (3) sequential recording of the measurement number performed on a specific graft, allowing monitoring of the evolution of bioelectrical properties; (4) selection of the target graft for the acquisition of new measurements, which enables interleaved operation of the device with multiple renal grafts. This last functionality is particularly useful in clinical scenarios where simultaneous evaluation of several grafts is required. To facilitate the handling of this interface, a user manual was developed and training sessions were conducted for the clinical team. The English translated version of the user manual, which highlights the different stages of the measurement process, is included in Figure A2 and Figure A3 of Appendix B.
- (c)
- Acoustic interface: The human-machine interface is complemented by an acoustic feedback system, designed to provide distinctive auditory signals that indicate the beginning and end of the bioimpedance measurement process. Additionally, this acoustic signaling system is used to communicate to the user relevant actions related to interaction with the graphical interface, such as confirmation of menu option selection or error notification. The implementation of this sensory feedback modality contributes to improving the usability of the device, especially in clinical environments where the user’s visual attention may be limited. The combination of visual and auditory signals ensures effective communication between the device and the user, facilitating the execution of measurement procedures and minimizing the possibility of operational errors.
- (d)
- Touch interface: The measurement probe incorporates an activation and signaling mechanism to optimize the data acquisition process. A push button, strategically located on one of the probe handles, allows the user to initiate the bioimpedance measurement once the electrodes have been correctly positioned on the renal organ. Additionally, an LED indicator light, integrated into the opposite handle, provides a visual signal of the measurement status. This LED emits an intermittent light signal during the 8 s that the data acquisition process lasts, alerting the user to the need to keep the probe in a static position. This functionality is crucial to minimize motion artifacts and ensure the accuracy and repeatability of bioimpedance measurements. The integration of these control and signaling elements into the measurement probe facilitates the operation of the device by clinical personnel, contributing to obtaining reliable data and optimizing workflow in the surgical environment, since all control is derived from the measurement probes at the moment the electrodes are placed on the renal organ for measurement.
- (e)
- Real-time clock: Each bioimpedance measurement is associated with a precise timestamp (date and time) thanks to the implementation of a real-time clock with its own battery based on the DS3231 module (Maxim Integrated, San Jose, CA, USA). This functionality facilitates the chronological correlation of bioimpedance data with relevant clinical information of the renal transplant during retrospective analysis and evolution studies. Considering that measurements can be performed simultaneously on several kidneys, and that, according to the measurement protocol, measurements are planned to be taken at two moments (after extraction and before implantation), adequate traceability of the measurements is ensured thanks to the real-time clock that assigns to each measurement the time at which it was carried out.
3.2. Feasibility of Using the Device for the Study of Renal Bioimpedance
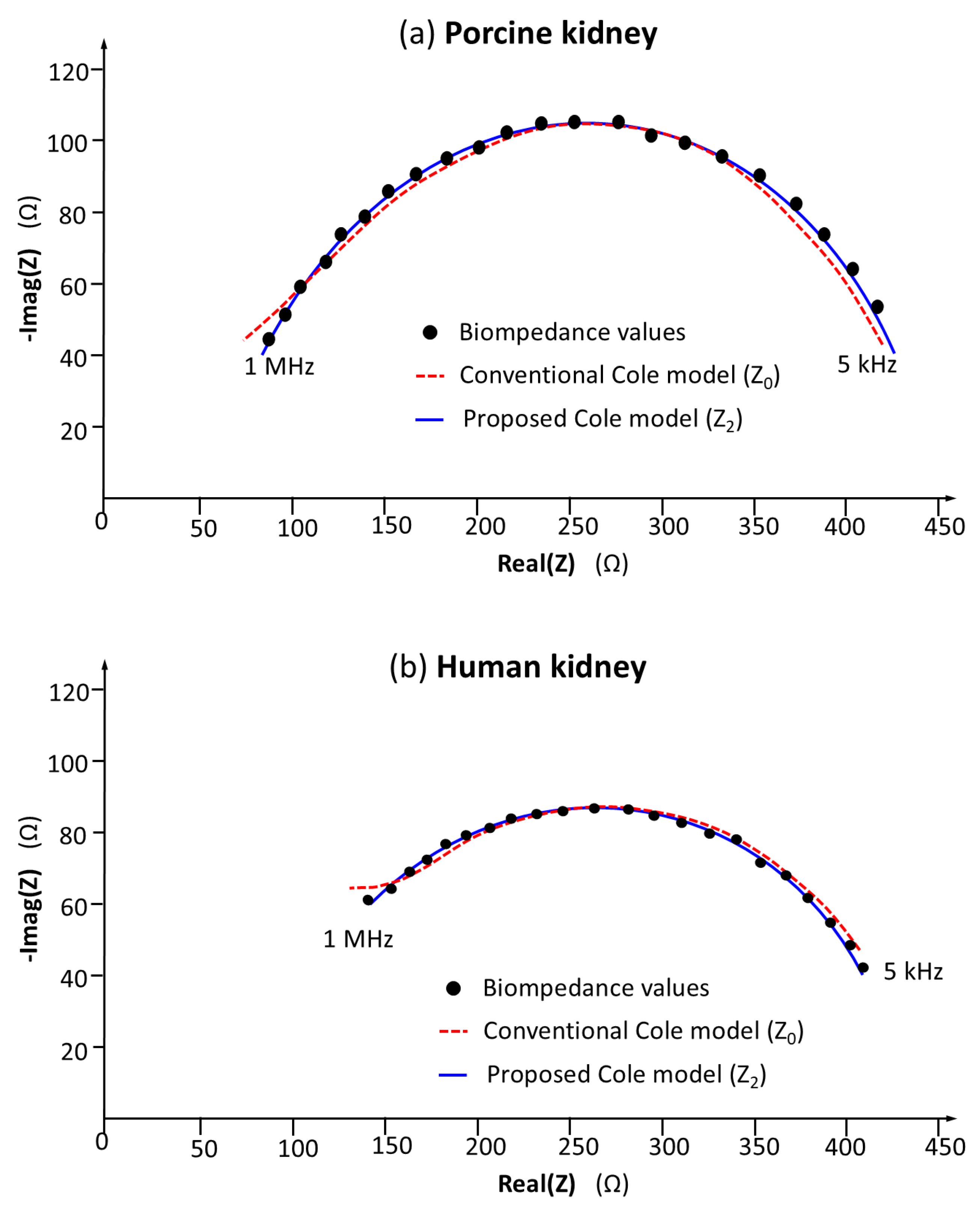
3.3. Validation of the Accuracy and Repeatability of Bioimpedance Measurements in Renal Models
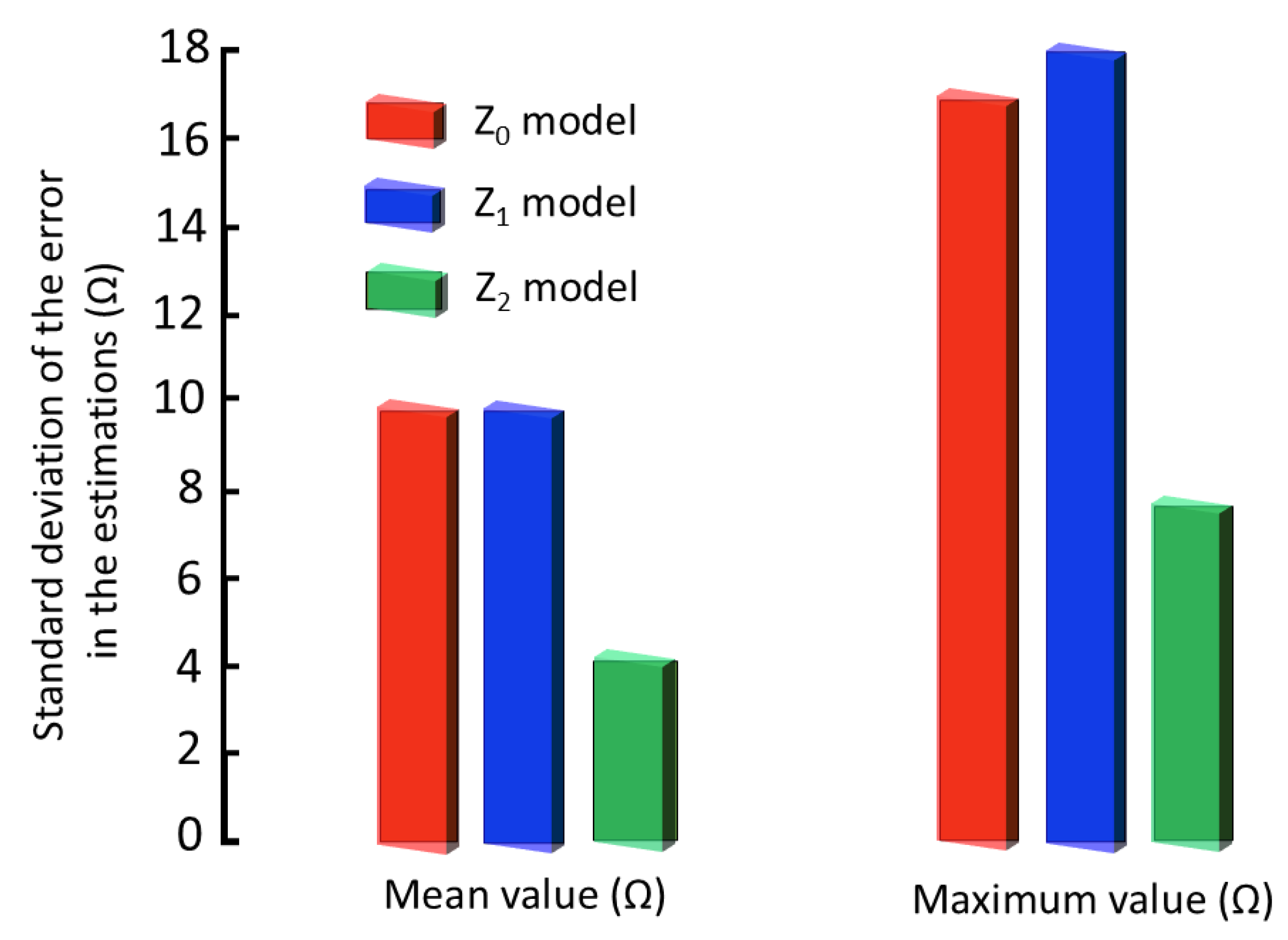
3.4. Comparative Discussion of Accuracy and Repeatability with Bioimpedance Models
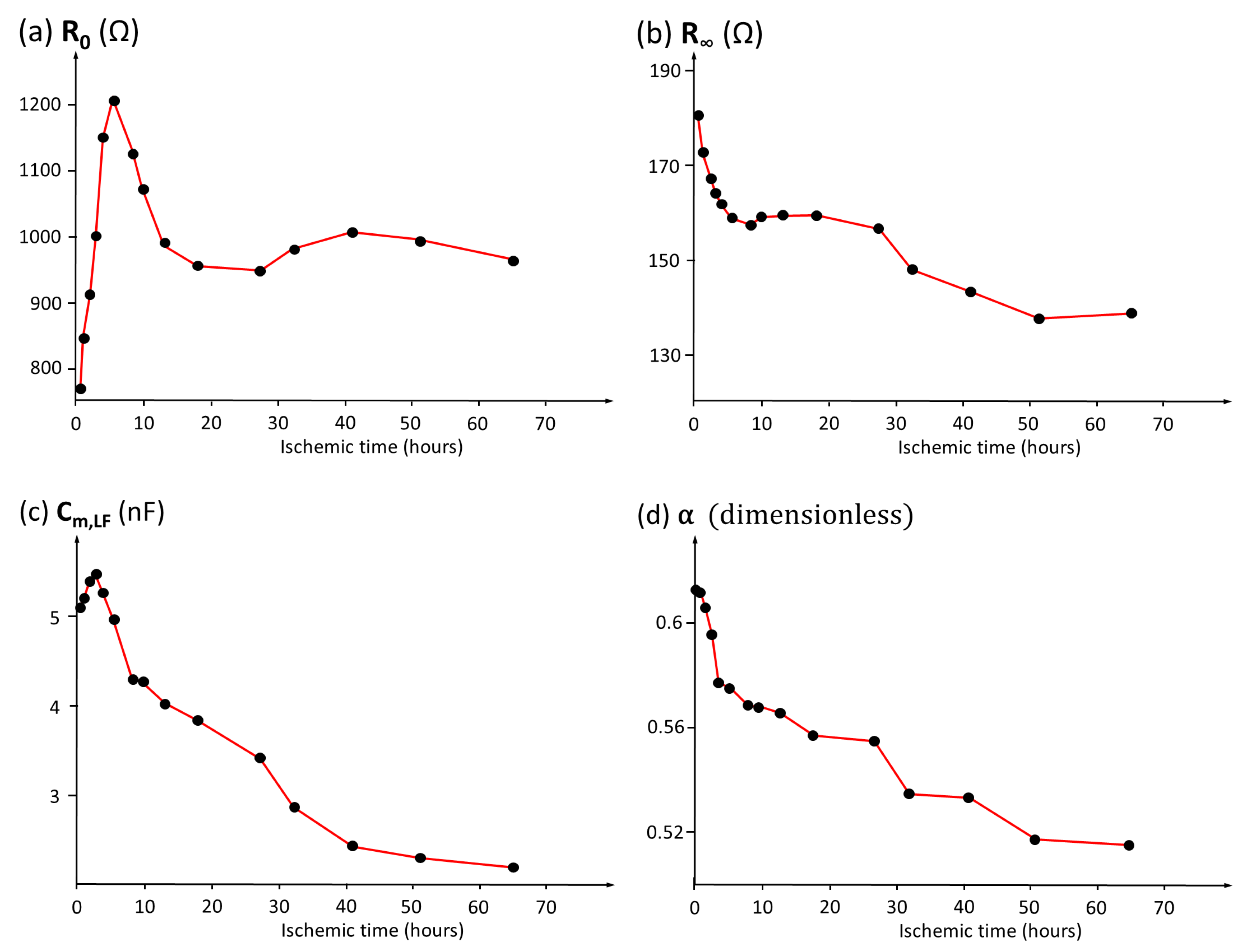
3.5. Analysis of the Temporal Evolution of Bioimpedance Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Design of the Measurement Protocol in the Operating Room
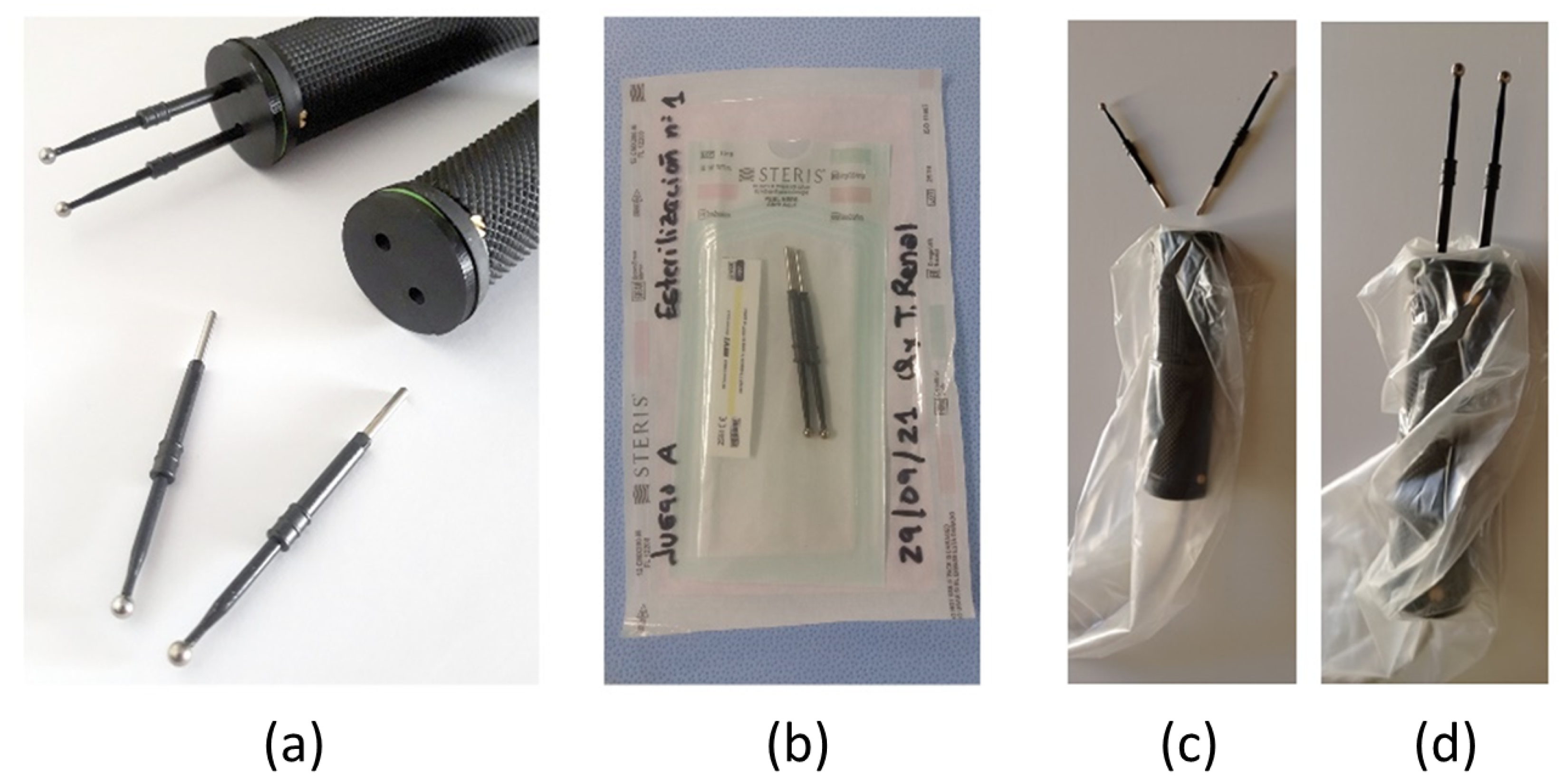
- Elements to be sterilized: The electrodes selected for use in the operating room are the FIAB F4068 commercial electrosurgery electrodes described in Section 3.1. Four new sterilized electrodes will be needed for each measurement (see Figure A1a). In successive measurements, carried out in a short period of time, the same set of electrodes can be used, but they will be renewed in the next measurement.
- Sterilization method: Following the recommendations of the manufacturer’s technical data sheet and in accordance with current regulations, the maximum number of sterilization cycles (20 cycles) and the exposure times, pressure, and temperatures of the autoclave sterilization process are established following the manufacturer’s instructions (for 134 °C, 2.05 bar, and 12 min; for 121°C, 1.05 bar, and 20 min).
- Packaging procedure: Each pair of electrodes will be packaged in self-adhesive bags for steam or ethylene oxide sterilization individually, and both bags will in turn be placed together in a second mixed bag. Each unit package of the electrode will carry an internal steam chemical control (see Figure A1b).
- Sterilization cycle control: In the Central Sterilization Department, a record will be kept with the control of the sterilization cycles carried out. Each package will be identified with the corresponding sterilization cycle number. After use and before sending them back to the Central Department, the operating room staff must identify on the package the next sterilization cycle that would correspond to it. If, in any case, the four electrodes of each package are sent to the sterilization department without the identification of the package and the number of previous sterilization cycles carried out, for safety reasons, their sterilization will not proceed, considering the useful life of said electrodes to be over.
Appendix B. User Manual

References
- National Transplant Organization. Kidney Donation and Transplant Activity Report 2024; Publications of the Ministry of Health, Government of Spain: Madrid, Spain, 2024.
- United States Renal Data System. 2024 Annual Data Report; U.S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2024.
- Lopez-Sanchez, P.; Portolés, J.; Rodríguez, L.M.; Tornero, F.; Martin-Vegue, A.J.R.; Herrero, J.A.; Bermúdez, J.L.C. Impact of first year renal replacement therapy on the hospital admissions of a regional public health system; [Impacto del primer año de tratamiento sustitutivo renal en la hospitalización de una comunidad autónoma]. Nefrologia 2019, 39, 653–663. [Google Scholar] [CrossRef]
- Zucman, N.; Uhel, F.; Verney, C.; Ricard, J.D.; Dreyfuss, D.; Roux, D. Water treatment-free prolonged intermittent kidney replacement therapy: A new approach for kidney replacement therapy in the ICU setting. A retrospective study. J. Crit. Care 2025, 87, 155014. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.B. Biopsy before transplant: Optimizing allocation or fueling discard? Kidney Int. 2024, 106, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, J.P.; Åsberg, A.; Heldal, K.; Jenssen, T.; Dörje, C.; Skauby, M.; Midtvedt, K. Long-term Outcomes After Kidney Transplantation From DBD Donors Aged 70 y and Older. Transplant. Direct 2024, 10, e1660. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Barreto, S.; Bravo, P.; Santos, J.P.; Ferreira, M.J.; Oliveira, C.; Ramos, A. Kidney Transplant From Elderly Donors: A Center Experience. Transplant. Proc. 2020, 52, 1265–1268. [Google Scholar] [CrossRef]
- Mella, A.; Calvetti, R.; Barreca, A.; Congiu, G.; Biancone, L. Kidney transplants from elderly donors: What we have learned 20 years after the Crystal City consensus criteria meeting. J. Nephrol. 2024, 37, 1449–1461. [Google Scholar] [CrossRef]
- Wellekens, K.; Naesens, M. Pretransplant biopsies for kidney allocation and discard: More questions than answers. Kidney Int. 2024, 106, 1032–1036. [Google Scholar] [CrossRef]
- McKenney, C.; Torabi, J.; Todd, R.; Akhtar, M.Z.; Tedla, F.M.; Shapiro, R.; Florman, S.S.; Holzner, M.L.; van Leeuwen, L.L. Wasted Potential: Decoding the Trifecta of Donor Kidney Shortage, Underutilization, and Rising Discard Rates. Transplantology 2024, 5, 51–64. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Stewart, D.E.; Bista, B.R.; Salkowski, N.; Snyder, J.J.; Israni, A.K.; Crary, G.S.; Rosendale, J.D.; Matas, A.J.; Delmonico, F.L. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin. J. Am. Soc. Nephrol. 2014, 9, 562–571. [Google Scholar] [CrossRef]
- Reese, P.P.; Aubert, O.; Naesens, M.; Huang, E.; Potluri, V.; Kuypers, D.; Bouquegneau, A.; Divard, G.; Raynaud, M.; Bouatou, Y.; et al. Assessment of the utility of kidney histology as a basis for discarding organs in the United States: A comparison of international transplant practices and outcomes. J. Am. Soc. Nephrol. 2021, 32, 397–409. [Google Scholar] [CrossRef]
- Carpenter, D.; Ali Husain, S.; Brennan, C.; Batal, I.; Hall, I.E.; Santoriello, D.; Rosen, R.; Crew, R.J.; Campenot, E.; Dube, G.K.; et al. Procurement biopsies in the evaluation of deceased donor kidneys. Clin. J. Am. Soc. Nephrol. 2018, 13, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xi, C.; Menon, M.C.; Cravedi, P.; Tedla, F.; Soto, A.; Sun, Z.; Liu, K.; Zhang, J.; Wei, C.; et al. A large-scale retrospective study enabled deep-learning based pathological assessment of frozen procurement kidney biopsies to predict graft loss and guide organ utilization. Kidney Int. 2024, 105, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Giorgakis, E.; Hardgrave, H.; Callais, N.; Wells, A. Machine learning-driven virtual biopsy system may increase organ discards at aggressive kidney transplant centers. Nat. Commun. 2024, 15, 10323. [Google Scholar] [CrossRef]
- Sung, R.; Christensen, L.; Leichtman, A.; Greenstein, S.; Distant, D.; Wynn, J.; Stegall, M.; Delmonico, F.; Port, F. Determinants of discard of expanded criteria donor kidneys: Impact of biopsy and machine perfusion. Am. J. Transplant. 2008, 8, 783–792. [Google Scholar] [CrossRef]
- Barah, M.; Kilambi, V.; Friedewald, J.J.; Mehrotra, S. Implications of Accumulated Cold Time for US Kidney Transplantation Offer Acceptance. Clin. J. Am. Soc. Nephrol. 2022, 17, 1353–1362. [Google Scholar] [CrossRef]
- Wellekens, K.; Koshy, P.; Naesens, M. Challenges in standardizing preimplantation kidney biopsy assessments and the potential of AI-Driven solutions. Curr. Opin. Nephrol. Hypertens. 2025, 34, 185–190. [Google Scholar] [CrossRef]
- Reeve, J.; Böhmig, G.A.; Eskandary, F.; Einecke, G.; Lefaucheur, C.; Loupy, A.; Halloran, P.F. Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2017, 2, e94197. [Google Scholar] [CrossRef] [PubMed]
- Loizeau, X.; Romanchikova, M.; Thomas, S.A.; Alsuleman, M.; Ayorinde, J.O.O.; Pettigrew, G.J. Quantifying measurement uncertainty in renal transplant biopsy assessment. Front. Nephrol. 2024, 4, 1458491. [Google Scholar] [CrossRef]
- Distefano, G.; Granata, S.; Morale, W.; Granata, A. Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives. Biomedicines 2024, 12, 2671. [Google Scholar] [CrossRef]
- Yoo, D.; Divard, G.; Raynaud, M.; Cohen, A.; Mone, T.D.; Rosenthal, J.T.; Bentall, A.J.; Stegall, M.D.; Naesens, M.; Zhang, H.; et al. A Machine Learning-Driven Virtual Biopsy System For Kidney Transplant Patients. Nat. Commun. 2024, 15, 554. [Google Scholar] [CrossRef]
- Talaminos Barroso, A.; Reina Tosina, J.; Roa, L.M.; Calvillo Arbizu, J.; Pérez Valdivia, M.A.; Medina, R.; Rocha Castilla, J.L.; Castro-de-la Nuez, P. Comparative Study of the Impact of Human Leukocyte Antigens on Renal Transplant Survival in Andalusia and the United States. Diagnostics 2023, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Liu, K.; Wu, H.; Wang, H.; Huang, J.; Li, J. Evaluation of electrical characteristics of biological tissue with electrical impedance spectroscopy. Electrophoresis 2020, 41, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Karpiel, I.; Urzeniczok, M.; Sobotnicka, E. Bioimpedance Spectroscopy—Niche Applications in Medicine: Systematic Review. In The Latest Developments and Challenges in Biomedical Engineering. Proceedings of the 23rd Polish Conference on Biocybernetics and Biomedical Engineering, Lodz, Poland, 27–29 September 2023; Springer: Berlin/Heidelberg, Germany, 2024; Volume 746, pp. 311–323. [Google Scholar] [CrossRef]
- Wen, J.; Wu, P.; Li, J.; Xu, H.; Li, Y.; Chen, K.; Li, G.; Lv, Z.; Wang, X. Application of bioelectrical impedance detection techniques: Cells and tissues. Biosens. Bioelectron. 2025, 273, 117159. [Google Scholar] [CrossRef]
- Emran, S.; Lappalainen, R.; Kullaa, A.M.; Myllymaa, S. Concentric ring probe for bioimpedance spectroscopic measurements: Design and ex vivo feasibility testing on pork oral tissues. Sensors 2018, 18, 3378. [Google Scholar] [CrossRef] [PubMed]
- Yanko, R.; Safonov, S.; Levashov, M. Morphological features of white adipose tissue in rats with different levels of energy metabolism in visceral obesity. Rep. Morphol. 2024, 30, 44–51. [Google Scholar] [CrossRef]
- Amini, M.; Hisdal, J.; Kalvøy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 2018, 9, 142–158. [Google Scholar] [CrossRef]
- Saadé, K.; Hussain, M.A.; Bainbridge, S.A.; St-Gelais, R.; Variola, F.; Fenech, M. Cost-Effective Bioimpedance Spectroscopy System for Monitoring Syncytialization In Vitro: Experimental and Numerical Validation of BeWo Cell Fusion. Micromachines 2024, 15, 1506. [Google Scholar] [CrossRef]
- Strand-Amundsen, R.J.; Tronstad, C.; Kalvoy, H.; Ruud, T.E.; Hogetveit, J.O.; Martinsen, O.G.; Tonnessen, T.I. Small intestinal ischemia and reperfusion—Bioimpedance measurements. Physiol. Meas. 2018, 39, 025001. [Google Scholar] [CrossRef]
- Jaimes-Morales, S.; Aguirre-Cardona, V.; Gonzalez-Correa, C. Ex vivo electrical bioimpedance measurements and Cole modelling on the porcine colon and rectum. Sci. Rep. 2024, 14, 21266. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Cai, Z.; Han, S.; Lin, S.; He, L.; Chen, M.; Pan, D.; Deng, G.; Duan, S.; et al. Variation in the dielectric properties of freshly excised colorectal cancerous tissues at different tumor stages. Bioelectromagnetics 2017, 38, 522–532. [Google Scholar] [CrossRef]
- Kim, H.J.; Seong, E.Y.; Jung, H.J.; Song, S.H. The phase angle before transplantation can predict the status of low muscle mass after kidney transplantation. Clin. Exp. Nephrol. 2024, 28, 1319–1326. [Google Scholar] [CrossRef]
- Wołoszyk, P.; Małgorzewicz, S.; Chamienia, A.; Dębska-Ślizień, A. Obesity After Successful Kidney Transplantation. Transplant. Proc. 2020, 52, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Yan, Q.; You, F.; Fu, F.; Dong, X.; Shi, X.; Yang, M. Correlation between the dielectric properties and biological activities of human ex vivo hepatic tissue. Phys. Med. Biol. 2015, 60, 2603–2617. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, X.; You, F.; Wang, H.; Wang, H.; Cai, Z.; Dong, X. Preliminary research on relationship between dielectric properties and microstructure of rabbit liver. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Beijing, China, 26–31 May 2012; IFMBE. Volume 39, pp. 643–646. [Google Scholar] [CrossRef]
- Kaya, E.; Bakir, A.; Koseoglu, Y.K.; Velidedeoglu, M.; Trabulus, S.; Seyahi, N. Association of Nutritional Assessment by Phase Angle With Mortality in Kidney Transplant Patients in an 8-Year Follow-Up. Prog. Transplant. 2019, 29, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kasiviswanathan, U.; Mukherjee, S.; Kumar Mahto, S.; Sharma, N.; Patnaik, R. Changes in electrolyte concentrations alter the impedance during ischemia-reperfusion injury in rat brain. Physiol. Meas. 2019, 40, 105004. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Chen, R.; Zhang, G.; Li, W.; Fu, F.; Dong, X. In vivo bioimpedance spectroscopy characterization of healthy, hemorrhagic and ischemic rabbit brain within 10 Hz–1 MHz. Sensors 2017, 17, 791. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Cao, X.; Dong, X.; Yang, L. Discrimination between human normal renal tissue and renal cell carcinoma by dielectric properties using in-vitro BIA. Front. Physiol. 2023, 14, 1121599. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Ji, Z.; Dong, X.; Shi, X. Bivariate polynomial for time-dependent dielectric properties of ex-vivo porcine and human liver tissue at frequencies below 100 MHz. Meas. Sci. Technol. 2023, 34, 074003. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhu, C.; Han, R.; Steuer, A.; Kolb, J.F.; Shi, F. Uncertainty Quantification and Sensitivity Analysis for the Electrical Impedance Spectroscopy of Changes to Intercellular Junctions Induced by Cold Atmospheric Plasma. Molecules 2022, 27, 5861. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, F.; Guo, J.; Wang, Q.; Kolb, J.F.; Wang, W.; Wu, X.; Zhuang, J. Impedimetric characterization of normal and cancer cell responses after nano-pulse stimulation. J. Phys. D Appl. Phys. 2021, 54, 185401. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef]
- Roa-Romero, L.; Reina-Tosina, L.; Naranjo-Hernández, D.; Estudillo-Valderrama, M. Sensor Inteligente de Bioimpedancia para Aplicaciones Biomédicas. Spanish Patent ES2537351, 7 May 2015. [Google Scholar]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostán, G.; Aresté-Fosalba, N.; Lara-Ruiz, A.; Cejudo-Ramos, P.; Ortega-Ruiz, F. Smart bioimpedance spectroscopy device for body composition estimation. Sensors 2020, 20, 70. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostán, G.; Ortega-Ruiz, F.; Cejudo Ramos, P. Smart Bioimpedance Device for the Assessment of Peripheral Muscles in Patients with COPD. Sensors 2024, 24, 4648. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.; Pérez-Valdivia, M.; Salgueira-Lazo, M.; Medina-López, R. Dispositivo inteligente para el estudio de la viabilidad de riñones para trasplante (Smart device for the study of the viability of kidneys for transplant). In Proceedings of the 38th Annual Congress of the Spanish Society of Biomedical Engineering, Virtual Congress, Spain, 25–27 November 2020. [Google Scholar]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.; Pérez-Valdivia, M.; Salgueira-Lazo, M.; Medina-López, R. Adaptación de un dispositivo de medida de bioimpedancia renal para uso en quirófano (Adaptation of a renal bioimpedance measurement device for use in the operating room). In Proceedings of the 36th National Symposium of the International Scientific Union of Radio, Vigo, Spain, 20–24 September 2021. [Google Scholar]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.; Calvillo-Arbizu, J.; Pérez-Valdivia, M.; Salgueira-Lazo, M.; Medina-López, R. Nuevo modelo de Cole para medidas de bioimpedancia en injertos renales (New Cole model for bioimpedance measurements in kidney grafts). In Proceedings of the 38th National Symposium of the International Scientific Union of Radio, Cáceres, Spain, 13–15 September 2023. [Google Scholar]
- Ferreira, J.; Pau, I.; Lindecrantz, K.; Seoane, F. A Handheld and Textile-Enabled Bioimpedance System for Ubiquitous Body Composition Analysis. An Initial Functional Validation. IEEE J. Biomed. Health Inform. 2017, 21, 1224–1232. [Google Scholar] [CrossRef]
- Genescà, M.; Ivorra, A.; Sola, A.; Palacios, L.; Goujon, J.M.; Hauet, T.; Villa, R.; Aguiló, J.; Hotter, G. Electrical bioimpedance measurement during hypothermic rat kidney preservation for assessing ischemic injury. Biosens. Bioelectron. 2005, 20, 1866–1871. [Google Scholar] [CrossRef]
- Allegri, D.; Donida, A.; Malcovati, P.; Barrettino, D. CMOS-Based Multifrequency Impedance Analyzer for Biomedical Applications. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Ramanathan, T.; Machado, D.; Sundararajan, R. Electrical impedance spectroscopy study of biological tissues. J. Electrost. 2008, 66, 165–177. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Yang, L.; Shi, X.; Wen, Z.; Dong, X. Exploring the relationship between the dielectric properties and viability of human normal hepatic tissues from 10 Hz to 100 MHz based on grey relational analysis and BP neural network. Comput. Biol. Med. 2021, 134, 104494. [Google Scholar] [CrossRef]
- Gómez, R.; Ivorra, A.; Villa, R.; Godignon, P.; Millán, J.; Erill, I.; Solà, A.; Hotter, G.; Palacios, L. A SiC microdevice for the minimally invasive monitoring of ischemia in living tissues. Biomed. Microdevices 2006, 8, 43–49. [Google Scholar] [CrossRef]
- Hou, J.; Strand-Amundsen, R.; Hødnebø, S.; Tønnessen, T.I.; Olav Høgetveit, J. Assessing ischemic injury in human intestine ex vivo with electrical impedance spectroscopy. J. Electr. Bioimpedance 2021, 12, 82–88. [Google Scholar] [CrossRef]
- Spottorno, J.; Multigner, M.; Rivero, G.; Álvarez, L.; De La Venta, J.; Santos, M. Time dependence of electrical bioimpedance on porcine liver and kidney under a 50 Hz ac current. Phys. Med. Biol. 2008, 53, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Yun, S.; Shin, H.; Kim, J.; Choi, J. Measurement of bioimpedance and cell viability during ischemia-reperfusion in the rat liver. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; VOLS. Volume 7, pp. 1945–1947. [Google Scholar] [CrossRef]
- Eduardo, P.M.; Mario, G.L.; Carlos César, P.M.; Mayra, M.A.; Sara, H.Y.; Beltran Nohra, E. Bioelectric, tissue, and molecular characteristics of the gastric mucosa at different times of ischemia. Exp. Biol. Med. 2021, 246, 1968–1980. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Shin, H.; Ahn, H.; Yun, S.; Shin, S.; Hwang, Y. Measurement of bioelectrical impedance during ischemia in the rat liver. In Proceedings of the Orld Congress of Medical Physics and Biomedical Engineering, Seoul, Republic of Korea, 27 August–1 September 2006; Volume 14, pp. 753–755. [Google Scholar] [CrossRef]
- Mellert, F.; Winkler, K.; Schneider, C.; Dudykevych, T.; Welz, A.; Osypka, M.; Gersing, E.; Preusse, C.J. Detection of (Reversible) myocardial ischemic injury by means of electrical bioimpedance. IEEE Trans. Biomed. Eng. 2011, 58, 1511–1518. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostán, G.; Calvillo-Arbizu, J.; Talaminos-Barroso, A.; Pérez-Valdivia, M.Á.; Medina-López, R.A. Development and Validation in Porcine and Human Models of a Bioimpedance Spectroscopy System for the Objective Assessment of Kidney Graft Viability. Sensors 2025, 25, 2871. https://doi.org/10.3390/s25092871
Naranjo-Hernández D, Reina-Tosina J, Roa LM, Barbarov-Rostán G, Calvillo-Arbizu J, Talaminos-Barroso A, Pérez-Valdivia MÁ, Medina-López RA. Development and Validation in Porcine and Human Models of a Bioimpedance Spectroscopy System for the Objective Assessment of Kidney Graft Viability. Sensors. 2025; 25(9):2871. https://doi.org/10.3390/s25092871
Chicago/Turabian StyleNaranjo-Hernández, David, Javier Reina-Tosina, Laura M. Roa, Gerardo Barbarov-Rostán, Jorge Calvillo-Arbizu, Alejandro Talaminos-Barroso, Miguel Ángel Pérez-Valdivia, and Rafael A. Medina-López. 2025. "Development and Validation in Porcine and Human Models of a Bioimpedance Spectroscopy System for the Objective Assessment of Kidney Graft Viability" Sensors 25, no. 9: 2871. https://doi.org/10.3390/s25092871
APA StyleNaranjo-Hernández, D., Reina-Tosina, J., Roa, L. M., Barbarov-Rostán, G., Calvillo-Arbizu, J., Talaminos-Barroso, A., Pérez-Valdivia, M. Á., & Medina-López, R. A. (2025). Development and Validation in Porcine and Human Models of a Bioimpedance Spectroscopy System for the Objective Assessment of Kidney Graft Viability. Sensors, 25(9), 2871. https://doi.org/10.3390/s25092871






