The Screening of Aptamers and the Development of a Colorimetric Detection Method for the Pesticide Deltamethrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Reagents
2.2. Screening of Del Aptamer
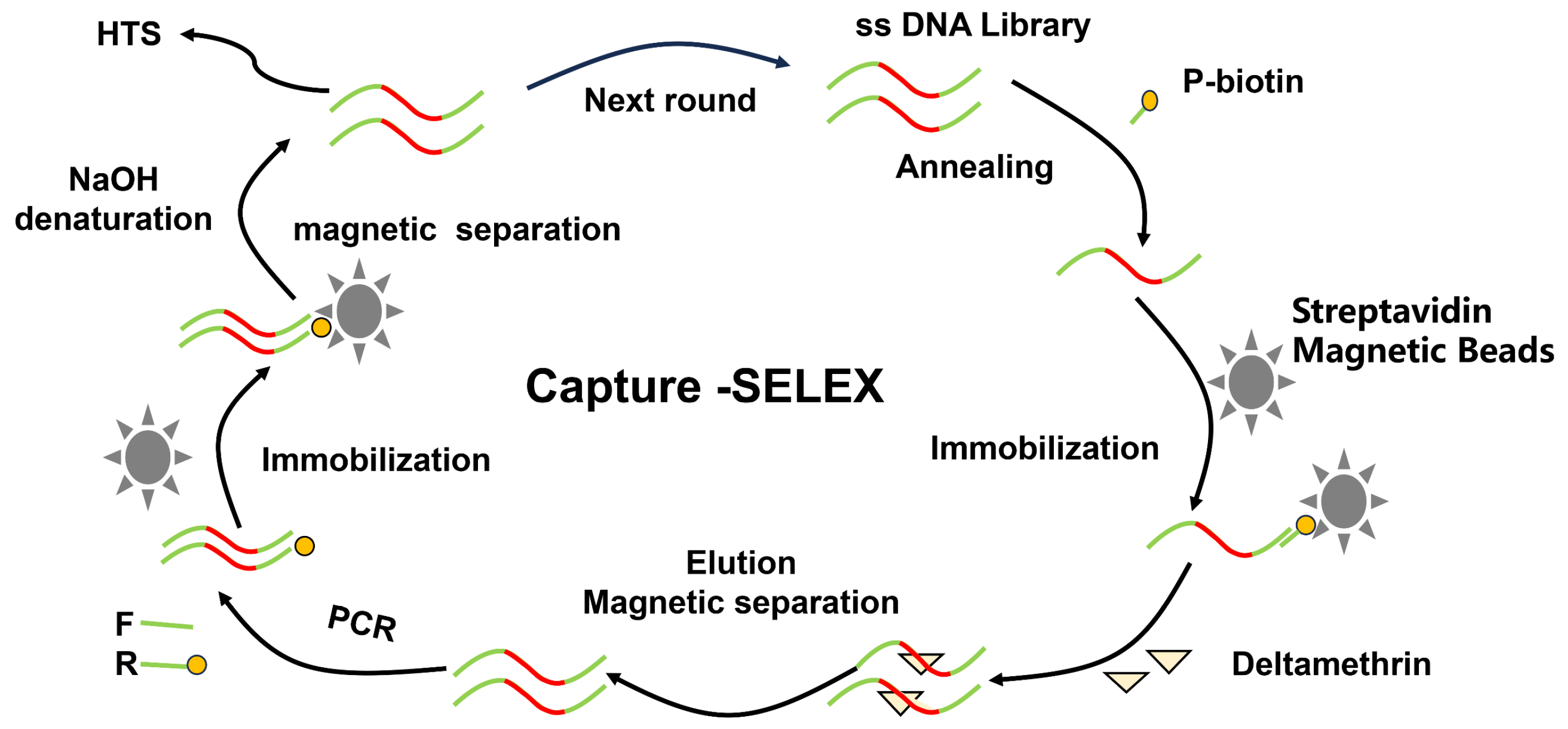
2.2.1. The Strategy for Screening Del Aptamers
2.2.2. Fixing of Random ssDNA
2.2.3. Elution of Affinity ssDNA Sequences
2.2.4. PCR Amplification and Electrophoretic Characterization
2.2.5. Preparation of the Next ssDNA Library
2.3. High-Throughput Sequencing and Secondary Structure Prediction of Del Aptamers
2.4. Affinity and Specificity Analysis of Del Aptamers
2.5. Molecular Docking
2.6. Construction of AuNPs for Colorimetric Detection of Del
2.7. Testing of Actual Samples
3. Results
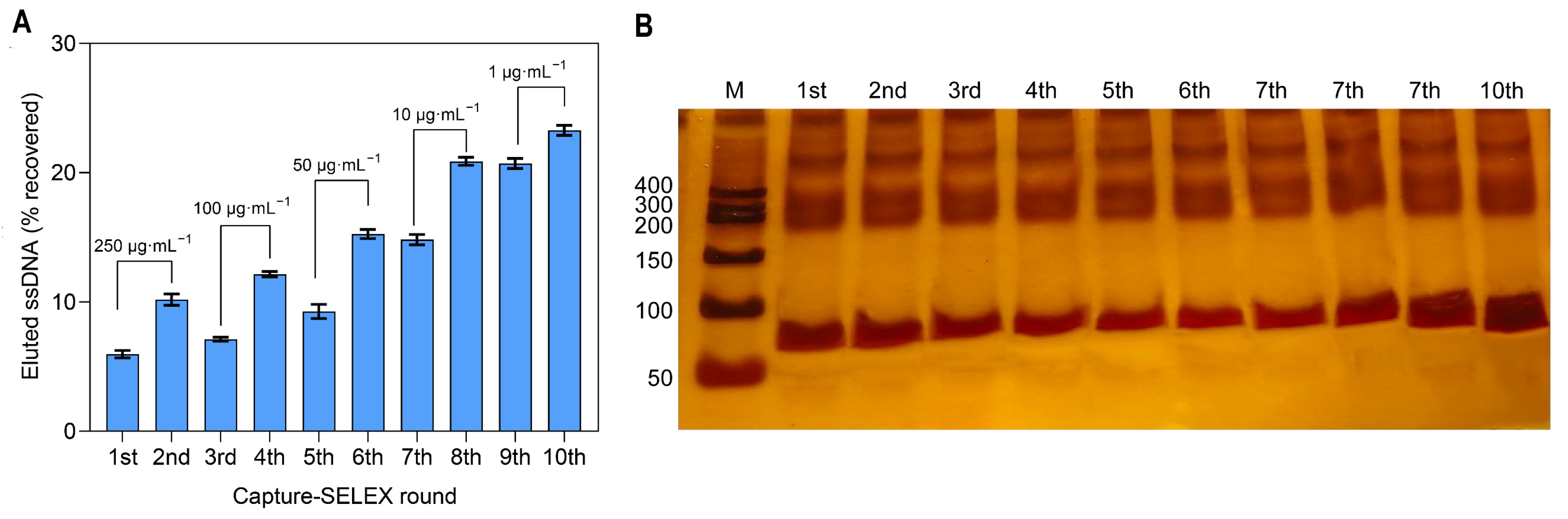
3.1. Screening of Del Aptamers
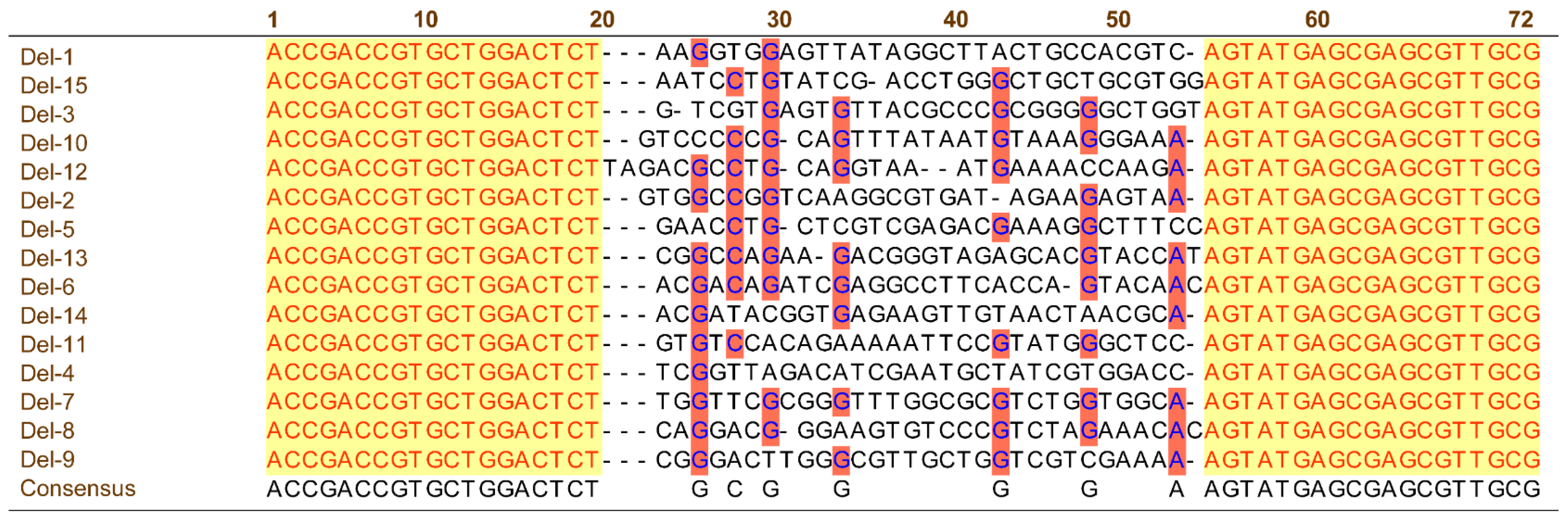
3.2. Sequence Analysis of Del Aptamers
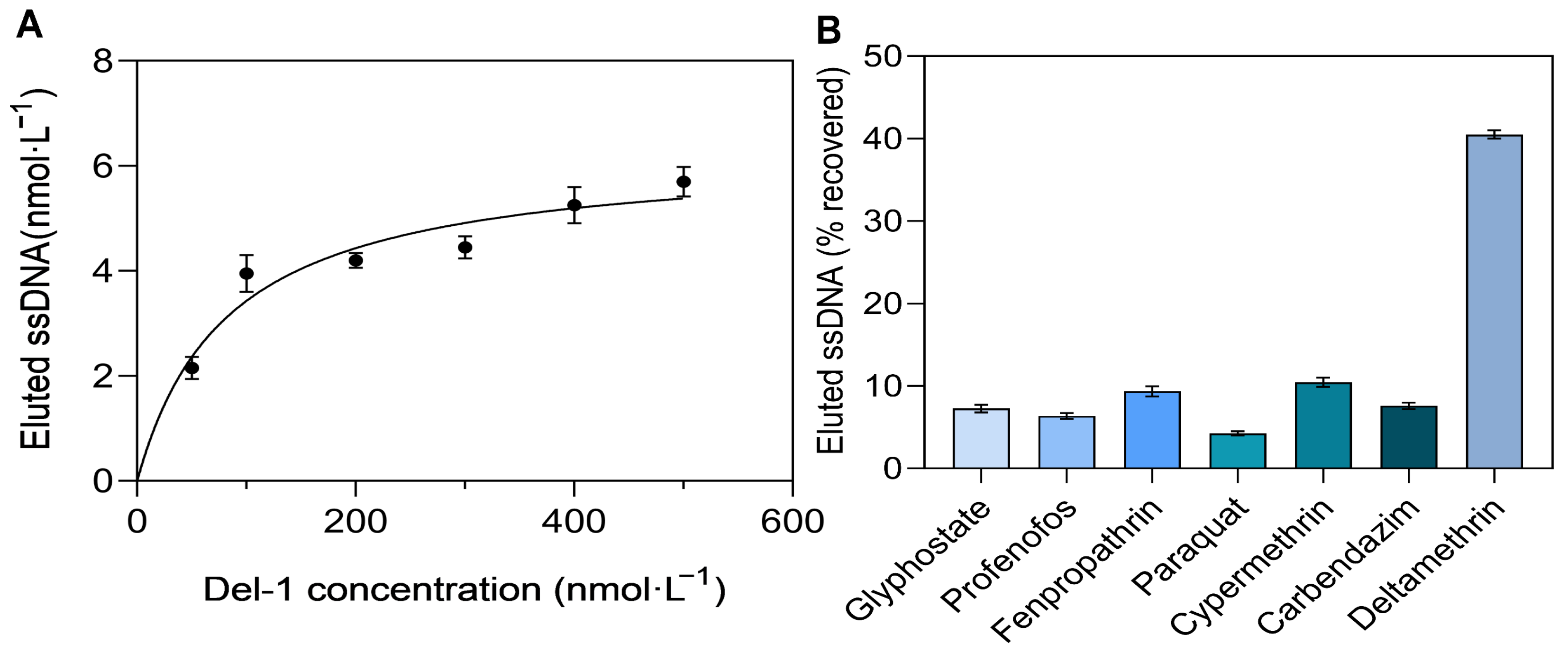
3.3. Kd Value Determination and Specificity Analysis of the Aptamer
3.4. Del- and Aptamer-Binding Mechanism
3.5. The Principle and Feasibility Analysis of the Colorimetric Method
3.6. The Optimization of the Conditions of the Colorimetric Method
3.7. Performance Analysis of the Colorimetric Method
3.8. Detection of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markus, V.; Teralı, K.; Dalmizrak, O.; Ozer, N. Assessment of the inhibitory activity of the pyrethroid pesticide deltamethrin against human placental glutathione transferase P1-1: A combined kinetic and docking study. Environ. Toxicol. Pharmacol. 2018, 61, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S.; Furey, A.; Crooks, S.; Danaher, M. A review of methodology for the analysis of pyrethrin and pyrethroid residues in food of animal origin. Food Addit. Contam. Part A 2018, 35, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sun, Y.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.E.; Yuan, Z.; Wang, X.; Martínez, M.A. Deltamethrin toxicity: A review ofoxidative stress and metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Joho, Y.; Vogel, M.; Fent, K. Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environ. Pollut. 2019, 244, 247–256. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, Q.; Chen, X.; Mao, G.; Feng, W.; Yang, L.; Zhao, T.; Wu, X.; Chen, Y. Overview of deltamethrin residues and toxic effects in the global environment. Environ. Geochem. Health 2024, 46, 271. [Google Scholar] [CrossRef]
- Xi, C.; Yang, Z.; Yu, Y.; Li, S.; He, J.; El-Aziz, T.M.A.; Zhao, F.; Cao, Z. Influence of perinatal deltamethrin exposure at distinct developmental stages on motor activity, learning and memory. Ecotoxicol. Environ. Saf. 2022, 236, 113460. [Google Scholar] [CrossRef]
- List of Carcinogens of the International Research Agency of the World Health Organization Group 3 Carcinogens; Shaoxing Market Supervision and Administration Bureau: Shaoxing, China, 2022; p. 592.
- GB 2763–2021; Maximum Residue Limits for Pesticides in Food. National Standards of the People’s Republic of China: Beijing, China, 2021.
- Yan, Y.; Chang, X.; Wang, X.; Luo, P.; Zhao, Y.; Wang, Y. Methods for simultaneous determination of methylchlor, hexachlorobenzene and deltamethrin in drinking water by GC-MS/MS. J. China Urban Water Assoc. 2024, 4, 33–38. [Google Scholar]
- Yan, Z.; Chen, X. Validation of methods for determining bifenthrin, cyhalopitrin and deltamethrin in fruits and vegetables by gas chromatography. South. Hortic. 2023, 34, 19–23. [Google Scholar]
- Xu, Z.H.; Li, L.; Chen, Z.J.; Wang, B.Z.; Yan, Y.Y.; Sun, Y.M.; Xu, Z.L. Study and application of broad spectrum immunochromatographic strips for pyrethroids. Anal. Chem. 2016, 44, 1900–1906. [Google Scholar]
- Klutz, S.; Holtmann, L.; Lobedann, M.; Schembecker, G. Cost evaluation of antibody production processes in different operation modes. Chem. Eng. Sci. 2016, 141, 63–74. [Google Scholar] [CrossRef]
- Ma, H.; Ó’Fágáin, C.; O’Kennedy, R. Antibody stability: A key to performance—Analysis, influences and improvement. Biochimie 2020, 177, 213–225. [Google Scholar] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [PubMed]
- Ellington, A.D.; Szostak, J.W. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 1992, 355, 850–852. [Google Scholar] [PubMed]
- Reid, R.; Chatterjee, B.; Das, S.J.; Ghosh, S.; Sharma, T.K. Application of aptamers as molecular recognition elements in lateral flow assays. Anal. Biochem. 2022, 593, 113574. [Google Scholar]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar]
- Stoltenburg, R.; Nikolaus, N.; Strehlitz, B. Capture-SELEX: Selection of DNA aptamers for aminoglycoside antibiotics. J. Anal. Methods. Chem. 2012, 1, 415697. [Google Scholar]
- Yang, Y.; Tang, Y.; Wang, C.; Liu, B.; Wu, Y. Selection and identification of a DNA aptamer for ultrasensitive and selective detection of λ-cyhalothrin residue in food. Anal. Chim. Acta. 2021, 1179, 338837. [Google Scholar]
- Yang, H.Q.; Xia, L.; Zheng, J.; Xie, Z.G.; Zhou, J.L.; Wu, Y.J. Screening and identification of a DNA aptamer to construct the label-free fluorescent aptasensor for ultrasensitive and selective detection of clothianidin residue in agricultural products. Talanta 2023, 262, 124712. [Google Scholar]
- Wu, Y.; Zhan, S.; Wang, L.; Zhou, P. Selection of a DNA aptamer for detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar]
- Ran, X.D.; Wu, Y.G. Screening of paraquat nucleic acid aptamer and its colorimetric detection study. Anal. Chem. 2019, 47, 567–575. [Google Scholar]
- Xia, L.; Yang, Y.; Yang, H.; Tang, Y.; Zhou, J.; Wu, Y. Screening and identification of an aptamer as novel recognition molecule in the test strip and its application for visual detection of ethyl carbamate in liquor. Anal. Chim. Acta 2022, 1226, 340289. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Fu, Z.; Sun, X.; Lin, C.; Yang, S.; Xu, J.; Zhang, M.; Zhang, H.; Ning, F.; Hu, P. A colorimetric aptasensor based on a hemin/EpCAM aptamer DNAzyme for sensitive exosome detection. Analyst 2022, 147, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xie, W.; Jia, Q.; Zhao, F.; Wu, W.; Yang, Q.; Hou, X. An aptamer and flower-shaped AuPtRh nanoenzyme-based colorimetric biosensor for the detection of profenofos. Analyst 2022, 147, 4105–4115. [Google Scholar] [CrossRef]
- Pires, T.A.; Narovec, C.M.; Whelan, R.J. Effects of Cationic Proteins on Gold Nanoparticle/Aptamer Assays. ACS Omega 2017, 2, 8222–8226. [Google Scholar] [CrossRef]
- Wang, S.; Su, L.; Wang, L.; Zhang, D.; Shen, G.; Ma, Y. Colorimetric determination of carbendazim based on the specific recognition of aptamer and the poly-diallyldimethylammonium chloride aggregation of gold nanoparticles. Spectrochim. Acta A 2020, 228, 117809. [Google Scholar]
- Duan, N.; Gong, W.; Wu, S.; Wang, Z. Selection and Application of ssDNA Aptamers against Clenbuterol Hydrochloride Based on ssDNA Library Immobilized SELEX. J. Agric. Food Chem. 2017, 65, 1771–1777. [Google Scholar]
- Kang, D.; Wang, J.; Zhang, W.; Song, Y.; Li, X.; Zou, Y.; Zhu, M.; Zhu, Z.; Chen, F.; Yang, C.J. Selection of DNA aptamers against glioblastoma cells with high affinity and specificity. PLoS ONE 2012, 7, e42731. [Google Scholar]
- Wang, L.; Liu, X.; Zhang, Q.; Zhang, C.H.; Liu, Y.; Tu, K.; Tu, J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol. Lett. 2012, 34, 869–874. [Google Scholar] [CrossRef]
- Yang, S.N.; Zhao, D.; Cai, M.; Han, Z.W.; Zhang, J.; Qiu, Y. A study on the immunomodulatory mechanism of Hibiscus sabdariffa flavonoids based on network pharmacology and molecular docking probes. Chin. J. Anim. Husb. 2024, 60, 309–318. [Google Scholar]
- Du, J.; Yan, H.; She, D.; Liu, B.; Yang, G. Simultaneous determination of cypermethrin and permethrin in pear juice by ultrasound-assisted dispersive liquid-liquid microextraction combined with gas chromatography. Talanta 2010, 82, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Bissacot, D.Z.; Vassilieff, I. HPLC determination of flumethrin, deltamethrin, cypermethrin, and cyhalothrin residues in the milk and blood of lactating dairy cows. J. Anal. Toxicol. 1997, 21, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Shan, G.; Watanabe, T.; Stoutamire, D.W.; Gee, S.J.; Hammock, B.D. Enzyme-linked immunosorbent assay for the pyrethroid deltamethrin. J. Agric. Food Chem. 2002, 50, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Chen, C.; Wang, R.Q.; Waterhouse, G.I.N.; Qiao, X.G.; Sun, Y.F.; Xu, Z.X. Internal standard-based self-assembly Ag@4-MBN@Ag nanoarray SERS ratio sensor for sensitive detection of deltamethrin in foods. Sens. Actuators B Chemical. 2024, 412, 135786. [Google Scholar] [CrossRef]
- Mutlu, E.; Şenocak, A.; Demirbaş, E.; Koca, A.; Akyüz, D. Selective and sensitive molecularly imprinted polymer-based electrochemical sensor for detection of deltamethrin. Food Chem. 2025, 463, 141121. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yin, L.F.; Zhang, W.Y.; Chen, M.L.; Feng, D.S.; Zhao, Y.; Zhu, Y.G. Colorimetric Measurement of Deltamethrin Pesticide Using a Paper Sensor Based on Aggregation of Gold Nanoparticles. Coatings 2022, 12, 38. [Google Scholar] [CrossRef]
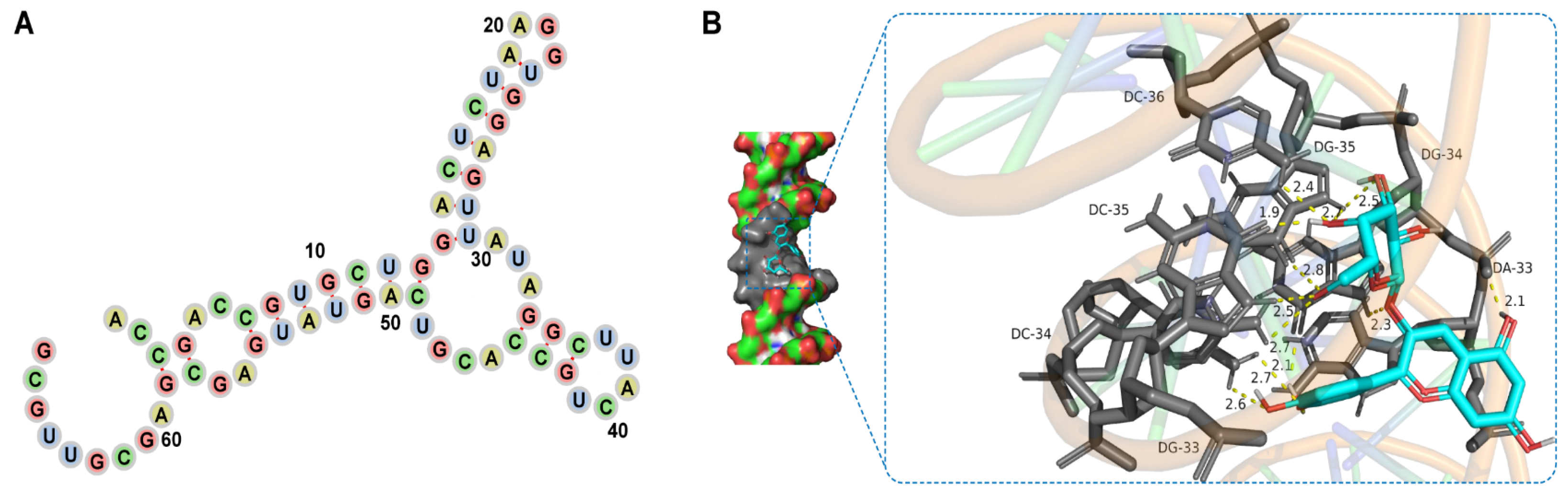
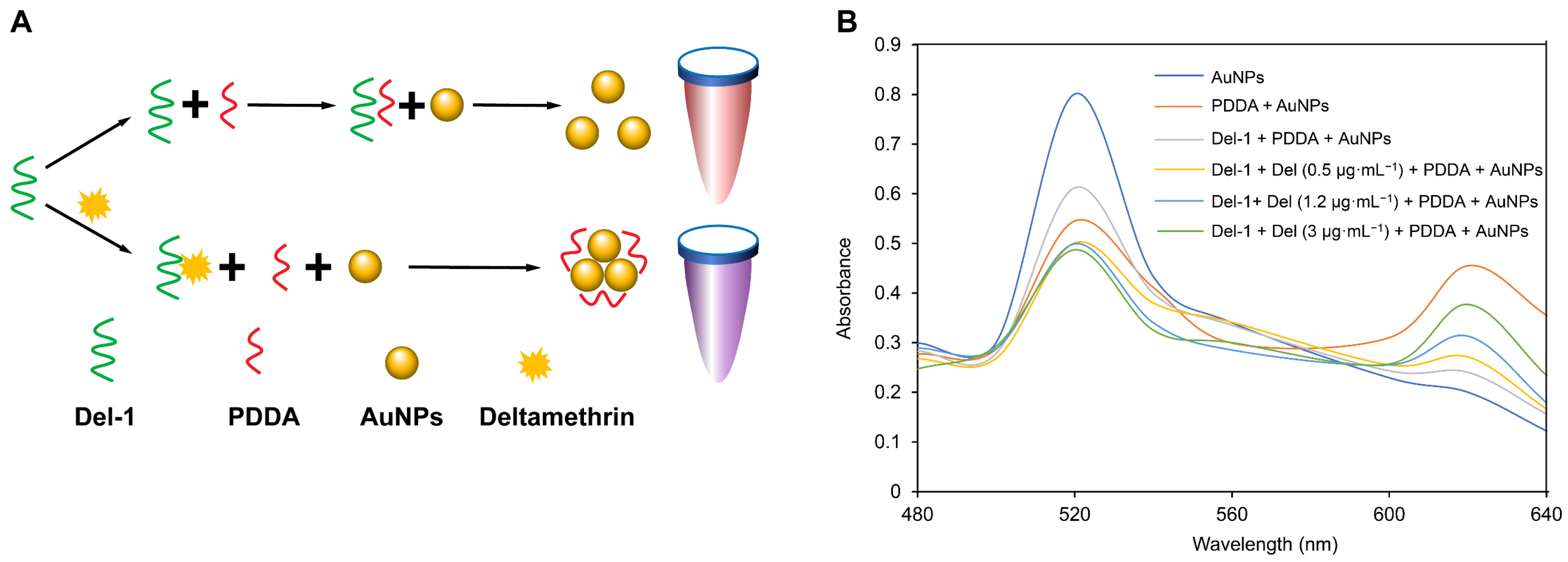

| Aptamers | Sequence of Aptamers (5′→3′) | GC (%) | ΔG (KJ/mol) |
|---|---|---|---|
| Del-1 | P1-AAGGTGGAGTTATAGGCTTACTGCCACGTC-P2 | 50 | ΔG = −12.333 |
| Del-2 | P1-GTGGCCGGTCAAGGCGTGATAGAAGAGTAA-P2 | 44 | ΔG = −6.908 |
| Del-3 | P1-GTCGTGAGTGTTACGCCCGCGGGGGCTGGT-P2 | 70 | ΔG = −17.50 |
| Del-4 | P1-TCGGTTAGACATCGAATGCTATCGTGGACC-P2 | 50 | ΔG = −4.605 |
| Del-5 | P1-TAGACGCCTGCAGGTAAATGAAAACCAAGA-P2 | 44 | ΔG = −2.595 |
| Del-6 | P1-ACGACAGATCGAGGCCTTCACCAGTACAAC-P2 | 54 | ΔG = −6.698 |
| Del-7 | P1-TGGTTCGCGGGTTTGGCGCGTCTGGTGGCA-P2 | 67 | ΔG = −17.626 |
| Del-8 | P1-CAGGACGGGAAGTGTCCCGTCTAGAAACAC-P2 | 60 | ΔG = −33.368 |
| Del-9 | P1-CGGGACTTGGGCGTTGCTGGTCGTCGAAAA-P2 | 57 | ΔG = −1.297 |
| Del-10 | P1-GTCCCCCGCAGTTTATAATGTAAAGGGAAA-P2 | 44 | ΔG = −6.908 |
| Del-11 | P1-GTGTCCACAGAAAAATTCCGTATGGGCTCC-P2 | 60 | ΔG = −3.475 |
| Del-12 | P1-GAACCTGCTCGTCGAGACGAAAGGCTTTCC-P2 | 57 | ΔG = −19.761 |
| Del-13 | P1-CGGCCAGAAGACGGGTAGAGCACGTACCAT-P2 | 60 | ΔG = −11.513 |
| Del-14 | P1-ACGATACGGTGAGAAGTTGTAACTAACGCA-P2 | 44 | ΔG = −1.925 |
| Del-15 | P1-AATCCTGTATCGACCTGGGCTGCTGCGTGG-P2 | 70 | ΔG = −2.386 |
| Sample | Added (ng·mL−1) | Mean found (ng·mL−1) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | |
| Apple | 500 | 510 | 102 | 0.13 |
| 1000 | 1180 | 118 | 6.12 | |
| 0 | 0 | 0 | 0 | |
| Pears | 500 | 370 | 74 | 2.23 |
| 1000 | 760 | 76 | 3.00 |
| Method | LOD (ng·mL−1) | Equipment Required | Cost of Detection | Time Required |
|---|---|---|---|---|
| GC-FID [33] | 0.01 | General Purpose GC System | high | 2–3 h |
| HPLC [34] | 1 | HPLC System | high | 1–3 h |
| ELISA [35] | 1.1 ± 0.5 | Spectrometer | high | 0.5–1 h |
| SERS [36] | 0.0316 × 10−3 | Raman Microscope System | high | 1–2 h |
| Electrochemical [37] | 77.3 | Potentiostat | medium | 15 min |
| Colorimetric [38] | 173 | No | low | 10–20 min |
| This work | 54.57 | No | low | 10–30 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Li, W.; Wang, J.; Li, S. The Screening of Aptamers and the Development of a Colorimetric Detection Method for the Pesticide Deltamethrin. Sensors 2025, 25, 2060. https://doi.org/10.3390/s25072060
Wu C, Li W, Wang J, Li S. The Screening of Aptamers and the Development of a Colorimetric Detection Method for the Pesticide Deltamethrin. Sensors. 2025; 25(7):2060. https://doi.org/10.3390/s25072060
Chicago/Turabian StyleWu, Caixia, Wenwei Li, Jiafu Wang, and Sheng Li. 2025. "The Screening of Aptamers and the Development of a Colorimetric Detection Method for the Pesticide Deltamethrin" Sensors 25, no. 7: 2060. https://doi.org/10.3390/s25072060
APA StyleWu, C., Li, W., Wang, J., & Li, S. (2025). The Screening of Aptamers and the Development of a Colorimetric Detection Method for the Pesticide Deltamethrin. Sensors, 25(7), 2060. https://doi.org/10.3390/s25072060






