The Effect of the Position of a Phenyl Group on the Luminescent and TNP-Sensing Properties of Cationic Iridium(III) Complexes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
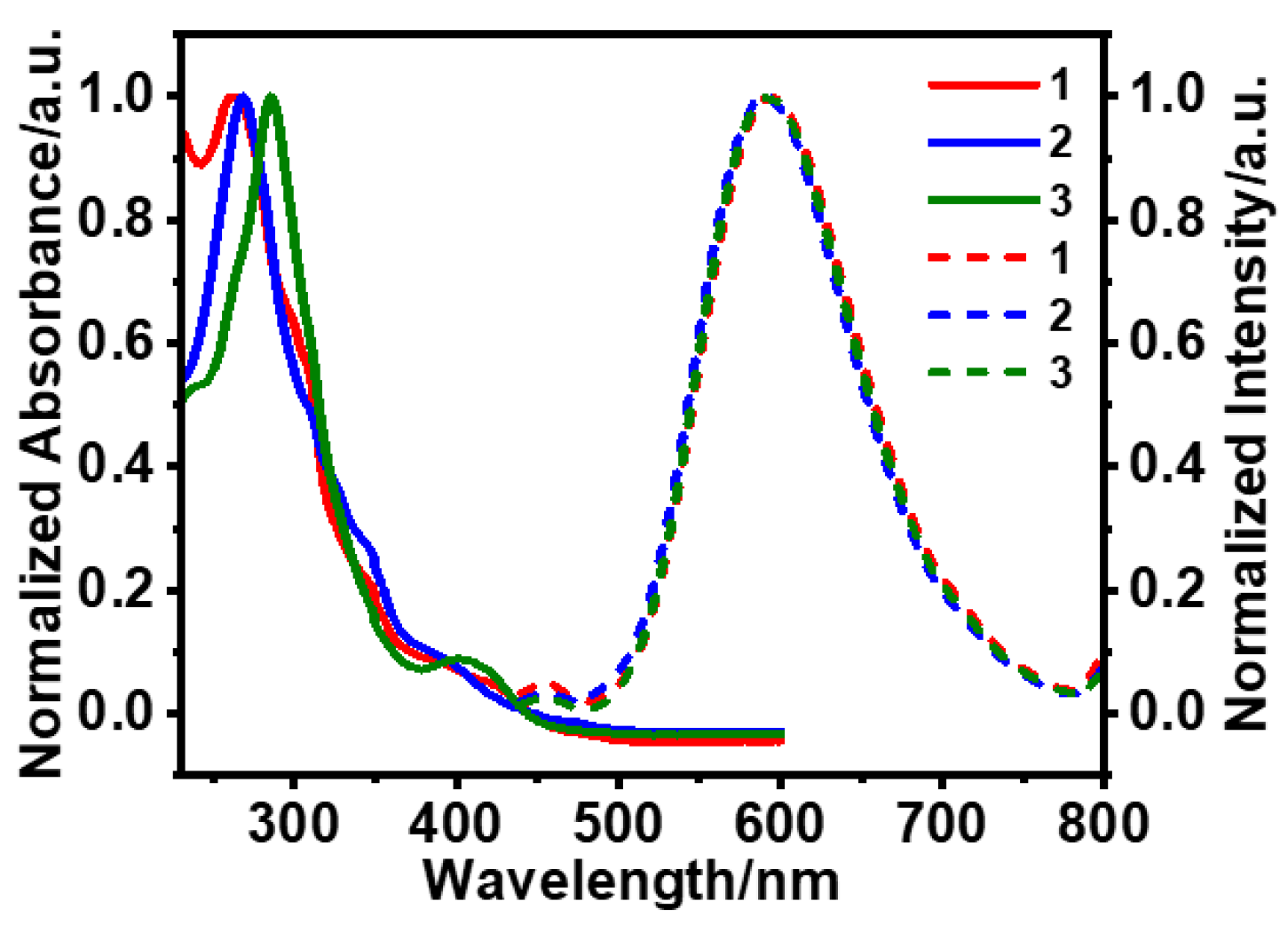
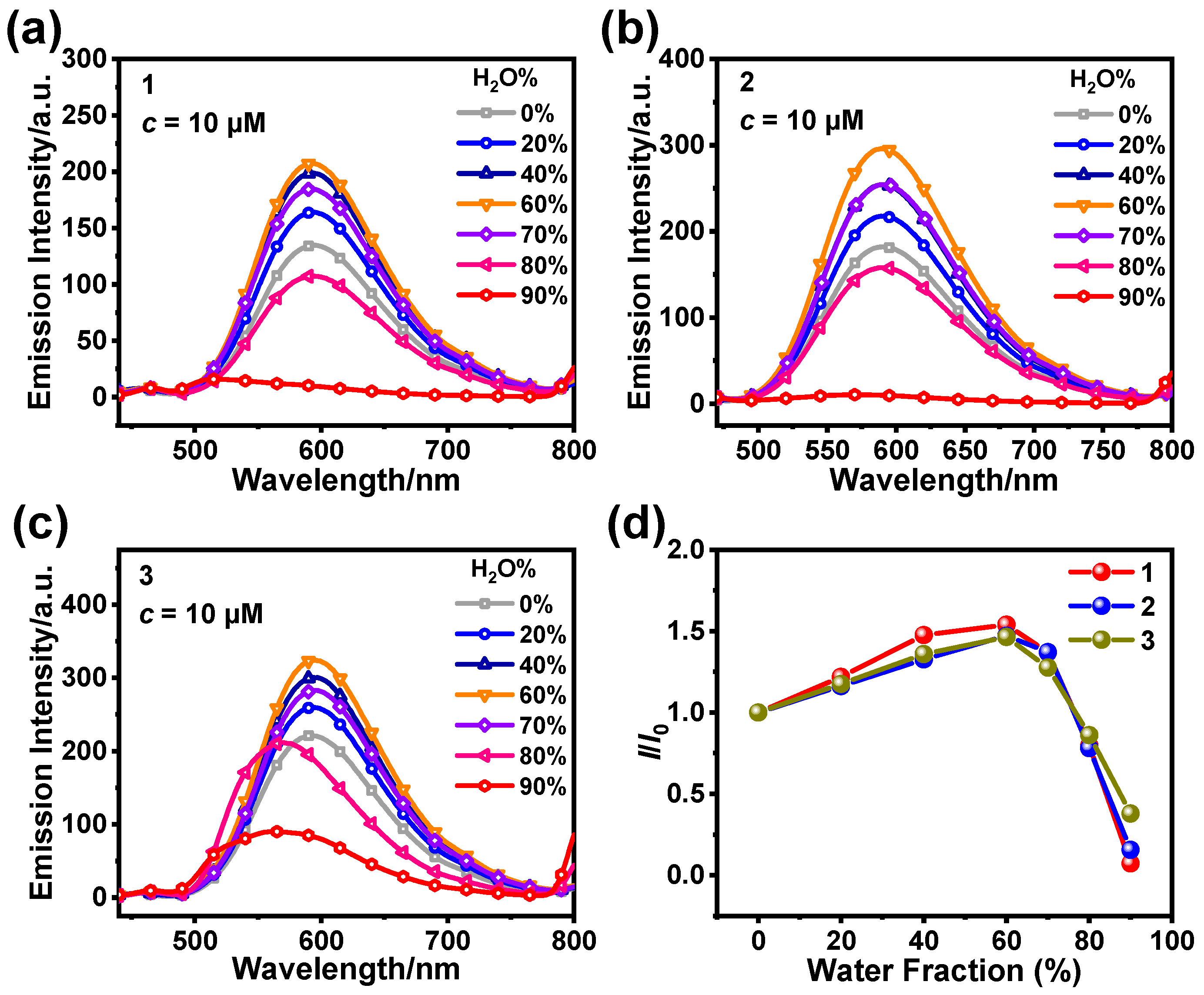
3.1. Photophysical and AIPE Properties
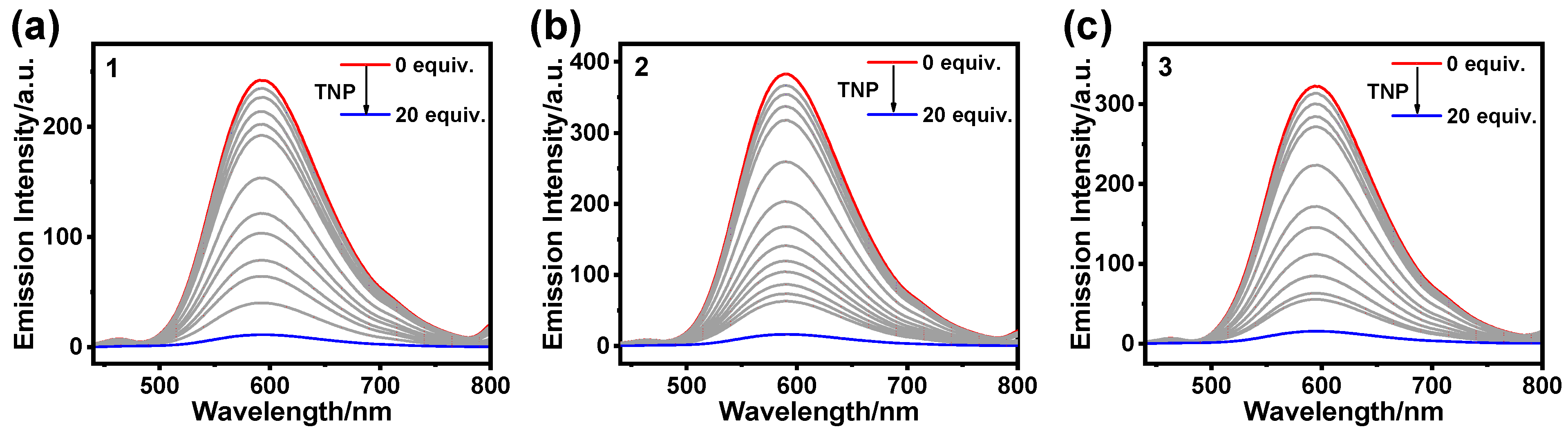
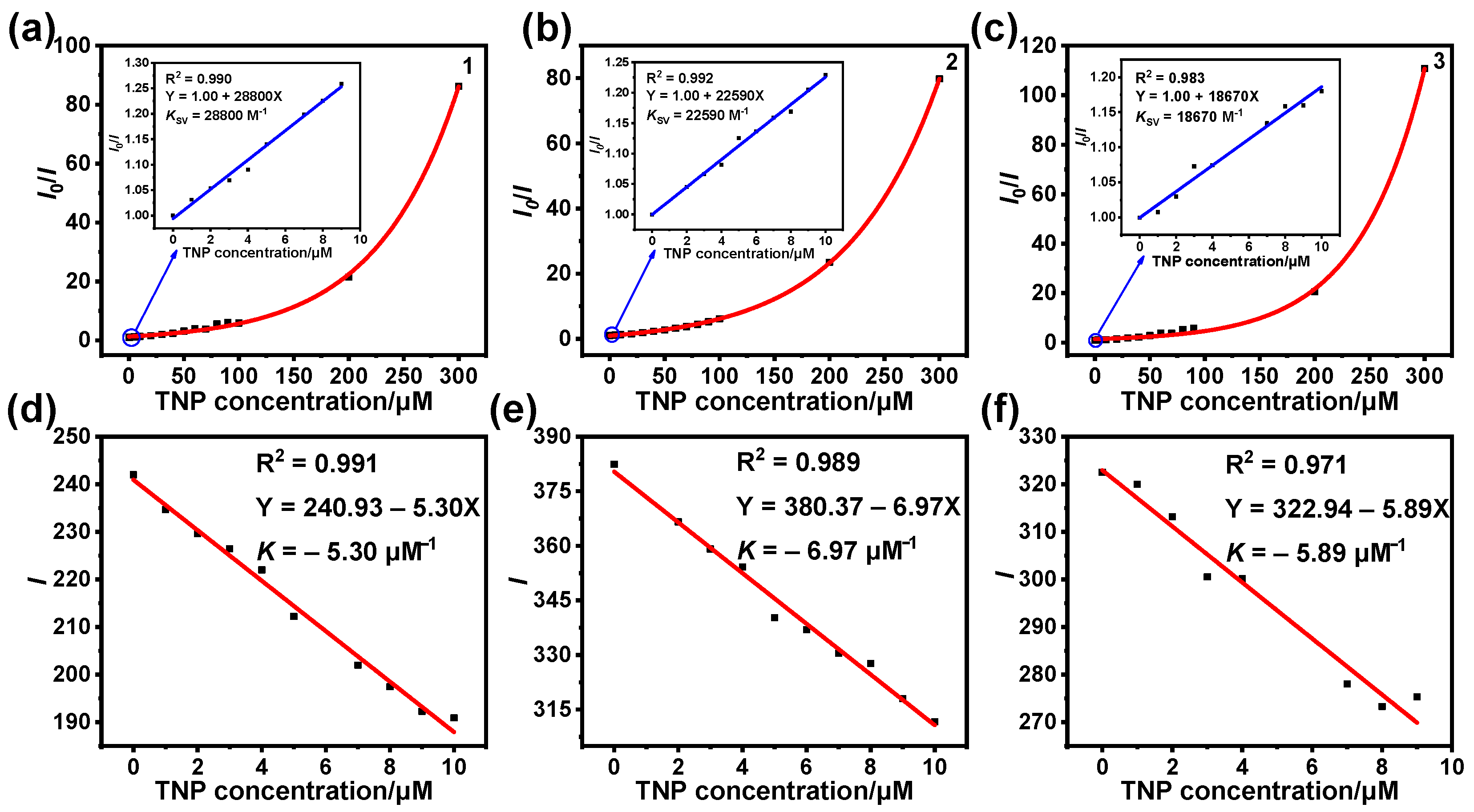
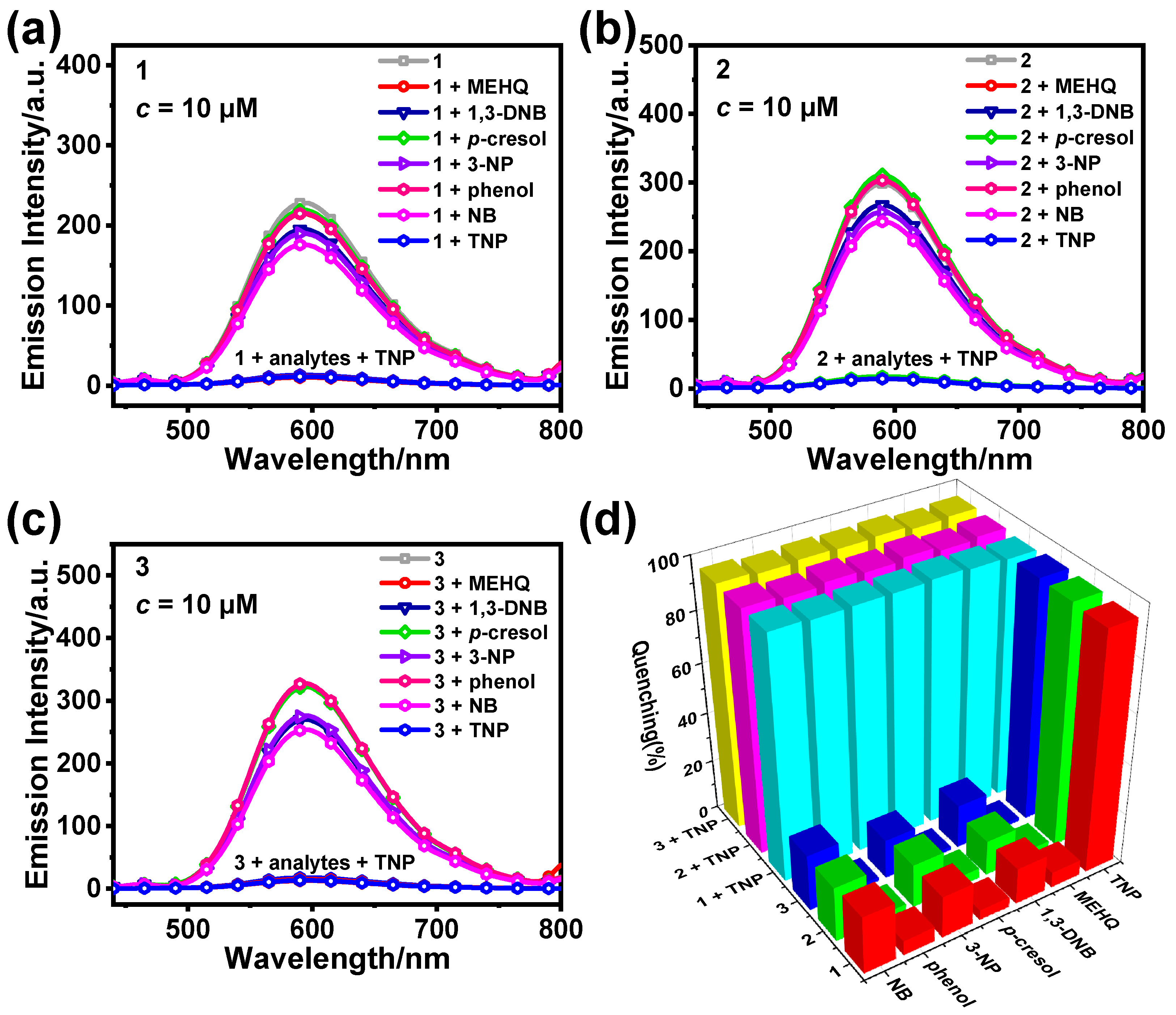
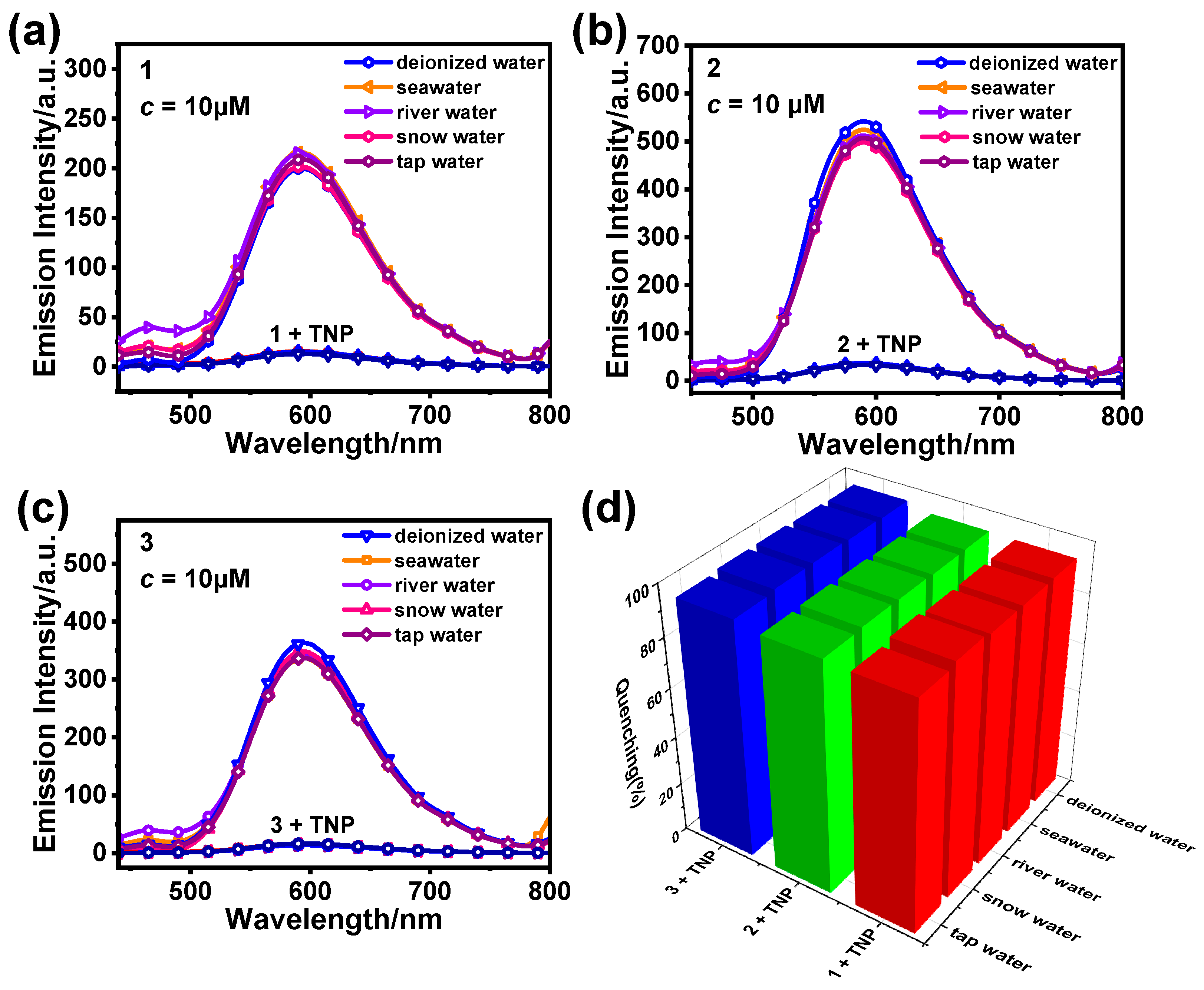
3.2. Detection of TNP
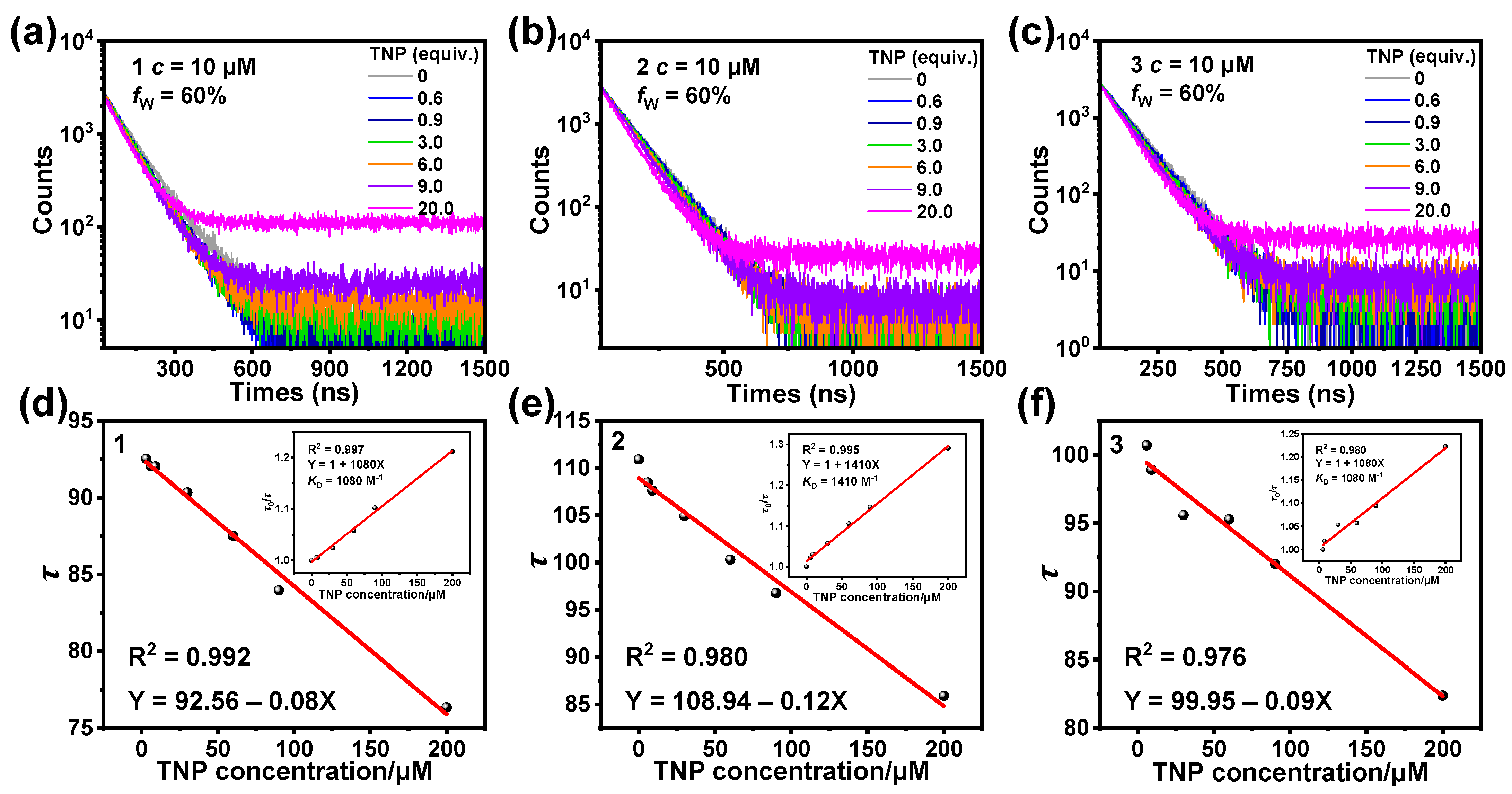
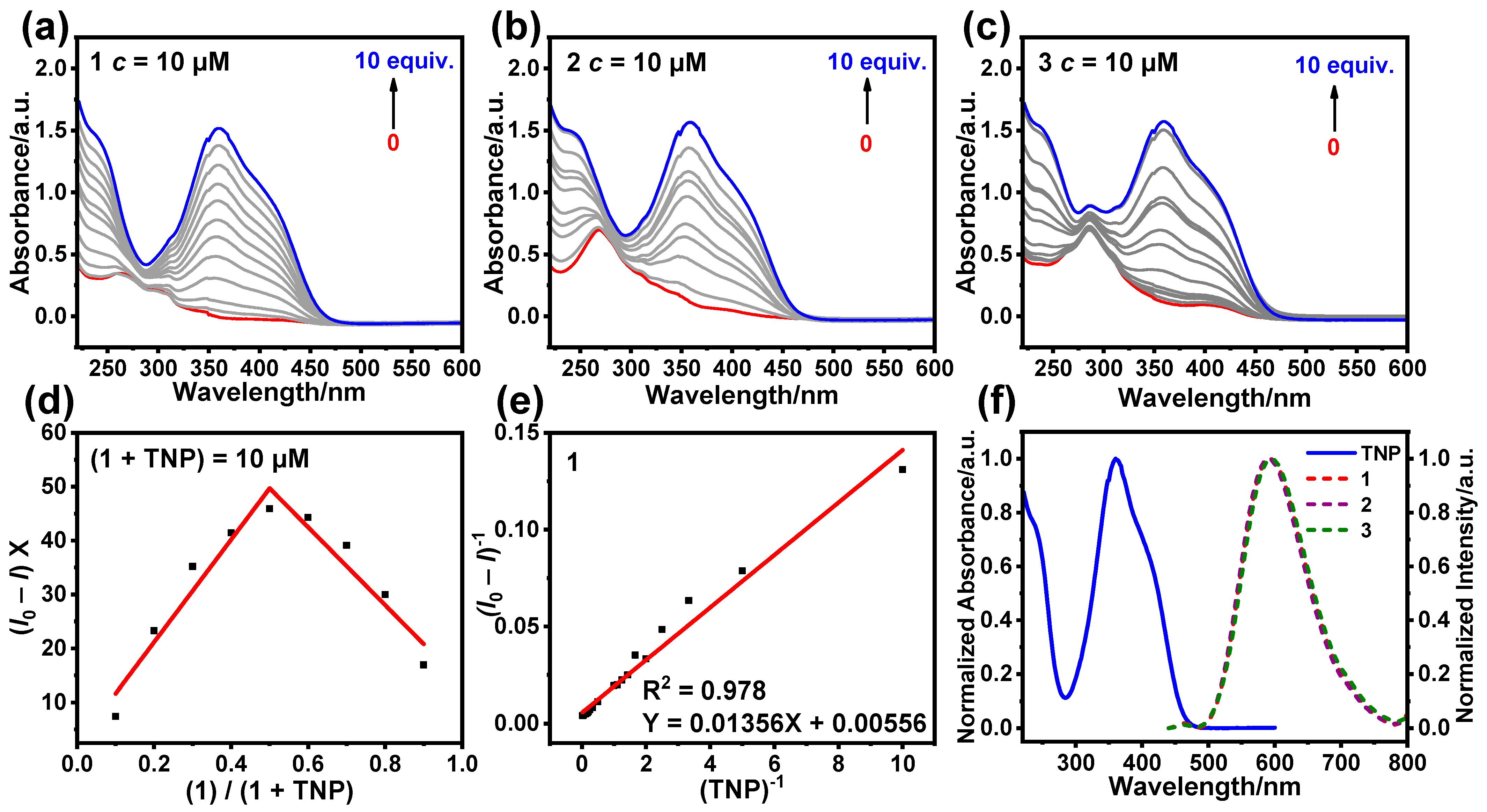
3.3. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Bhatta, R.P.; Kachwal, V.; Laskar, I.R. Controlling the sensitivity and selectivity for the detection of nitro-based explosives by modulating the electronic substituents on the ligand of AIPE-active cyclometalated iridium(iii) complexes. Dalton Trans. 2023, 52, 14182–14193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, L.-G.; Yuan, Y.-P.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 2012, 221–222, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, C.; Liu, Y.; Wu, Y.; Yuan, Y.; Zhou, Q. A highly sensitive and selective chemosensor for 2,4,6-trinitrophenol based on L-cysteine-coated cadmium sulfide quantum dots. Talanta 2019, 198, 242–248. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Peng, H.N.; Liu, T.H.; Yang, M.N.; Zhang, Y.; Fang, Y. A novel picric acid film sensor via combination of the surface enrichment effect of chitosan films and the aggregation-induced emission effect of siloles. J. Mater. Chem. 2009, 19, 7347–7353. [Google Scholar] [CrossRef]
- Burton, D.T.; Cooper, K.R.; Goodfellow, W.L.; Rosenblatt, D.H. Uptake, elimination, and metabolism of 14C-picric acid and 14C-picramic acid in the American oyster (Crassostrea virginica). Arch. Environ. Contam. Toxicol. 1984, 13, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Kayser, E.G.; Burlinson, N.E. Migration of explosives in soil: Analysis of rdx, tnt, and tetryl from a 14c lysimeter study. J. Energetic Mater. 1988, 6, 45–71. [Google Scholar] [CrossRef]
- Thorne, P.G.; Jenkins, T.F. A field method for quantifying ammonium picrate and picric acid in soil. Field Anal. Chem. Technol. 1997, 1, 165–170. [Google Scholar] [CrossRef]
- Wyman, J.F.; Serve, M.P.; Hobson, D.W.; Lee, L.H.; Uddin, D.E. Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer 344 rats. J. Toxicol. Environ. Health 1992, 37, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.S. Instrumentation for trace detection of high explosives. Rev. Sci. Instrum. 2004, 75, 2499–2512. [Google Scholar] [CrossRef]
- Sylvia, J.M.; Janni, J.A.; Klein, J.D.; Spencer, K.M. Surface-Enhanced Raman Detection of 2,4-Dinitrotoluene Impurity Vapor as a Marker To Locate Landmines. Anal. Chem. 2000, 72, 5834–5840. [Google Scholar] [CrossRef]
- Estevanes, J.; Monjardez, G. Detection of organic explosive residues from outdoor detonations using confocal Raman microscopy. Forensic Sci. Int. 2024, 365, 112292. [Google Scholar] [CrossRef] [PubMed]
- Hakonen, A.; Andersson, P.O.; Stenbæk Schmidt, M.; Rindzevicius, T.; Käll, M. Explosive and chemical threat detection by surface-enhanced Raman scattering: A review. Anal. Chim. Acta 2015, 893, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krausa, M.; Schorb, K. Trace detection of 2,4,6-trinitrotoluene in the gaseous phase by cyclic voltammetry. J. Electroanal. Chem. 1999, 461, 10–13. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Chen, W. Recent advances in electrochemical sensors for the detection of 2, 4, 6-trinitrotoluene. Curr. Opin. Electrochem. 2019, 17, 16–22. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Wu, X.; Hang, H.; Li, H.; Chen, Y.; Tong, H.; Wang, L. Water-dispersible hyperbranched conjugated polymer nanoparticles with sulfonate terminal groups for amplified fluorescence sensing of trace TNT in aqueous solution. Mater. Chem. Front. 2017, 1, 1875–1880. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Fluorescent Calix [4]arene-Carbazole-Containing Polymers as Sensors for Nitroaromatic Explosives. Chemosensors 2020, 8, 128. [Google Scholar] [CrossRef]
- Noh, D.; Oh, E. Estimation of Environmental Effects and Response Time in Gas-Phase Explosives Detection Using Photoluminescence Quenching Method. Polymers 2024, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Miao, C.; Li, D.; Zhang, Y.; Yu, J.; Xu, R. AIE luminogen functionalized mesoporous silica nanoparticles as efficient fluorescent sensor for explosives detection in water. Microporous Mesoporous Mater. 2014, 196, 46–50. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens. Actuators B Chem. 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, X.; Zhang, Q.; Kong, L.; Tian, Y.; Li, G.; Zhang, X.; Yang, J. AIE-active luminogen for highly sensitive and selective detection of picric acid in water samples: Pyridyl as an effective recognition group. Dye. Pigment. 2019, 163, 1–8. [Google Scholar] [CrossRef]
- Cheng, C.; Roberto, M.; Destro, G.; Stefania, R.; Carrera, C.; Terreno, E. A boron-nitrogen heterocyclic AIE probe for sensitive detection of picric acid. J. Photochem. Photobiol. A Chem. 2024, 450, 115462. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Manimaran, B.; Thanasekaran, P.; Rajendran, T.; Lin, R.J.; Chang, I.J.; Lee, G.H.; Peng, S.M.; Rajagopal, S.; Lu, K.L. Luminescence enhancement induced by aggregation of alkoxy-bridged rhenium(I) molecular rectangles. Inorg. Chem. 2002, 41, 5323–5325. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, Y.; Liu, C. Carbazolyl-Modified Neutral Ir(III) Complexes for Efficient Detection of Picric Acid in Aqueous Media. Sensors 2024, 24, 4074. [Google Scholar] [CrossRef]
- He, P.; Chen, Y.; Li, X.-N.; Yan, Y.-Y.; Liu, C. Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water. Chemosensors 2023, 11, 177. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, X.; MacMillan, D.W.C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 2018, 559, 83–88. [Google Scholar] [CrossRef]
- Wang, W.; Yung, T.-L.; Cheng, S.-S.; Chen, F.; Liu, J.-B.; Leung, C.-H.; Ma, D.-L. A long-lived luminogenic iridium(III) complex for acetylacetone detection in environmental samples. Sens. Actuators B Chem. 2020, 321, 128486. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, M.; Liu, J.; Wu, H.; Chao, H. Cyclometalated iridium(iii) tetrazine complexes for mitochondria-targeted two-photon photodynamic therapy. Dalton Trans. 2024, 53, 12917–12926. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Chen, Y.; Li, X.-N.; Yan, Y.-Y.; Liu, C. AIPE-active cationic Ir(iii) complexes for efficient detection of 2,4,6-trinitrophenol and oxygen. Dalton Trans. 2023, 52, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Xing, Y.; Yang, Z.; Qiao, C.; Xia, Z. Photostable aggregation-induced emission of iridium(III) complex realizing robust and high-resolution imaging of latent fingerprints. Sens. Actuators B Chem. 2023, 375, 132898. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wang, X.; Wang, L.; Zhang, J. The influence of different cyclometalated ligand substituents and ancillary ligand on the phosphorescent properties of iridium(III) complexes. Mater. Chem. Phys. 2016, 177, 179–189. [Google Scholar] [CrossRef]
- Jang, J.-H.; Park, H.J.; Park, J.Y.; Kim, H.U.; Hwang, D.-H. Orange phosphorescent Ir(III) complexes consisting of substituted 2-phenylbenzothiazole for solution-processed organic light-emitting diodes. Org. Electron. 2018, 60, 31–37. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Wang, X.; Qi, H. Electrogenerated Chemiluminescence from Heteroleptic Iridium(III) Complexes with Multicolor Emission. Inorg. Chem. 2015, 54, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhou, K.; Li, M.; Sun, H.; Chen, Y.; Su, S.; Pu, X.; Huang, Y.; Lu, Z. Iridium(iii) complexes with enhanced film amorphism as guests for efficient orange solution-processed single-layer PhOLEDs with low efficiency roll-off. Dalton Trans. 2013, 42, 10559–10571. [Google Scholar] [CrossRef] [PubMed]
- Rabha, M.; Lyngkhoi, D.L.; Patra, S.K.; Khatua, S. AIE-active cyclometalated iridium(iii) complexes for highly efficient picric acid detection in aqueous media. Inorg. Chem. Front. 2024, 11, 1434–1449. [Google Scholar] [CrossRef]
- Iqbal, A.; Tian, Y.; Wang, X.; Gong, D.; Guo, Y.; Iqbal, K.; Wang, Z.; Liu, W.; Qin, W. Carbon dots prepared by solid state method via citric acid and 1,10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sens. Actuators B Chem. 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Das, D.; Maity, A.; Shyamal, M.; Maity, S.; Mudi, N.; Misra, A. Aggregation induced emission of 9-Anthraldehyde microstructures and its selective sensing behavior towards picric acid. J. Mol. Liq. 2018, 261, 446–455. [Google Scholar] [CrossRef]
- Shi, Z.; Li, W.; Pi, H.; Liu, H.; Chen, H.; Li, P.; Jiang, X. Trace amounts of mercaptans with key roles in forming an efficient three-component photoinitiation system for holography. Mater. Today Chem. 2022, 26, 100999. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, L.; Che, G. Diphenyl imidazole-based supramolecular self-assembly system for an efficient fluorometric detection of picric acid. Dye. Pigment. 2022, 203, 110378. [Google Scholar] [CrossRef]
- Karuk Elmas, S.N.; Karagoz, A.; Arslan, F.N.; Yilmaz, I. Propylimidazole Functionalized Coumarin Derivative as Dual Responsive Fluorescent Chemoprobe for Picric Acid and Fe3+ Recognition: DFT and Natural Spring Water Applications. J. Fluoresc. 2022, 32, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- P, K.; Cherian, A.R.; Sirimahachai, U.; Thadathil, D.A.; Varghese, A.; Hegde, G. Detection of picric acid in industrial effluents using multifunctional green fluorescent B/N-carbon quantum dots. J. Environ. Chem. Eng. 2022, 10, 107209. [Google Scholar] [CrossRef]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Du, J.; Cai, R.; Liu, C. The Effect of the Position of a Phenyl Group on the Luminescent and TNP-Sensing Properties of Cationic Iridium(III) Complexes. Sensors 2025, 25, 839. https://doi.org/10.3390/s25030839
Yang X, Du J, Cai R, Liu C. The Effect of the Position of a Phenyl Group on the Luminescent and TNP-Sensing Properties of Cationic Iridium(III) Complexes. Sensors. 2025; 25(3):839. https://doi.org/10.3390/s25030839
Chicago/Turabian StyleYang, Xiaoran, Jiahao Du, Rui Cai, and Chun Liu. 2025. "The Effect of the Position of a Phenyl Group on the Luminescent and TNP-Sensing Properties of Cationic Iridium(III) Complexes" Sensors 25, no. 3: 839. https://doi.org/10.3390/s25030839
APA StyleYang, X., Du, J., Cai, R., & Liu, C. (2025). The Effect of the Position of a Phenyl Group on the Luminescent and TNP-Sensing Properties of Cationic Iridium(III) Complexes. Sensors, 25(3), 839. https://doi.org/10.3390/s25030839





