Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum–Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical Reagents
2.2. Pretreatment of PtZn NFs/CC Electrode
2.3. Characterization of Catalysts
2.4. Electrochemical Measurements
3. Results and Discussion
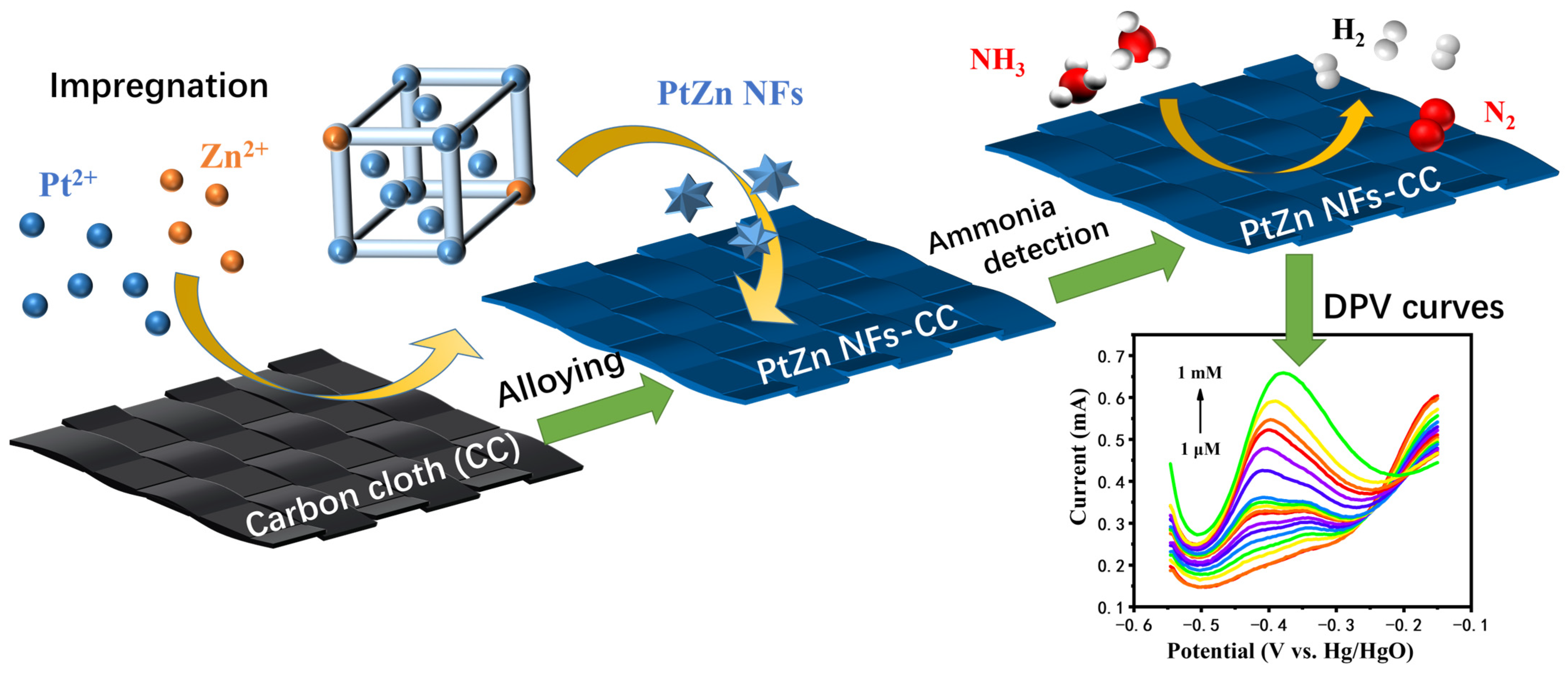
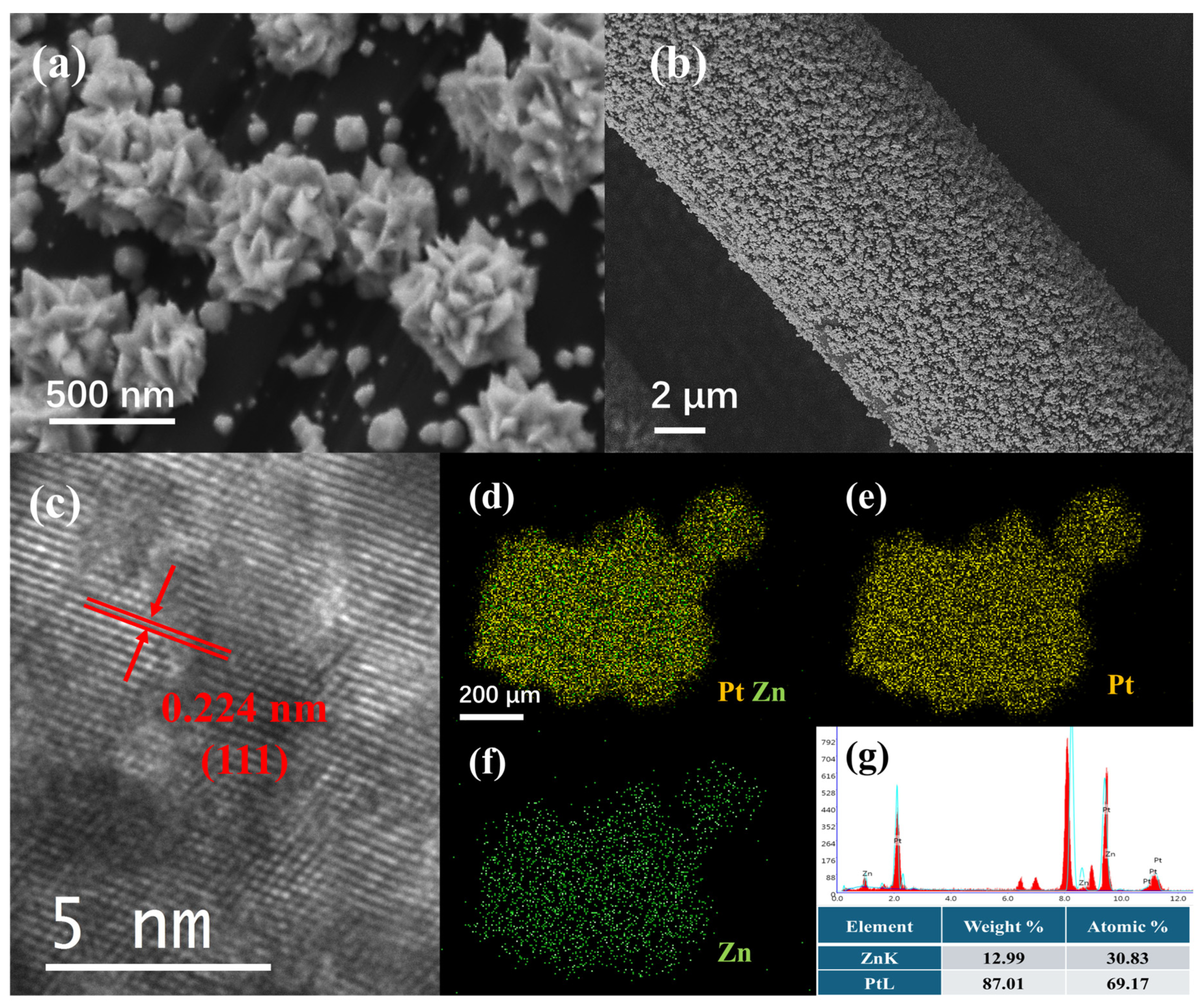
3.1. Material Characterization
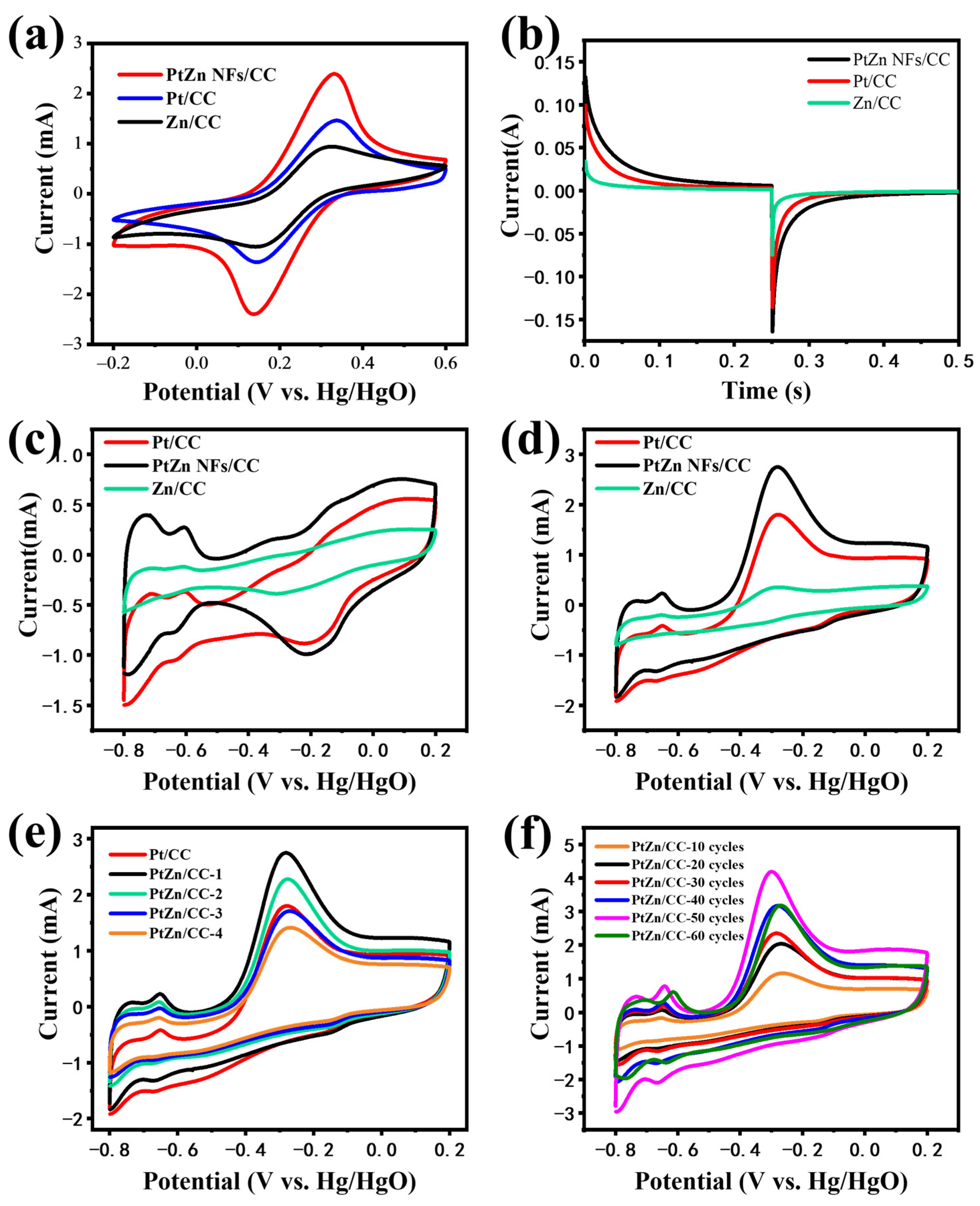
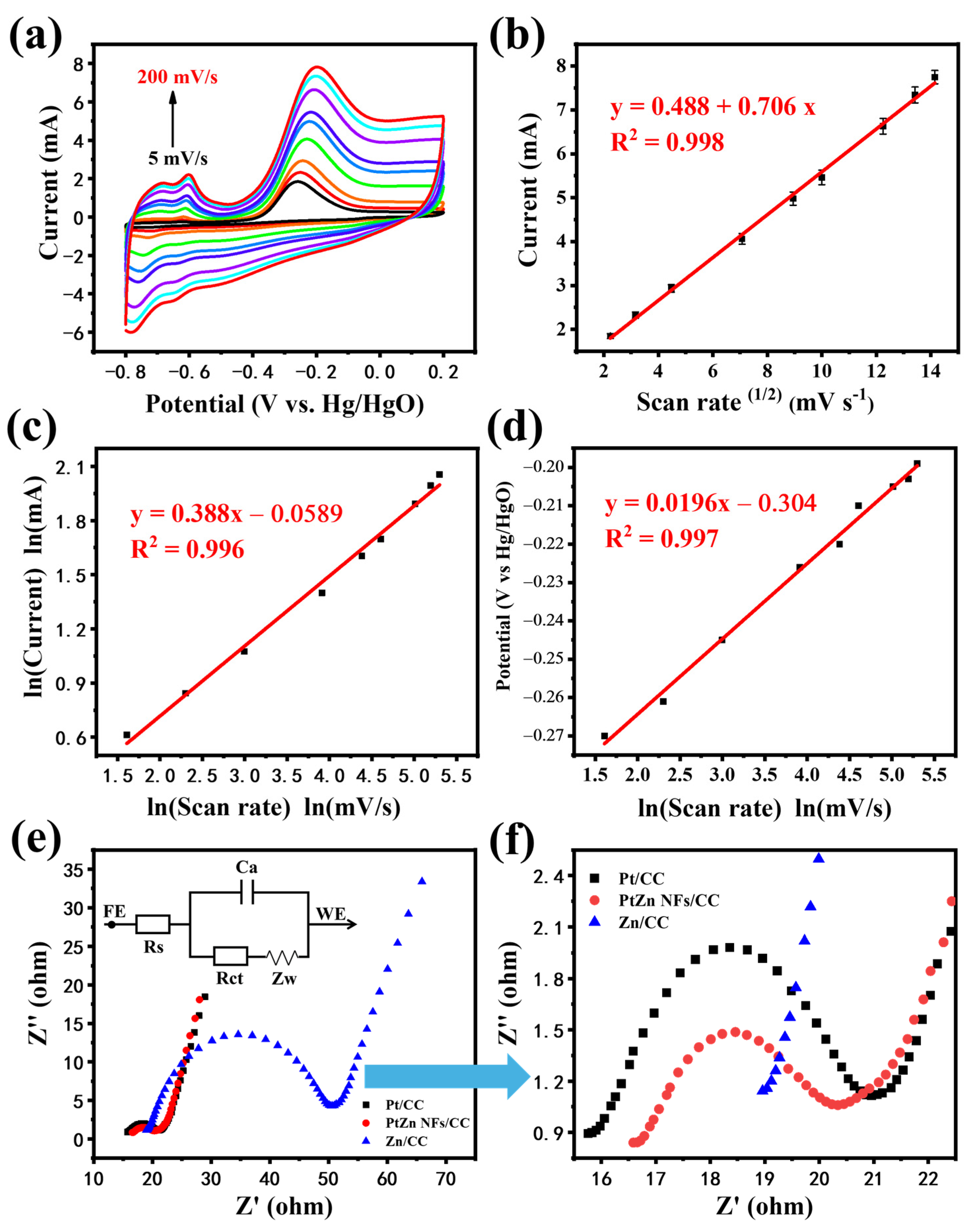
3.2. Electrochemical Characteristics of PtZn NFs/CC
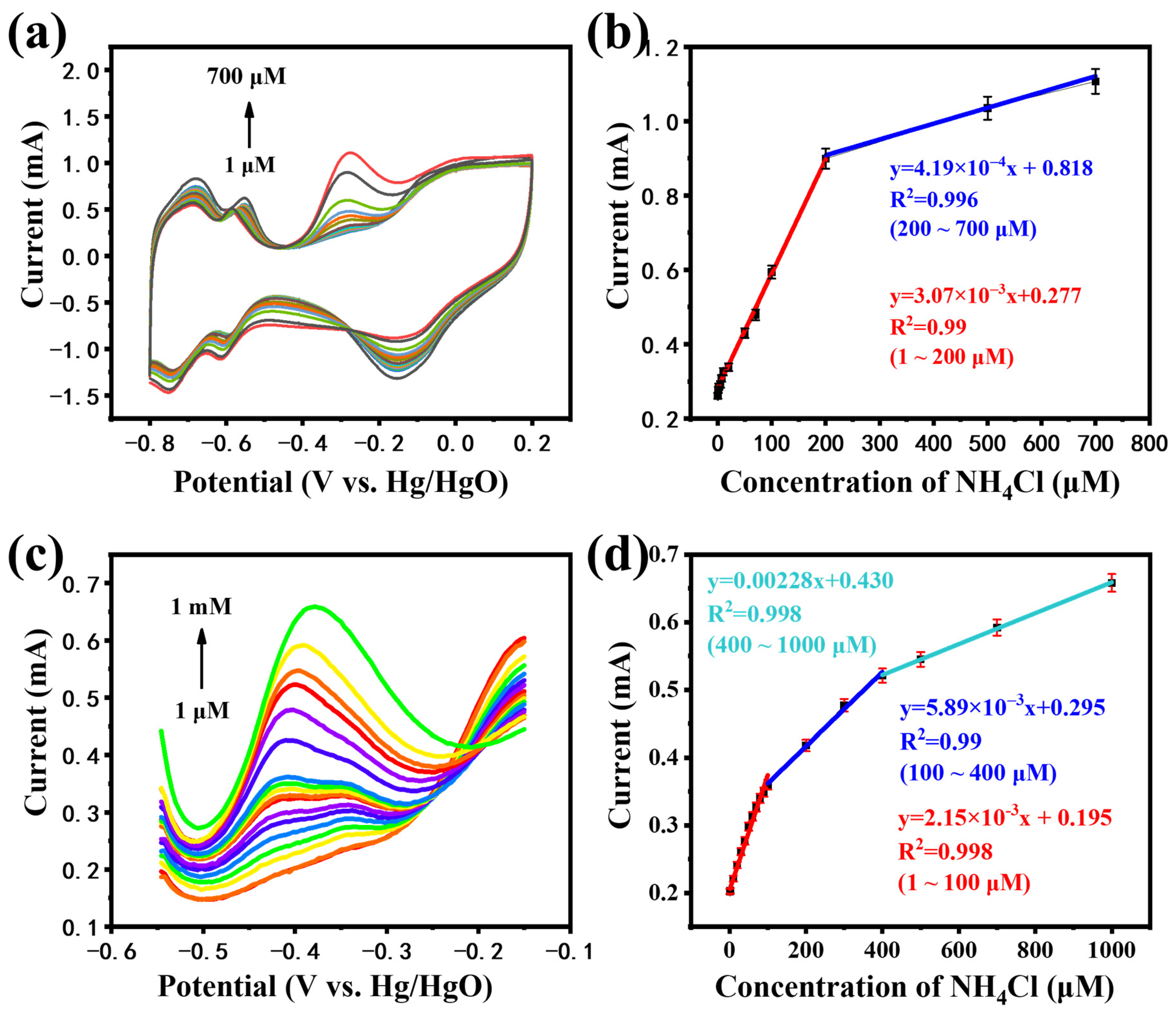
3.3. Determination of Ammonia
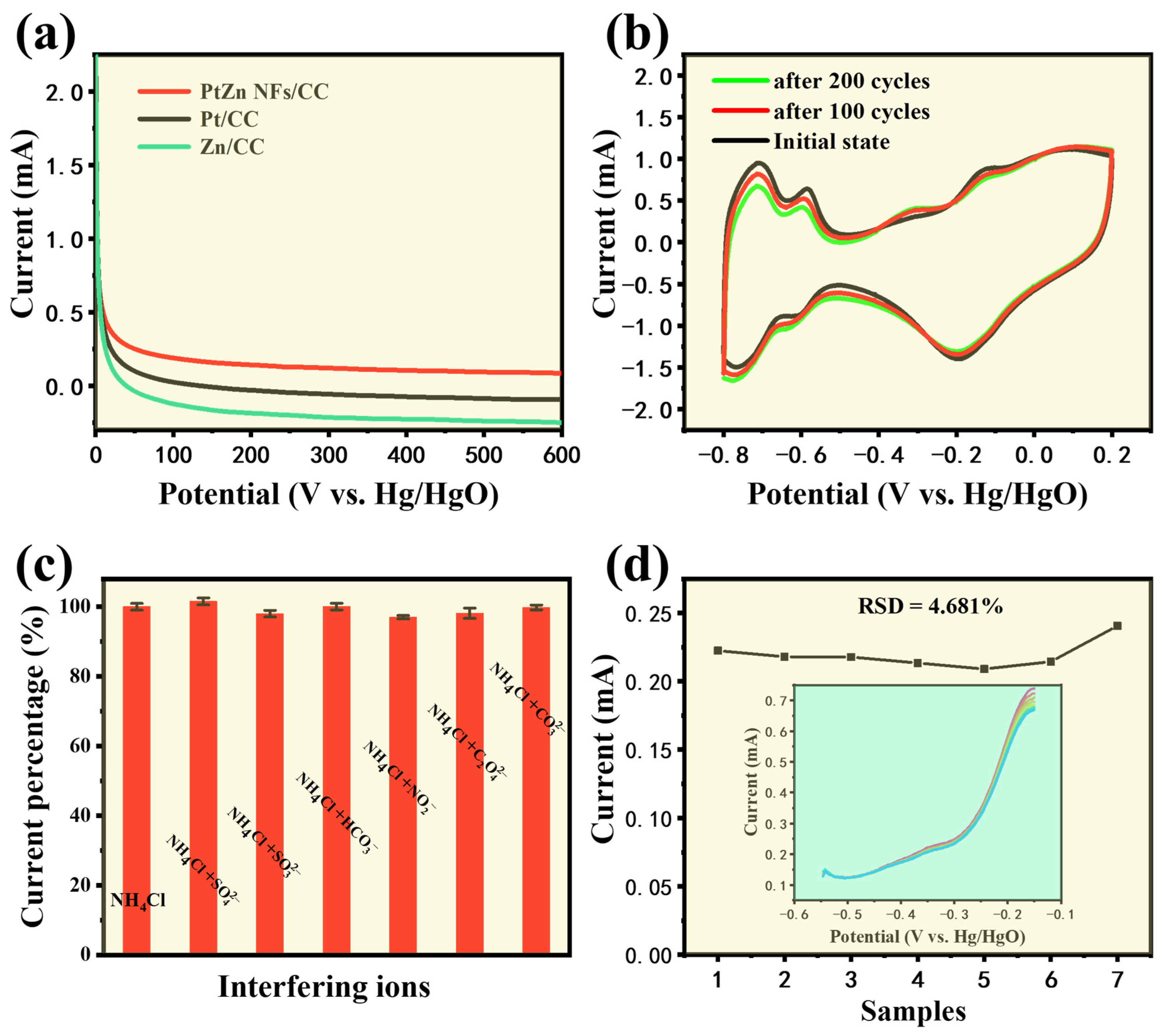
3.4. Comprehensive Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A Guide for Selecting the Most Appropriate Method for Ammonium Determination in Water Analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Zuffo, T.I.; Durigon, E.G.; Morselli, M.B.; Picoli, F.; Folmann, S.; Kinas, J.F.; Savaris, T.; Zampar, A.; Lopes, D.L.D.A. Lethal Temperature and Toxicity of Ammonia in Juveniles of Curimbatá (Prochilodus lineatus). Aquaculture 2021, 545, 737138. [Google Scholar] [CrossRef]
- Boardman, G.D.; Starbuck, S.M.; Hudgins, D.B.; Li, X.; Kuhn, D.D. Toxicity of Ammonia to Three Marine Fish and Three Marine Invertebrates. Environ. Toxicol. 2004, 19, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection Methods of Ammonia Nitrogen in Water: A Review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef]
- Phansi, P.; Sumantakul, S.; Wongpakdee, T.; Fukana, N.; Ratanawimarnwong, N.; Sitanurak, J.; Nacapricha, D. Membraneless Gas-Separation Microfluidic Paper-Based Analytical Devices for Direct Quantitation of Volatile and Nonvolatile Compounds. Anal. Chem. 2016, 88, 8749–8756. [Google Scholar] [CrossRef]
- Le, P.T.T.; Boyd, C.E. Comparison of Phenate and Salicylate Methods for Determination of Total Ammonia Nitrogen in Freshwater and Saline Water. J. World Aquac. Soc. 2012, 43, 885–889. [Google Scholar] [CrossRef]
- Goldshleger, N.; Grinberg, A.; Harpaz, S.; Shulzinger, A.; Abramovich, A. Real-Time Advanced Spectroscopic Monitoring of Ammonia Concentration in Water. Aquac. Eng. 2018, 83, 103–108. [Google Scholar] [CrossRef]
- Yang, S.; Zang, G.; Peng, Q.; Fan, J.; Liu, Y.; Zhang, G.; Zhao, Y.; Li, H.; Zhang, Y. In-Situ Growth of 3D Rosette-like Copper Nanoparticles on Carbon Cloth for Enhanced Sensing of Ammonia Based on Copper Electrodissolution. Anal. Chim. Acta 2020, 1104, 60–68. [Google Scholar] [CrossRef]
- Valentini, F.; Biagiotti, V.; Lete, C.; Palleschi, G.; Wang, J. The Electrochemical Detection of Ammonia in Drinking Water Based on Multi-Walled Carbon Nanotube/Copper Nanoparticle Composite Paste Electrodes. Sens. Actuators B Chem. 2007, 128, 326–333. [Google Scholar] [CrossRef]
- Baciu, A.; Manea, F.; Pop, A.; Pode, R.; Schoonman, J. Simultaneous Voltammetric Detection of Ammonium and Nitrite from Groundwater at Silver-Electrodecorated Carbon Nanotube Electrode. Process Saf. Environ. Prot. 2017, 108, 18–25. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, Y.; Chang, Q.; Xie, Y.; Jiang, G. Highly Sensitive Determination of Ammonia Nitrogen by Nanocubic Copper Preparedby Direct Electrochemical Deposition. Electrochemistry 2024, 92, 017002. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.; Kim, H.; Jun, Y.; Kim, S.Y.; Ahn, S.H. Recent Progress in Pt-Based Electrocatalysts for Ammonia Oxidation Reaction. Appl. Mater. Today 2022, 29, 101640. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Ren, R.; Zhang, J.; He, D.; Zhao, C.; Suo, H. In Situ Synthesis of Hierarchical Platinum Nanosheets-Polyaniline Array on Carbon Cloth for Electrochemical Detection of Ammonia. J. Hazard. Mater. 2020, 392, 122342. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Peng, X.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabrication of a Ni Foam-Supported Platinum Nanoparticles-Silver/Polypyrrole Electrode for Aqueous Ammonia Sensing. Synth. Met. 2020, 259, 116257. [Google Scholar] [CrossRef]
- Chan, Y.T.; Siddharth, K.; Shao, M. Investigation of Cubic Pt Alloys for Ammonia Oxidation Reaction. Nano Res. 2020, 13, 1920–1927. [Google Scholar] [CrossRef]
- Xi, X.; Fan, Y.; Zhang, K.; Liu, Y.; Nie, F.; Guan, H.; Wu, J. Carbon-Free Sustainable Energy Technology: Electrocatalytic Ammonia Oxidation Reaction. Chem. Eng. J. 2022, 435, 134818. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Yang, H.; Luo, Y.; Qun Tian, Z.; Kang Shen, P. Surface-Structure Tailoring of Dendritic PtCo Nanowires for Efficient Oxygen Reduction Reaction. J. Colloid. Interface Sci. 2023, 652, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Cheng, Y. Fabrication by Electrolytic Deposition of Pt–Ni Electrocatalyst for Oxidation of Ammonia in Alkaline Solution. Int. J. Hydrogen Energy 2008, 33, 6681–6686. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Wang, Z.; Zhu, X.; Zhu, J.; Chen, P.; Lyu, T.; Li, C.; Qun Tian, Z.; Kang Shen, P. Hyperbranched Concave Octahedron of PtIrCu Nanocrystals with High-Index Facets for Efficiently Electrochemical Ammonia Oxidation Reaction. J. Colloid. Interface Sci. 2021, 601, 1–11. [Google Scholar] [CrossRef]
- Endo, K.; Katayama, Y.; Miura, T. Pt–Ir and Pt–Cu Binary Alloys as the Electrocatalyst for Ammonia Oxidation. Electrochim. Acta 2004, 49, 1635–1638. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Wu, Y.; An, Y.; Xu, L.; Wan, C. Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride. Catalysts 2019, 9, 1009. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Wang, C.; Huang, W.; Liu, X. Dehydrogenation of Hydrous Hydrazine over Carbon Nanosphere- Supported PtNi Nanoparticles for on-Demand H2 Release. Fuel 2023, 332, 126116. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, X.; Han, Y.; Shen, L.; Yin, S.; Li, G.; Jiang, Y.; Sun, S. Engineering PtRu Bimetallic Nanoparticles with Adjustable Alloying Degree for Methanol Electrooxidation: Enhanced Catalytic Performance. Appl. Catal. B Environ. 2020, 263, 118345. [Google Scholar] [CrossRef]
- Jiang, J. Promotion of PtIr and Pt Catalytic Activity towards Ammonia Electrooxidation through the Modification of Zn. Electrochem. Commun. 2017, 75, 52–55. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.; Sun, B.; He, D.; Zhao, C.; Suo, H. Enhanced Ammonia Sensitivity Electrochemical Sensors Based on PtCu Alloy Nanoparticles In-Situ Synthesized on Carbon Cloth Electrode. J. Electroanal. Chem. 2022, 922, 116721. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, L.; Yan, Y.; Zhang, J.; Li, Y.; Li, W.; Yan, W.; Han, Y.; Zheng, J.; Li, G.; et al. Adjusting the Near-Surface Composition of Pt–Zn Intermetallic Nanoparticles to Enhance Methanol Oxidation Activity and Stability. J. Phys. Chem. C 2023, 127, 5366–5375. [Google Scholar] [CrossRef]
- Yan, W.; Cao, S.; Liu, H.; Xing, Q.; Ren, J.; Li, Z.; Li, X.; Lu, X.; Chen, Y. Facile Solid-Phase Method for Preparing a Highly Active and Stable PtZn-Based Oxygen Reduction/Hydrogen Evolution Bifunctional Electrocatalyst: Effect of Bi-Facet Lattice Strain on Catalytic Activity. ACS Appl. Energy Mater. 2022, 5, 13791–13801. [Google Scholar] [CrossRef]
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-Performance Ammonia Oxidation Catalysts for Anion-Exchange Membrane Direct Ammonia Fuel Cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Bai, J.; Wang, C.; Liu, K.; Wang, H.; Liu, Y.; Liu, F.; Suo, H.; Liang, X.; Zhang, C.; Liu, F.; et al. Enhanced Gas Sensing Performance Based on the PtCu Octahedral Alloy Nanocrystals Decorated SnO2 Nanoclusters. Sens. Actuators B Chem. 2021, 330, 129375. [Google Scholar] [CrossRef]
- Wang, G.; Ma, G.; Gao, J.; He, D.; Zhao, C.; Suo, H. Enhanced Sensitivity of Electrochemical Sensors for Ammonia-Nitrogen via In-Situ Synthesis PtNi Nanoleaves on Carbon Cloth. Sensors 2024, 24, 387. [Google Scholar] [CrossRef]
- Zhang, B.; Li, G.; Liu, S.; Qin, Y.; Song, L.; Wang, L.; Zhang, X.; Liu, G. Boosting Propane Dehydrogenation over PtZn Encapsulated in an Epitaxial High-Crystallized Zeolite with a Low Surface Barrier. ACS Catal. 2022, 12, 1310–1314. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Montiel, V.; Feliu, J.M.; Aldaz, A. Ammonia Selective Oxidation on Pt(100) Sites in an Alkaline Medium. J. Phys. Chem. B 2005, 109, 12914–12919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Gao, J.; Zhang, L.; Zhao, C.; Suo, H. In Situ Fabrication of Copper Oxide Nanoparticles Decorated Carbon Cloth for Efficient Electrocatalytic Detection of Nitrite. Microchem. J. 2023, 194, 109302. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, W.B.; Cheng, Y.F. Recent Advances in Electrocatalysts for Electro-Oxidation of Ammonia. J. Mater. Chem. A 2013, 1, 3216–3238. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Li, J.; Li, Q.; Liu, S.; Lei, Z. A Highly Efficient Pt-NiO/C Electrocatalyst for Ammonia Electro-Oxidation. J. Electrochem. Soc. 2017, 164, F958–F965. [Google Scholar] [CrossRef]
- Novell-Leruth, G.; Valcárcel, A.; Clotet, A.; Ricart, J.M.; Pérez-Ramírez, J. DFT Characterization of Adsorbed NHxSpecies on Pt(100) and Pt(111) Surfaces. J. Phys. Chem. B 2005, 109, 18061–18069. [Google Scholar] [CrossRef]
- Novell-Leruth, G.; Valcárcel, A.; Pérez-Ramírez, J.; Ricart, J.M. Ammonia Dehydrogenation over Platinum-Group Metal Surfaces. Structure, Stability, and Reactivity of Adsorbed NHxSpecies. J. Phys. Chem. C 2007, 111, 860–868. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Xu, G.-R.; Li, F.-M.; Hong, Q.-L.; Jin, P.-J.; Chen, P.; Chen, Y. Hydrogen Generation from Ammonia Electrolysis on Bifunctional Platinum Nanocubes Electrocatalysts. J. Energy Chem. 2020, 47, 234–240. [Google Scholar] [CrossRef]
- Khan, S.I.; Motghare, R.V. Electrochemical Determination of Chlorophenaramine Based on RTIL/CNT Composite Modified Glassy Carbon Electrode in Pharmaceutical Samples. J. Electrochem. Soc. 2019, 166, B1202–B1208. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Xu, H.; Nie, P. Gold Nanoparticle-Modified Electrodes for Electrochemical Sensing of Ammonia Nitrogen in Water. ACS Appl. Nano Mater. 2024, 7, 577–593. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, F.; Miao, Y.; Zhou, C.; Xu, N.; Shi, R.; Wu, L.; Tang, J.; Zhang, T. Revealing Ammonia Quantification Minefield in Photo/Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 21728–21731. [Google Scholar] [CrossRef]
- Karakuş, S.; Baytemir, G.; Özeroğlu, C.; Taşaltın, N. An Ultra-Sensitive Smartphone-Integrated Digital Colorimetric and Electrochemical Camellia Sinensis Polyphenols Encapsulated CuO Nanoparticles-Based Ammonia Biosensor. Inorg. Chem. Commun. 2022, 143, 109733. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, L.; Liu, J.; Gu, J.; Suo, H.; Zhao, C. One Step and in Situ Synthesis of Ni Foam-Supported Pt-Ni(OH)2 Nanosheets as Electrochemical Sensor for Ammonia–Nitrogen Detection. Mater. Lett. 2022, 318, 132197. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Zhang, S.; Long, M.; Tang, A. Cuprous Oxide and Ag Modified Titanium Dioxide Electrode for Ultra-Sensitive Detection of Ammonia Nitrogen. J. Electroanal. Chem. 2023, 949, 117842. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Cong, A.; Tong, J.; Bian, C. Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water. Micromachines 2023, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Lahari, S.A.; Amreen, K.; Dubey, S.K.; Ponnalagu, R.N.; Goel, S. Modified Ultra Micro-Carbon Electrode for Efficient Ammonia Sensing for Water Quality Assessment. IEEE Trans. Nanobiosci. 2023, 22, 301–307. [Google Scholar] [CrossRef]
- Ahmed, M.; Zhao, R.; Xing, T.; Du, J. Constructing Netlike Nanosheets of ZnO/BiOCl with Heterojunction as Robust Material for Electrochemical Amine Detection. Chem. A Eur. J. 2023, 29, e202202658. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Wei, L.; Cai, H.; Gu, J.; Wang, X. Anchoring Platinum Nanofibers on Nickel Hydroxide Nanosheets for Electrochemical Detection of Ammonia-Nitrogen in the Aqueous Environment. Mater. Lett. 2023, 336, 133868. [Google Scholar] [CrossRef]
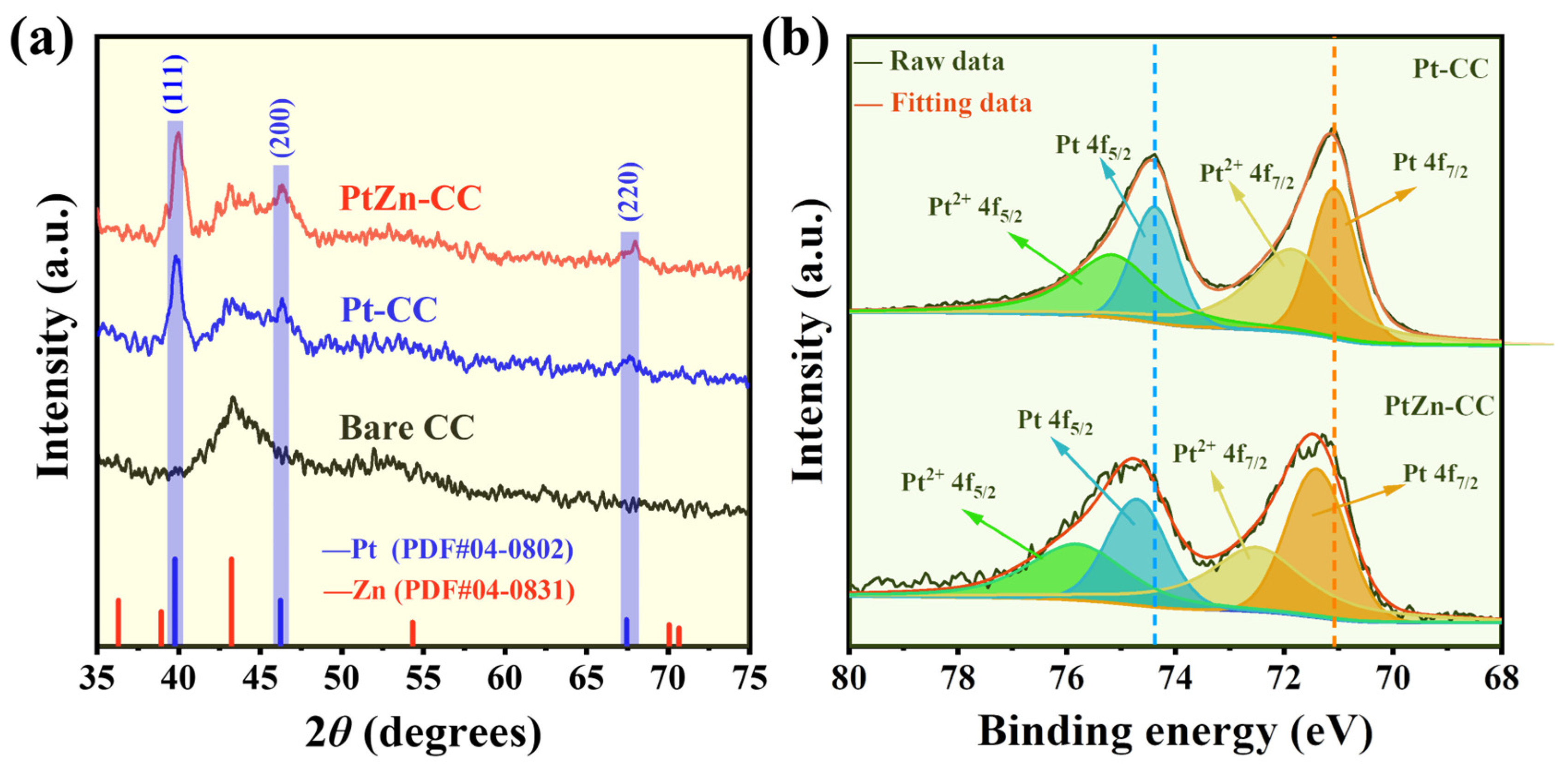
| Species | Pt 4f 7/2 (eV) | Pt 4f 5/2 (eV) | Relative Concentrations (%) |
|---|---|---|---|
| Pt/CC | |||
| Pt | 71.08 | 74.38 | 42.36% |
| Pt2+ | 71.86 | 75.16 | 57.64% |
| PtZn NFs/CC | |||
| Pt | 71.41 | 74.71 | 51.48% |
| Pt2+ | 72.55 | 75.85 | 48.52% |
| Electrode Materials | Linear Range (μM) | Sensitivity (μA μM−1) | LOD | Method | Ref. |
|---|---|---|---|---|---|
| PPS-CuO NPs | 12.5–100 | 8.48 | 40.6 nM | CV | [45] |
| Cu NPs/CC | 5–9425 | 0.0062 | 1.25 μM | i-t | [10] |
| Pt-Ni(OH)2 | 0.05–600 | 0.191 | 39.2 nM | DPV | [46] |
| Ag/Cu2O/TNTs | 0.1–101 | 4.940 | 74 nM | i-t | [47] |
| MEMS | 0.15 mg/L~2.0 mg/L | 0.4181 | / | DPV | [48] |
| UME/MWCNT | 10–60 | 0.049 | 8.69μM | i-t | [49] |
| ZnO/BiOCl | 200–1000 | 11.8 | 0.25 μM | CV | [50] |
| Pt-Ni(OH)2-NF | 5–500 | 12.27 | 2.74 μM | DPV | [51] |
| Pt-Ag/PPy | 0.05–50 | 8.9 | 37 nM | CV/LSV | [16] |
| Au NPs/CC | 0.001–10,000 | 513.577 | 1.0310 nM | CV | [43] |
| Pt7Cu1 | 0.5–500 | 9.4 | 8.6 nM | DPV | [31] |
| PtNi/CC | 150–500 0.5–150 | 0.945 7.83 | 24 nM | DPV | [32] |
| PtZn NFs/CC | 400–1000 100–400 1–100 | 2.28 5.89 21.5 | 27.81 nM | DPV | This work |
| Sample | Initial (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| Lake water | 17.61 | 10 | 28.03 | 104.22 | 3.27 |
| 30 | 49.21 | 105.33 | 4.38 | ||
| Tap water | 0 | 10 | 9.97 | 99.70 | 1.05 |
| 30 | 31.07 | 103.57 | 1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Zhou, G.; Zhang, Q.; He, D.; Zhao, C.; Suo, H. Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum–Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode. Sensors 2024, 24, 915. https://doi.org/10.3390/s24030915
Wang G, Zhou G, Zhang Q, He D, Zhao C, Suo H. Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum–Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode. Sensors. 2024; 24(3):915. https://doi.org/10.3390/s24030915
Chicago/Turabian StyleWang, Guanda, Guangfeng Zhou, Qingze Zhang, Dong He, Chun Zhao, and Hui Suo. 2024. "Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum–Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode" Sensors 24, no. 3: 915. https://doi.org/10.3390/s24030915
APA StyleWang, G., Zhou, G., Zhang, Q., He, D., Zhao, C., & Suo, H. (2024). Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum–Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode. Sensors, 24(3), 915. https://doi.org/10.3390/s24030915






