Cellulose-Based Electrochemical Sensors
Abstract
1. Introduction
2. Cellulose-Based Materials for Electrochemical Sensors
2.1. Types of Cellulose Used in Electrochemical Sensors
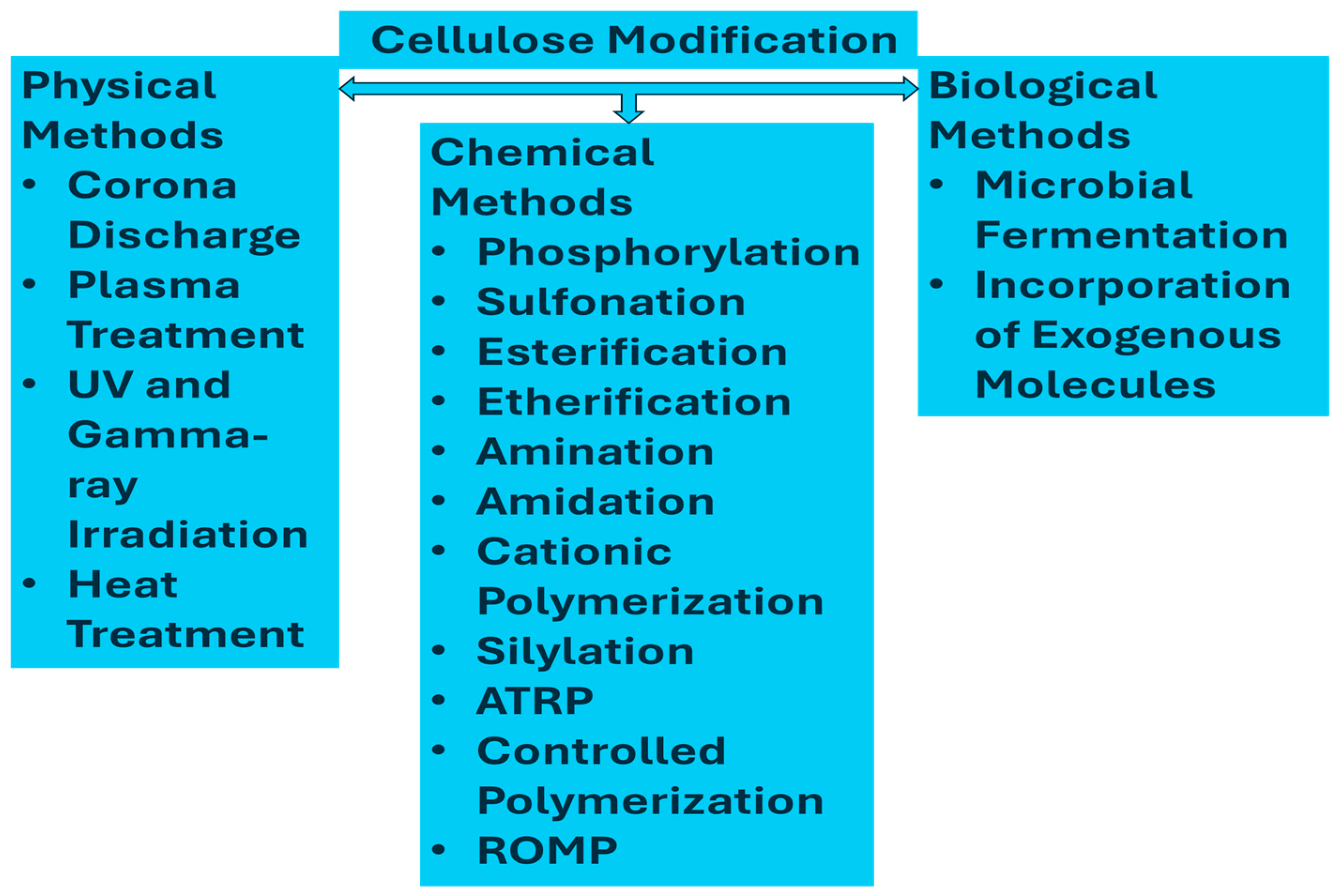
2.2. Modifications of Cellulose
3. Fabrication of Cellulose-Based Electrochemical Sensors
3.1. Paper-Based Electrochemical Sensors
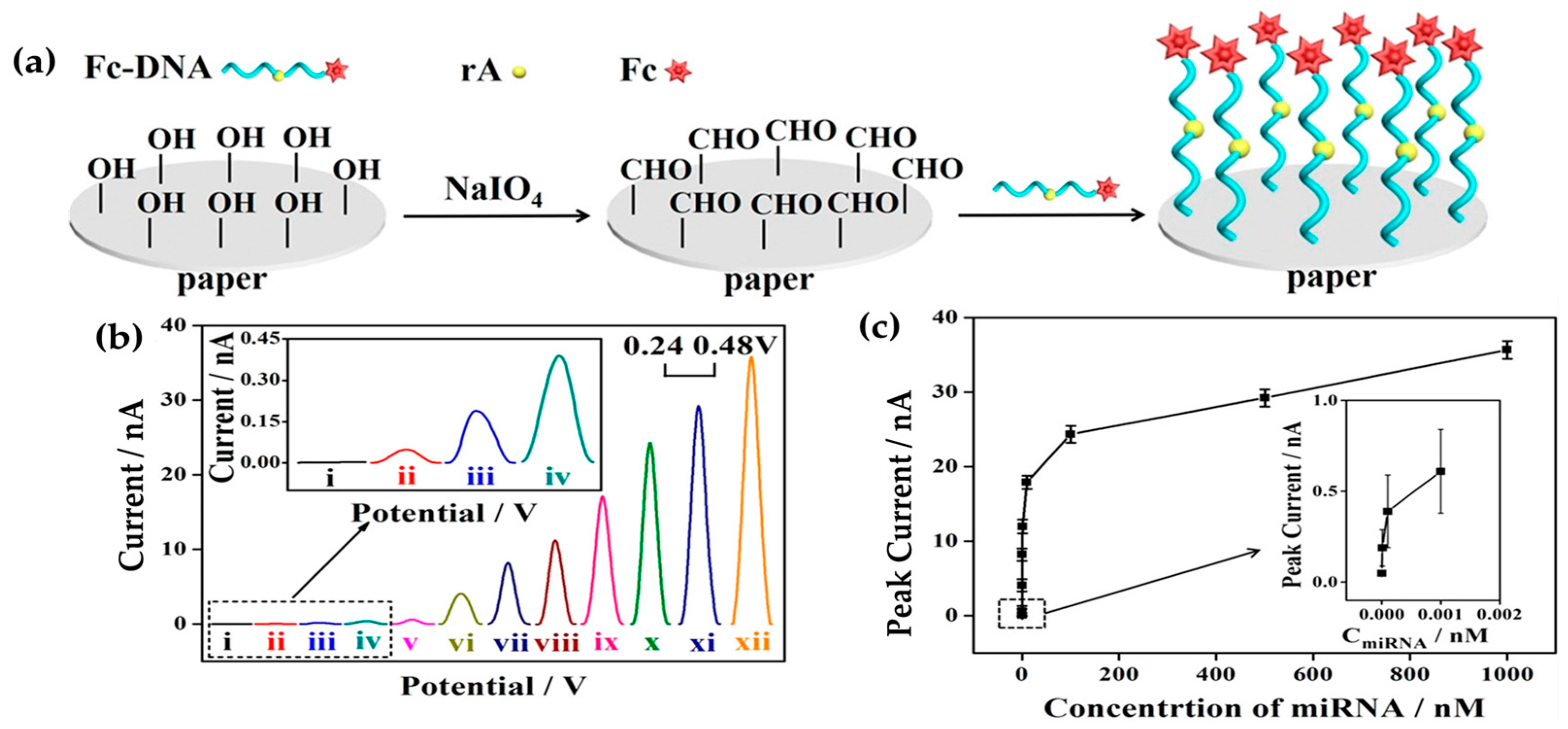
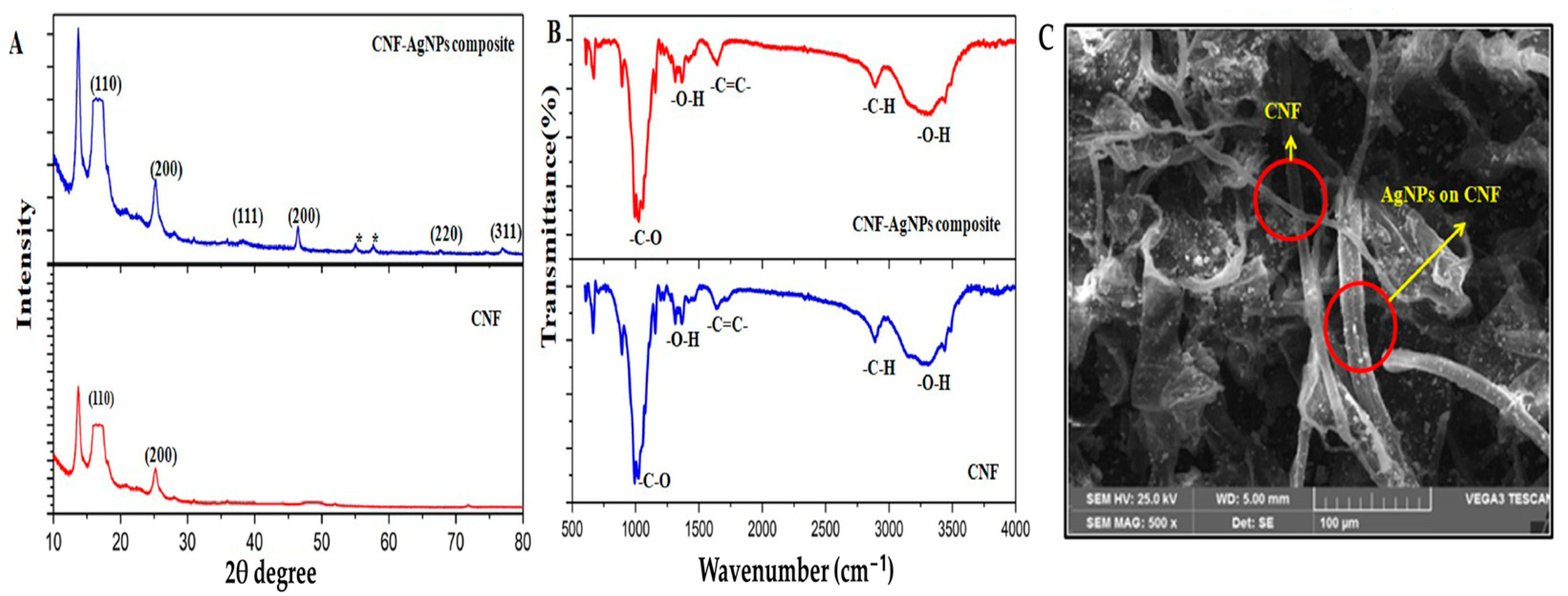
3.2. Nanocellulose-Based Nanocomposite Electrochemical Sensors
4. Electrochemical Sensors
4.1. Medical and Healthcare Sensors
4.2. Environmental Sensors
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M.; Perale, G.; Hilborn, J. 2—Natural Polymers: A Source of Inspiration. In Bioresorbable Polymers for Biomedical Applications; Woodhead Publishing: Sawston, CA, USA, 2017; pp. 31–64. [Google Scholar]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent advances in cellulose-based biosensors for medical diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ngasainao, M.R.; Sharma, D.; Sengar, M.; Gahlot, A.P.S.; Shukla, S.; Kumari, P. Contemporary nanocellulose-composites: A new paradigm for sensing applications. Carbohydr. Polym. 2022, 298, 120052. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of bacterial nanocellulose-based electrochemical biosensors: Functionalization, challenges, and future perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Chaka, K.T. Extraction of cellulose nanocrystals from agricultural by-products: A review. Green Chem. Lett. Rev. 2022, 15, 582–597. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging cellulose-derived materials: A promising platform for the design of flexible wearable sensors toward health and environment monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Wang, D.-C.; Lei, S.-N.; Zhong, S.; Xiao, X.; Guo, Q.-H. Cellulose-based conductive materials for energy and sensing applications. Polymers 2023, 15, 4159. [Google Scholar] [CrossRef]
- Almeida, E.V.; Morgado, D.L.; Ramos, L.A.; Frollini, E. Sisal cellulose and its acetates: Generation of films and reinforcement in a one-pot process. Cellulose 2013, 20, 453–465. [Google Scholar] [CrossRef]
- Verlhac, C.; Dedier, J.; Chanzy, H. Availability of surface hydroxyl groups in Valonia and bacterial cellulose. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 1171–1177. [Google Scholar] [CrossRef]
- de Souza, S.S.; Berti, F.V.; de Oliveira, K.P.; Pittella, C.Q.; de Castro, J.V.; Pelissari, C.; Rambo, C.R.; Porto, L.M. Nanocellulose biosynthesis by Komagataeibacter hansenii in a defined minimal culture medium. Cellulose 2019, 26, 1641–1655. [Google Scholar] [CrossRef]
- Manian, A.P.; Cordin, M.; Pham, T. Extraction of cellulose fibers from flax and hemp: A review. Cellulose 2021, 28, 8275–8294. [Google Scholar] [CrossRef]
- Melesse, G.T.; Hone, F.G.; Mekonnen, M.A. Extraction of cellulose from sugarcane bagasse optimization and characterization. Adv. Mater. Sci. Eng. 2022, 2022, 1712207. [Google Scholar] [CrossRef]
- Ganguly, P.; Sengupta, S.; Das, P.; Bhowal, A. Valorization of food waste: Extraction of cellulose, lignin and their application in energy use and water treatment. Fuel 2020, 280, 118581. [Google Scholar] [CrossRef]
- Lai, V.W.X.; Jamaluddin, J.; Mat Nasir, N.A.F.; Baharulrazi, N.; Majid, R.A.; Lai, J.C.; Mohd Bohari, S.P.; Chua, L.S.; Hasham, R.; Rahmat, Z. Extraction of cellulose from rice straw for regeneration of hydrogels. Environ. Qual. Manag. 2022, 32, 333–341. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Danial, W.H.; Majid, Z.A.; Muhid, M.N.M.; Triwahyono, S.; Bakar, M.B.; Ramli, Z. The reuse of wastepaper for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2015, 118, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Manzato, L.; Rabelo, L.; De Souza, S.; Da Silva, C.; Sanches, E.; Rabelo, D.; Mariuba, L.; Simonsen, J. New approach for extraction of cellulose from tucumã’s endocarp and its structural characterization. J. Mol. Struct. 2017, 1143, 229–234. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhardwaj, S.; Tiwari, P.; Dev, K.; Ghosh, K.; Maji, P.K. Recent advances in cellulose nanocrystals-based sensors: A review. Mater. Adv. 2024, 5, 2622–2654. [Google Scholar] [CrossRef]
- Gabrielli, V.; Frasconi, M. Cellulose-Based Functional Materials for Sensing. Chemosensors 2022, 10, 352. [Google Scholar] [CrossRef]
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Pontié, M.; Mbokou, S.F.; Bouchara, J.-P.; Razafimandimby, B.; Egloff, S.; Dzilingomo, O.; Pontalier, P.-Y.; Tonle, I.K. Paracetamol sensitive cellulose-based electrochemical sensors. J. Renew. Mater. 2018, 6, 242–250. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Rodthongkum, N.; Ummartyotin, S. A critical review on cellulose wastes as the novel substrates for colorimetric and electrochemical sensors. Curr. Res. Green Sustain. Chem. 2021, 4, 100190. [Google Scholar] [CrossRef]
- Chen, G.; Chen, T.; Hou, K.; Ma, W.; Tebyetekerwa, M.; Cheng, Y.; Weng, W.; Zhu, M. Robust, hydrophilic graphene/cellulose nanocrystal fiber-based electrode with high capacitive performance and conductivity. Carbon 2018, 127, 218–227. [Google Scholar] [CrossRef]
- El Miri, N.; El Achaby, M.; Fihri, A.; Larzek, M.; Zahouily, M.; Abdelouahdi, K.; Barakat, A.; Solhy, A. Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. Carbohydr. Polym. 2016, 137, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, conductive, and printable composites consisting of TEMPO-oxidized nanocellulose and carbon nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, V.; Li, P.; Liljestrom, T.; Wester, N.; Etula, J.; Leppanen, I.; Ge, Y.; Kontturi, K.S.; Tammelin, T.; Laurila, T. Functionalized nanocellulose/multiwalled carbon nanotube composites for electrochemical applications. ACS Appl. Nano Mater. 2021, 4, 5842–5853. [Google Scholar] [CrossRef]
- Morimune-Moriya, S.; Salajkova, M.; Zhou, Q.; Nishino, T.; Berglund, L.A. Reinforcement effects from nanodiamond in cellulose nanofibril films. Biomacromolecules 2018, 19, 2423–2431. [Google Scholar] [CrossRef]
- Cheng, H.; Du, Y.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Jiang, W.; Wang, L.; Sui, X. Flexible cellulose-based thermoelectric sponge towards wearable pressure sensor and energy harvesting. Chem. Eng. J. 2018, 338, 1–7. [Google Scholar] [CrossRef]
- Du, H.; Parit, M.; Liu, K.; Zhang, M.; Jiang, Z.; Huang, T.-S.; Zhang, X.; Si, C. Multifunctional cellulose nanopaper with superior water-resistant, conductive, and antibacterial properties functionalized with chitosan and polypyrrole. ACS Appl. Mater. Interfaces 2021, 13, 32115–32125. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT: PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem. Eng. J. 2022, 428, 131994. [Google Scholar] [CrossRef]
- Guan, Y.; He, H.; Tang, D.; Guo, P.; Han, X.; Zhang, H.; Xu, J.; Dai, L.; Huang, Z.; Si, C. Functional cellulose paper with high transparency, high haze, and UV-blocking for perovskite solar cells. Adv. Compos. Hybrid Mater. 2024, 7, 12. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liang, Q.; Zhao, Q.; Zhao, D.; Si, C. Compressible cellulose nanofibrils/reduced graphene oxide composite carbon aerogel for solid-state supercapacitor. Adv. Compos. Hybrid Mater. 2022, 5, 1168–1179. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.; Xu, Y.; Gao, M.; Xu, T.; Si, C. Wood-based hierarchical porous nitrogen-doped carbon/manganese dioxide composite electrode materials for high-rate supercapacitor. Adv. Compos. Hybrid Mater. 2023, 6, 174. [Google Scholar] [CrossRef]
- Yang, G.; Tang, X.; Zhao, G.; Li, Y.; Ma, C.; Zhuang, X.; Yan, J. Highly sensitive, direction-aware, and transparent strain sensor based on oriented electrospun nanofibers for wearable electronic applications. Chem. Eng. J. 2022, 435, 135004. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalysis overview: Addressing the green credentials in the use of electroanalytical sensors. Green Carbon 2023, 1, 85–93. [Google Scholar] [CrossRef]
- Hindi, S.; Hindi, S. Microcrystalline cellulose: Its processing and pharmaceutical specifications. BioCrystals J. 2016, 1, 26–38. [Google Scholar]
- Yang, P.; Yan, M.; Tian, C.; Huang, X.; Lu, H.; Zhou, X. Solvent-free preparation of thermoplastic bio-materials from microcrystalline cellulose (MCC) through reactive extrusion. Int. J. Biol. Macromol. 2022, 217, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.R.; Esposito, D.; Gillette, W.; Zhu, J.; Baxa, U.; Mcneil, S.E. Enzymatic preparation of nanocrystalline and microcrystalline cellulose. Tappi J. 2014, 13, 35–42. [Google Scholar] [CrossRef]
- Ismail, F.; Othman, N.E.A.; Wahab, N.A.; Hamid, F.A.; Aziz, A.A. Preparation of microcrystalline cellulose from oil palm empty fruit bunch fibre using steam-assisted acid hydrolysis. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 81, 88–98. [Google Scholar] [CrossRef]
- Raudhatussyarifah, R.; Sediawan, W.; Azis, M.; Hartati, I. Microcrystalline cellulose production by acid hydrolysis of hydrotropic rice straw pulp. IOP Conf. Ser. Earth Environ. Sci. 2022, 963, 012055. [Google Scholar] [CrossRef]
- Hassan, T.M.; Hossain, M.S.; Kassim, M.H.M.; Ibrahim, M.; Rawi, N.F.M.; Hussin, M.H. Optimizing the acid hydrolysis process for the isolation of microcrystalline cellulose from oil palm empty fruit bunches using response surface methods. Waste Biomass Valorization 2020, 11, 2755–2770. [Google Scholar] [CrossRef]
- Nurhadi, B.; Angeline, A.; Sukri, N.; Masruchin, N.; Arifin, H.R.; Saputra, R.A. Characteristics of microcrystalline cellulose from nata de coco: Hydrochloric acid versus maleic acid hydrolysis. J. Appl. Polym. Sci. 2022, 139, 51576. [Google Scholar] [CrossRef]
- Abdi, M.M.; Md Tahir, P.; Liyana, R.; Javahershenas, R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules 2018, 23, 2470. [Google Scholar] [CrossRef] [PubMed]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Zhang, M.; Yuan, Z. Enhanced removal of prometryn using copper modified microcrystalline cellulose (Cu-MCC): Optimization, isotherm, kinetics and regeneration studies. Cellulose 2019, 26, 6241–6258. [Google Scholar] [CrossRef]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline cellulose as pharmaceutical excipient. In Pharmaceutical Formulation Design-Recent Practices; IntechOpen: London, UK, 2019. [Google Scholar]
- Ahmed, A.E.S.I.; Hassan, M.L.; El-Masry, A.M.; El-Gendy, A.M. Testing of medical tablets produced with microcrystalline cellulose prepared from agricultural wastes. Polym. Compos. 2014, 35, 1343–1349. [Google Scholar] [CrossRef]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline cellulose for active food packaging applications: A review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Safety and efficacy of microcrystalline cellulose for all animal species. EFSA J. 2020, 18, e06209. [Google Scholar] [PubMed]
- Zhong, F.; Nsor-Atindana, J. Microcrystalline cellulose and nanocrystalline cellulose. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 509–536. [Google Scholar]
- Lupidi, G.; Pastore, G.; Marcantoni, E.; Gabrielli, S. Recent developments in chemical derivatization of microcrystalline cellulose (MCC): Pre-treatments, functionalization, and applications. Molecules 2023, 28, 2009. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, H.; Cai, H.; Han, X.; Lin, X.; Qian, M.; Zhao, Y.; Huo, E.; Villota, E.M.; Mateo, W. Improvement on the properties of microcrystalline cellulose/polylactic acid composites by using activated biochar. J. Clean. Prod. 2020, 252, 119898. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Kumar, A.; Katiyar, V. Microcrystalline cellulose, polylactic acid and polypropylene biocomposites and its morphological, mechanical, thermal and rheological properties. Compos. Part B Eng. 2020, 184, 107717. [Google Scholar] [CrossRef]
- Ramires, E.C.; Megiatto, J.D.; Dufresne, A.; Frollini, E. Cellulose Nanocrystals versus Microcrystalline Cellulose as Reinforcement of Lignopolyurethane Matrix. Fibers 2020, 8, 21. [Google Scholar] [CrossRef]
- Kurniawan, T.W.; Sulistyarti, H.; Rumhayati, B.; Sabarudin, A. Cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs) as adsorbents of heavy metal ions. J. Chem. 2023, 2023, 5037027. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167, 167–176. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, T.; Makarem, M.; Santiago Cintrón, M.; Cheng, H.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H. Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Amiralian, N.; Annamalai, P.K.; Memmott, P.; Martin, D.J. Isolation of cellulose nanofibrils from Triodia pungens via different mechanical methods. Cellulose 2015, 22, 2483–2498. [Google Scholar] [CrossRef]
- Yang, H.; Tejado, A.; Alam, N.; Antal, M.; van de Ven, T.G. Films prepared from electrosterically stabilized nanocrystalline cellulose. Langmuir 2012, 28, 7834–7842. [Google Scholar] [CrossRef]
- Gallardo-Sánchez, M.A.; Diaz-Vidal, T.; Navarro-Hermosillo, A.B.; Figueroa-Ochoa, E.B.; Ramirez Casillas, R.; Anzaldo Hernández, J.; Rosales-Rivera, L.C.; Soltero Martínez, J.F.A.; García Enríquez, S.; Macías-Balleza, E.R. Optimization of the obtaining of cellulose nanocrystals from agave Tequilana weber var. Azul Bagasse by acid hydrolysis. Nanomaterials 2021, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.; Dungani, R.; Paridah, M.; Sarker, M.Z.I.; Fazita, M.N.; Syakir, M. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess. 2019, 6, 45. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A. Preparation and characterization of nanocrystalline cellulose using ultrasonication combined with a microwave-assisted pretreatment process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Cornelis, F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-based materials for water remediation: Adsorption, catalysis, and antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Li, D.; Xu, Y.; Xu, F. Flexible and sensitivity-adjustable pressure sensors based on carbonized bacterial nanocellulose/wood-derived cellulose nanofibril composite aerogels. ACS Appl. Mater. Interfaces 2021, 13, 8754–8763. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, K.; Kim, B.; Lee, K.-J.; Kang, J.-W.; Jeon, S. Vertically stacked nanocellulose tactile sensor. Nanoscale 2017, 9, 17212–17219. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, T.; Cai, C.; Liu, K.; Liu, W.; Zhang, M.; Du, H.; Si, C.; Zhang, K. Multifunctional superelastic, superhydrophilic, and ultralight nanocellulose-based composite carbon aerogels for compressive supercapacitor and strain sensor. Adv. Funct. Mater. 2022, 32, 2113082. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Liu, W.; Zhang, M.; Wang, Y.; Liu, H.; Zhang, X.; Xu, T.; Si, C. Strong, flexible, and highly conductive cellulose nanofibril/PEDOT: PSS/MXene nanocomposite films for efficient electromagnetic interference shielding. Nanoscale 2022, 14, 14902–14912. [Google Scholar] [CrossRef] [PubMed]
- Passornraprasit, N.; Siripongpreda, T.; Ninlapruk, S.; Rodthongkum, N.; Potiyaraj, P. γ-Irradiation crosslinking of graphene oxide/cellulose nanofiber/poly (acrylic acid) hydrogel as a urea sensing patch. Int. J. Biol. Macromol. 2022, 213, 1037–1046. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Zhang, M.; Xu, T.; Du, H.; Pang, B.; Si, C. Sustainable polysaccharide-based materials for intelligent packaging. Carbohydr. Polym. 2023, 313, 120851. [Google Scholar] [CrossRef]
- Langari, M.M.; Antxustegi, M.M.; Labidi, J. Nanocellulose-based sensing platforms for heavy metal ions detection: A comprehensive review. Chemosphere 2022, 302, 134823. [Google Scholar] [CrossRef]
- Langari, M.M.; Nikzad, M.; Labidi, J. Nanocellulose-based sensors in medical/clinical applications: The state-of-the-art review. Carbohydr. Polym. 2023, 304, 120509. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Knight, V.F.; Nurazzi, N.M.; Jenol, M.A.; Misenan, M.S.M.; Janudin, N.; Kasim, N.A.M.; Shukor, M.F.A.; Ilyas, R.A.; Asyraf, M.R.M. The frontiers of functionalized nanocellulose-based composites and their application as chemical sensors. Polymers 2022, 14, 4461. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Gao, M.; Guo, Y.; Xu, T.; Jiang, H.; Zhang, Z.; Ji, X.; Si, C. Nanocellulose-based functional materials for physical, chemical, and biological sensing: A review of materials, properties, and perspectives. Ind. Crops Prod. 2024, 212, 118326. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.; Correa, D.S. A review on the role and performance of cellulose nanomaterials in sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A. Bacterial cellulose as a potential biopolymer in biomedical applications: A state-of-the-art review. J. Mater. Chem. B 2022, 10, 3199–3241. [Google Scholar] [CrossRef]
- Poddar, M.K.; Dikshit, P.K. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Sel. 2021, 2, 1605–1628. [Google Scholar] [CrossRef]
- Pandit, A.; Kumar, R. A review on production, characterization and application of bacterial cellulose and its biocomposites. J. Polym. Environ. 2021, 29, 2738–2755. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kuzmenko, T.P.; Kurgaeva, I.V.; Revin, V.D.; Ullah, M.W. Bacterial Cellulose-Based Polymer Nanocomposites: A Review. Polymers 2022, 14, 4670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S. Bacterial cellulose production and its industrial applications. J. Bioprocess. Biotechnol. 2014, 4, 150. [Google Scholar] [CrossRef]
- Son, H.J.; Heo, M.S.; Kim, Y.G.; Lee, S.J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter. Biotechnol. Appl. Biochem. 2001, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ccorahua, R.; Troncoso, O.P.; Rodriguez, S.; Lopez, D.; Torres, F.G. Hydrazine treatment improves conductivity of bacterial cellulose/graphene nanocomposites obtained by a novel processing method. Carbohydr. Polym. 2017, 171, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose–starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.; Won, K.; Kim, H.J.; Lee, S.H. Bacterial cellulose–carbon nanotube composite as a biocompatible electrode for the direct electron transfer of glucose oxidase. J. Chem. Technol. Biotechnol. 2013, 88, 1067–1070. [Google Scholar] [CrossRef]
- Sanchis, M.; Carsí, M.; Gómez, C.; Culebras, M.; Gonzales, K.; Torres, F. Monitoring molecular dynamics of bacterial cellulose composites reinforced with graphene oxide by carboxymethyl cellulose addition. Carbohydr. Polym. 2017, 157, 353–360. [Google Scholar] [CrossRef]
- Torres, F.; Arroyo, J.; Troncoso, O. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Torres, F.G.; Ccorahua, R.; Arroyo, J.; Troncoso, O.P. Enhanced conductivity of bacterial cellulose films reinforced with NH4I-doped graphene oxide. Polym.-Plast. Technol. Mater. 2019, 58, 1585–1595. [Google Scholar] [CrossRef]
- Li, D.; Ao, K.; Wang, Q.; Lv, P.; Wei, Q. Preparation of Pd/bacterial cellulose hybrid nanofibers for dopamine detection. Molecules 2016, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, K.; Li, D.; Lv, P.; Wang, Q.; Huang, F.; Wei, Q. Biosensor based on bacterial cellulose-Au nanoparticles electrode modified with laccase for hydroquinone detection. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 408–414. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.Y.; Zhang, D.W.; Jiang, J.; Cui, Y.R.; Qiu, S.; Zhou, Y.L.; Zhang, X.X. Fabrication of bienzymatic glucose biosensor based on novel gold nanoparticles-bacteria cellulose nanofibers nanocomposite. Electroanalysis 2010, 22, 2543–2550. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated synthesis of gold nanoparticle–bacteria cellulose nanofiber nanocomposites and their application in biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Rebelo, A.; Liu, Y.; Liu, C.; Schäfer, K.-H.; Saumer, M.; Yang, G. Poly (4-vinylaniline)/polyaniline bilayer functionalized bacterial cellulose membranes as bioelectronics interfaces. Carbohydr. Polym. 2019, 204, 190–201. [Google Scholar] [CrossRef]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. Conductive bacterial cellulose/multiwall carbon nanotubes nanocomposite aerogel as a potentially flexible lightweight strain sensor. Carbohydr. Polym. 2018, 201, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial cellulose-based biosensors. Med. Devices Sens. 2020, 3, e10102. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Madhushree, M.; Vairavel, P.; Mahesha, G.; Bhat, K.S. A Comprehensive Review of Cellulose and Cellulose-Based Materials: Extraction, Modification, and Sustainable Applications. J. Nat. Fibers 2024, 21, 2418357. [Google Scholar] [CrossRef]
- Hassani, F.O.; Merbahi, N.; Oushabi, A.; Elfadili, M.; Kammouni, A.; Oueldna, N. Effects of corona discharge treatment on surface and mechanical properties of Aloe Vera fibers. Mater. Today Proc. 2020, 24, 46–51. [Google Scholar] [CrossRef]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface modification of bacterial cellulose membrane by oxygen plasma treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Pertile, R.A.; Andrade, F.K.; Alves, C., Jr.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Vosmanska, V.; Kolarova, K.; Rimpelova, S.; Svorcik, V. Surface modification of oxidized cellulose haemostat by argon plasma treatment. Cellulose 2014, 21, 2445–2456. [Google Scholar] [CrossRef]
- Mohit, H.; Arul Mozhi Selvan, V. A comprehensive review on surface modification, structure interface and bonding mechanism of plant cellulose fiber reinforced polymer based composites. Compos. Interfaces 2018, 25, 629–667. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Zia, K.M.; Ali, Z.; Zuber, M. Modification of cellulosic fibers to enhance their dyeability using UV-irradiation. Carbohydr. Polym. 2012, 89, 783–787. [Google Scholar] [CrossRef]
- Lenfeld, P.; Brdlík, P.; Borůvka, M.; Běhálek, L.; Habr, J. Effect of radiation crosslinking and surface modification of cellulose fibers on properties and characterization of biopolymer composites. Polymers 2020, 12, 3006. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B. Pine wood modification by heat treatment in air. BioResources 2008, 3, 142–154. [Google Scholar] [CrossRef]
- Yildiz, S.; Gümüşkaya, E. The effects of thermal modification on crystalline structure of cellulose in soft and hardwood. Build. Environ. 2007, 42, 62–67. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Natalio, F.; Fuchs, R.; Cohen, S.R.; Leitus, G.; Fritz-Popovski, G.; Paris, O.; Kappl, M.; Butt, H.-J. Biological fabrication of cellulose fibers with tailored properties. Science 2017, 357, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Said, H.A.; Bourhim, I.A.; Ouarga, A.; Iraola-Arregui, I.; Lahcini, M.; Barroug, A.; Noukrati, H. Sustainable phosphorylated microcrystalline cellulose toward enhanced removal performance of methylene blue. Int. J. Biol. Macromol. 2023, 225, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Lander, S.; Vagin, M.; Gueskine, V.; Erlandsson, J.; Boissard, Y.; Korhonen, L.; Berggren, M.; Wågberg, L.; Crispin, X. Sulfonated cellulose membranes improve the stability of aqueous organic redox flow batteries. Adv. Energy Sustain. Res. 2022, 3, 2200016. [Google Scholar] [CrossRef]
- Baraka, F.; Robles, E.; Labidi, J. Microwave-assisted esterification of bleached and unbleached cellulose nanofibers. Ind. Crops Prod. 2023, 191, 115970. [Google Scholar] [CrossRef]
- Li, B.; Xu, C.; Yu, J.; Liu, L.; Zhang, X.; Fan, Y. One-pot cellulose etherification and self-crosslinking via a mild hydroxyl–yne click reaction in a homogeneous system. Green Chem. 2023, 25, 2608–2619. [Google Scholar] [CrossRef]
- Yang, H.; Tan, X.; Du, G.; Ni, K.; Wu, Y.; Li, Z.; Ran, X.; Gao, W.; Li, J.; Yang, L. Development of biomass adhesives based on aminated cellulose and oxidized sucrose reinforced with epoxy functionalized wood interface. Compos. Part B Eng. 2023, 263, 110872. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Solvent-free synthesis of amidated carboxymethyl cellulose derivatives: Effect on the thermal properties. Polymers 2019, 11, 1227. [Google Scholar] [CrossRef]
- Sang, Y.; Xiao, H. Preparation and application of cationic cellulose fibers modified by in situ grafting of cationic PVA. Colloids Surf. A Physicochem. Eng. Asp. 2009, 335, 121–127. [Google Scholar] [CrossRef]
- Pacaphol, K.; Seraypheap, K.; Aht-Ong, D. Extraction and Silylation of Cellulose Nanofibers from Agricultural Bamboo Leaf Waste for Hydrophobic Coating on Paper. J. Nat. Fibers 2023, 20, 2178581. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Qi, H.; Xu, D.; Huang, H.; Liu, M.; Wen, Y.; Deng, F.; Zhang, X.; Wei, Y. Preparation of fluorescent cellulose nanocrystal polymer composites with thermo-responsiveness through light-induced ATRP. Cellulose 2020, 27, 743–753. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Surface modification of cellulose via conventional and controlled radiation-induced grafting. Radiat. Phys. Chem. 2019, 160, 1–8. [Google Scholar] [CrossRef]
- Carlsson, L.; Malmström, E.; Carlmark, A. Surface-initiated ring-opening metathesis polymerisation from cellulose fibres. Polym. Chem. 2012, 3, 727–733. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D.; Palleschi, G. How cutting-edge technologies impact the design of electrochemical (bio) sensors for environmental analysis. A review. Anal. Chim. Acta 2017, 959, 15–42. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Shaw, J.L. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155. [Google Scholar]
- Matzeu, G.; Florea, L.; Diamond, D. Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B Chem. 2015, 211, 403–418. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Wang, J. Wearable electrochemical sensors and biosensors: A review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Gao, X.; Ge, L.; Sun, X.; Li, F. A universal paper-based electrochemical sensor for zero-background assay of diverse biomarkers. ACS Appl. Mater. Interfaces 2019, 11, 15381–15388. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, R.; Ling, H.; Zhao, Z.; Han, W.; Shi, X.; Payne, G.F.; Wang, X. Toward scalable fabrication of electrochemical paper sensor without surface functionalization. Npj Flex. Electron. 2022, 6, 12. [Google Scholar] [CrossRef]
- Santhosh, A.; Sandeep, S.; Bound, D.J.; Nandini, S.; Nalini, S.; Suresh, G.; Swamy, N.K.; Rajabathar, J.R.; Selvaraj, A. A multianalyte electrochemical sensor based on cellulose fibers with silver nanoparticles composite as an innovative nano-framework for the simultaneous determination of ascorbic acid, dopamine and paracetamol. Surf. Interfaces 2021, 26, 101377. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Basirun, W.J.; Bagheri, S.; Anuar, N.S.; Johan, M.R. Hybrid nanocellulose/f-MWCNTs nanocomposite for the electrochemical sensing of diclofenac sodium in pharmaceutical drugs and biological fluids. Electrochim. Acta 2019, 304, 323–333. [Google Scholar] [CrossRef]
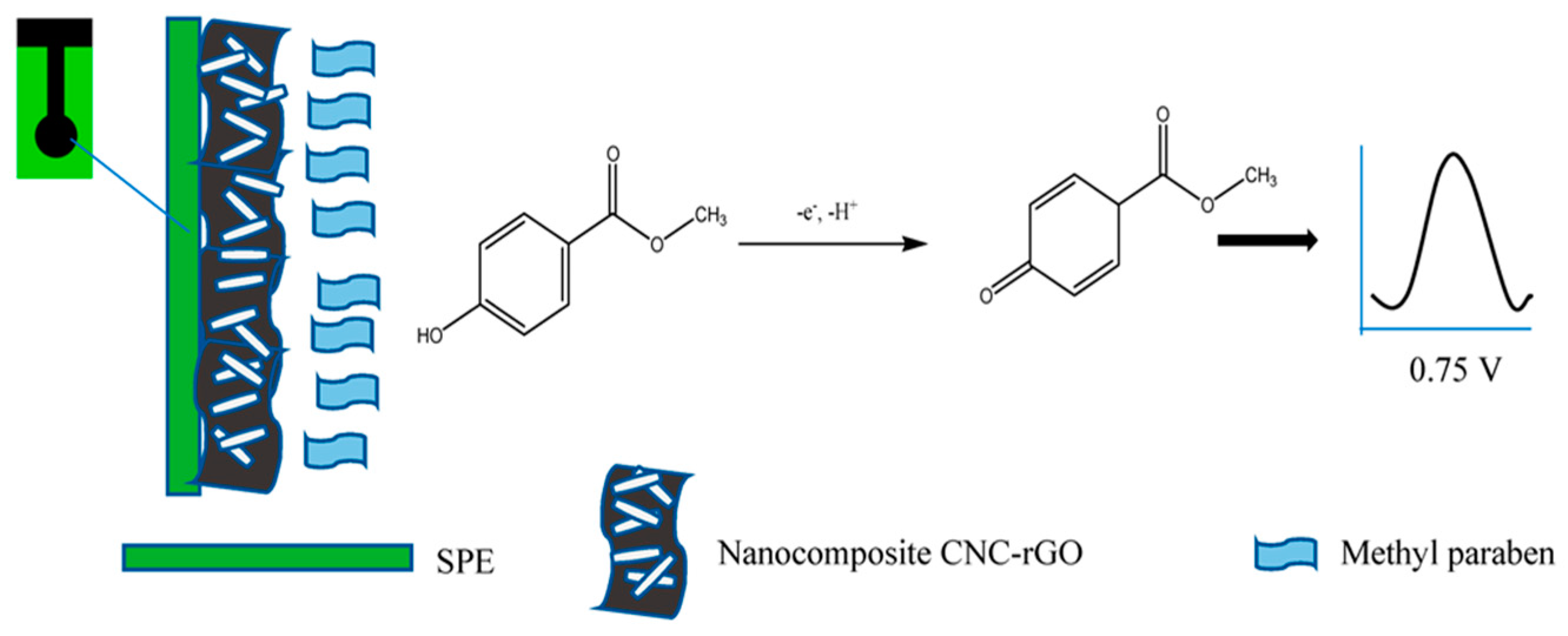
- Wan Khalid, W.E.F.; Mat Arip, M.N.; Jasmani, L.; Lee, Y.H. A new sensor for methyl paraben using an electrode made of a cellulose nanocrystal–reduced graphene oxide nanocomposite. Sensors 2019, 19, 2726. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.H.M.; Abdullah, J.; Yusof, N.A.; Wasoh, H.; Sulaiman, Y.; Noh, M.F.M.; Issa, R. Reduced graphene oxide/TEMPO-nanocellulose nanohybrid-based electrochemical biosensor for the determination of mycobacterium tuberculosis. J. Sens. 2020, 2020, 4051474. [Google Scholar] [CrossRef]
- Anirudhan, T.; Deepa, J. Electrochemical sensing of cholesterol by molecularly imprinted polymer of silylated graphene oxide and chemically modified nanocellulose polymer. Mater. Sci. Eng. C 2018, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Abd Manan, F.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater. Sci. Eng. C 2019, 99, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Petropoulos, K.; Kurth, F.; Gao, H.; Migliorelli, D.; Guenat, O.; Generelli, S. Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring. Biosensors 2020, 10, 125. [Google Scholar] [CrossRef]
- Burrs, S.; Bhargava, M.; Sidhu, R.; Kiernan-Lewis, J.; Gomes, C.; Claussen, J.; McLamore, E. A paper based graphene-nanocauliflower hybrid composite for point of care biosensing. Biosens. Bioelectron. 2016, 85, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose. Sensors 2015, 15, 24681–24697. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, J.; Waterhouse, G.I.; Cheng, Z.; Ai, S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2018, 101, 110–115. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Migliorini, F.L.; Facure, M.H.; Correa, D.S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercury (II). Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Nitin, A.; Jain, R. Cellulose fabricated pencil graphite sensor for the quantification of hazardous herbicide atrazine. Diam. Relat. Mater. 2020, 105, 107788. [Google Scholar]
- Das, J.; Mishra, H.N. Electrochemical biosensor for monitoring fish spoilage based on nanocellulose as enzyme immobilization matrix. J. Food Meas. Charact. 2023, 17, 3827–3844. [Google Scholar] [CrossRef]
- Fan, J.; Xu, M.; Xu, Y.-T.; Hamad, W.Y.; Meng, Z.; MacLachlan, M.J. A visible multi-response electrochemical sensor based on cellulose nanocrystals. Chem. Eng. J. 2023, 457, 141175. [Google Scholar] [CrossRef]
- Nekoueian, K.; Kontturi, K.S.; Meinander, K.; Quliyeva, U.; Kousar, A.; Durairaj, V.; Tammelin, T.; Laurila, T. Advanced nanocellulose-based electrochemical sensor for tetracycline monitoring. Electrochim. Acta 2024, 500, 144639. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Li, J.; Shen, M.; Liu, H.; Xu, X.; Shang, S. Self-healing, self-adhesive, and stretchable conductive hydrogel for multifunctional sensor prepared by catechol modified nanocellulose stabilized poly (α-thioctic acid). Carbohydr. Polym. 2023, 313, 120813. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ling, Z.; Liu, X.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Nanocomposite hydrogels flexible sensors with functional cellulose nanocrystals for monitoring human motion and lactate in sweat. Chem. Eng. J. 2023, 466, 143306. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, Y.; Mário, B.J.L.; Wang, S. Cellulose-derived hierarchical porous carbon based electrochemical sensor for simultaneous detection of catechol and hydroquinone. Ionics 2024, 30, 1089–1100. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, D.; Zheng, Y.; Lin, X.; Yang, F.; Liu, C.; Sun, H. Preparation of Conductive Cellulose Coated with Conductive Polymer and Its Application in the Detection of pH and Characteristic Substances in Sweat. Int. J. Mol. Sci. 2024, 25, 6393. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, N. Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat. Molecules 2024, 29, 1256. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, I.S.; Almughathawi, R.; Madkhali, M.M.; Alshammari, N.M.; Alaysuy, O.; Mogharbel, A.T.; Hosni, M.; El-Metwaly, N.M. Sustainable waste-derived cellulose-based nanosensor for cobalt ion detection, removal, and recovery from industrial effluents and battery wastes. J. Water Process Eng. 2025, 70, 106974. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Z.; Li, W.; Xiao, D.; Liu, G.; Yu, Z.; Yin, S.; Liang, M. A high-sensitivity continuous glucose sensor using porous 3D cellulose/carbon nanotube network. Talanta 2025, 283, 127201. [Google Scholar] [CrossRef]


| Materials | Analytes | Method of Detection | Reference |
|---|---|---|---|
| Microbial nanocellulose (MNC)/screen-printed carbon electrodes (SPCEs) | Pb2+, Cd2+, uric acid, and 17β-estradiol in artificial sweat | DPV | [154] |
| Reduced graphene oxide (NH2-rGO)/2,2,6,6-tetramethylpiperidin-1-yl) oxyl nanocrystalline cellulose (TEMPO-NCC)/screen-printed carbon electrode | Mycobacterium tuberculosis | DPV, EIS, CV | [155] |
| Silylated graphene oxide-grafted chemically modified nanocellulose (Si-GO-g-CMNC)/ZnO incorporated in CMNC/glassy carbon electrode | Cholesterol | CV, DPV | [156] |
| NCC/CdS quantum dots (QDs)/cetyltriammonium bromide (CTAB)/3-mercaptopropionic acid (3-MPA)/tyrosinase enzyme (Tyr) | Phenol | DPV | [157] |
| TEMPO-oxidized CNCs/glucose oxidase (GOx)/screen-printed electrode | Glucose | CV, chronoamperometry | [158] |
| Graphene paper/rGO/graphene oxide-coated nanocellulose/glucose oxidase (GOx)/RNA aptamer/chitosan | E. coli O157:H7, glucose | EIS, CV | [159] |
| Polypyrrole–cellulose nanocrystal/GOx | Glucose | CV, DPV | [160] |
| Graphene-perylene-3,4,9,10-tetracarboxylic acid nanocomposites (GR-PTCA)/nanocellulose–Au NP composites (NC-Au)/the alkaline phosphatase (ALP) | Avian leukosis virus subgroup J (ALV-J) | CV, DPV | [161] |
| Cellulose nanowhiskers (CNWs)/rGO/polyamide 6 (PA6) electrospun nanofibers | Hg2+ | CV | [162] |
| Cellulose/pencil graphite electrode | Atrazine (ATZ) | SWV, CV | [163] |
| Nanocellulose/xanthine oxidase (XO)/glassy carbon electrode | Xanthine (XA) | DPV, EIS | [164] |
| CNCs/polyaniline (PANI)/glucose | Humidity, pH, organic solvents | Stimuli-responsive CNC/PANI composite films, electrochromic device | [165] |
| TEMPO-oxidized cellulose nanofibers-polyethyleneimine hybrids/single-walled carbon nanotubes (SWCNTs) | Tetracycline | CV, DPV | [166] |
| Thioctic acid (TA)/dopamine-grafted cellulose nanofibers (DCNF)/Li+ | Strain, conductivity | Electrical conductivity measurement | [167] |
| CNCs/polydopamine (PDA)/AuNPs/polyvinyl alcohol/lactate oxidase (LOx) | Lactate in sweat | Lactate sweat sensor | [168] |
| Cellulose/KOH | Hydroquinone, catechol | CV, amperometry | [169] |
| Cellulose/polystyrene sulfonate sodium (PSS) | Ion concentration in sweat, pH | Cellulose-based conductive membrane | [170] |
| Cellulose hydrogel/AgNPs/ferrocyanide redox probe | Sulfamethoxazole | SWV | [171] |
| Cellulose nanofibers from wastepaper/1-(2-hydroxy-1-naphthylazo)-2-naphthol-4-sulfonic acid (HNNSA) | Co2+ | Cobalt nanosensor | [172] |
| Cellulose/carbon nanotubes/glucose oxidase/Pt/Ag/AgCl | Glucose | Continuous glucose sensor | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheraz, M.; Sun, X.-F.; Siddiqui, A.; Wang, Y.; Hu, S.; Sun, R. Cellulose-Based Electrochemical Sensors. Sensors 2025, 25, 645. https://doi.org/10.3390/s25030645
Sheraz M, Sun X-F, Siddiqui A, Wang Y, Hu S, Sun R. Cellulose-Based Electrochemical Sensors. Sensors. 2025; 25(3):645. https://doi.org/10.3390/s25030645
Chicago/Turabian StyleSheraz, Muhammad, Xiao-Feng Sun, Adeena Siddiqui, Yongke Wang, Sihai Hu, and Ran Sun. 2025. "Cellulose-Based Electrochemical Sensors" Sensors 25, no. 3: 645. https://doi.org/10.3390/s25030645
APA StyleSheraz, M., Sun, X.-F., Siddiqui, A., Wang, Y., Hu, S., & Sun, R. (2025). Cellulose-Based Electrochemical Sensors. Sensors, 25(3), 645. https://doi.org/10.3390/s25030645










