The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
- -
- Patients aged over 16 years;
- -
- Patients able to give informed consent to participate;
- -
- Patients that completed colonic examination;
- -
- Patients that returned both stool and urine samples.
- -
- Patients under 16 years of age;
- -
- Pregnant patients.
3. Results
3.1. Reproducibility and Calibration of the Electronic Nose System
3.1.1. Reproducibility over Time
3.1.2. Calibration
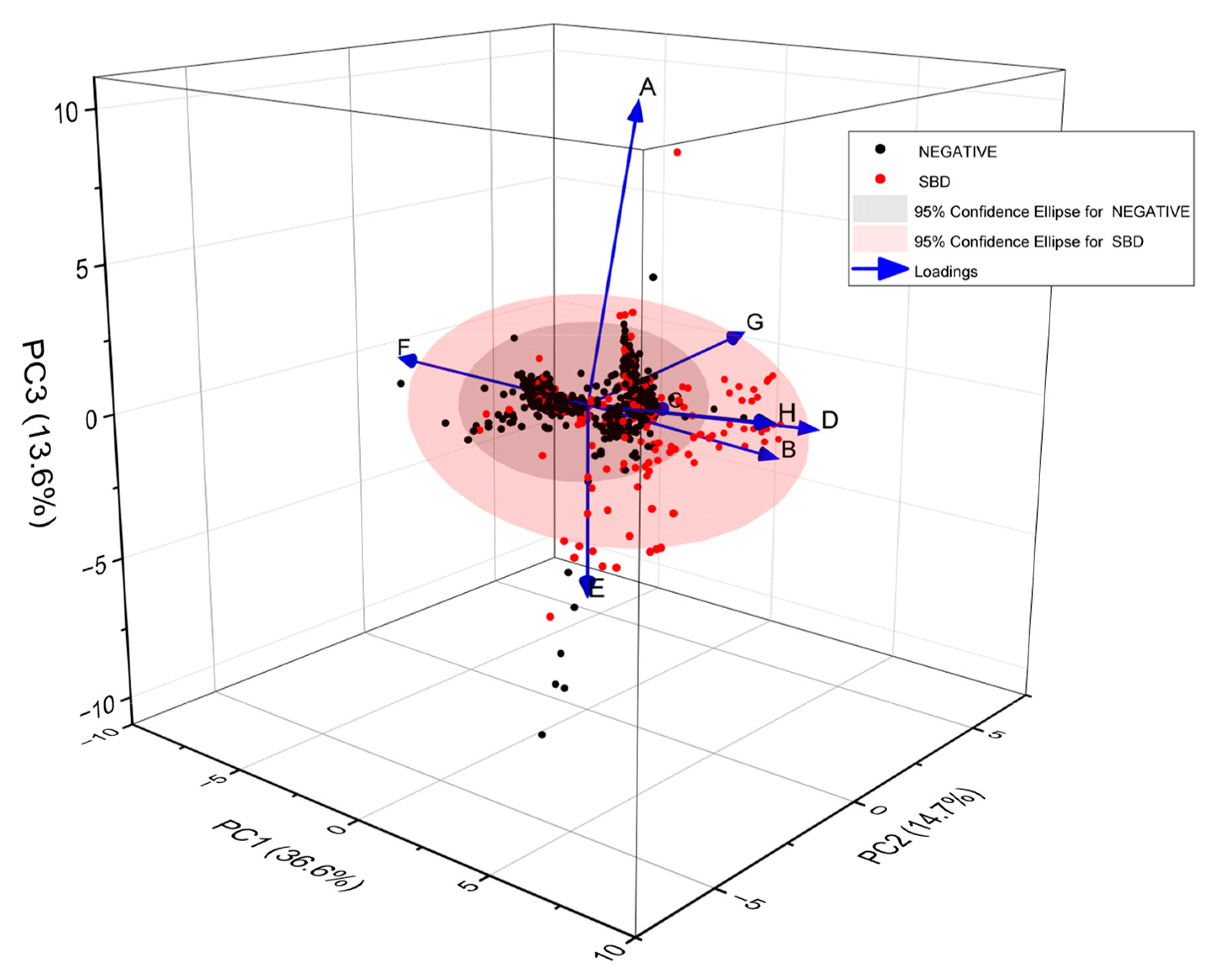
3.2. Blinded Data
3.3. Unblinded Data
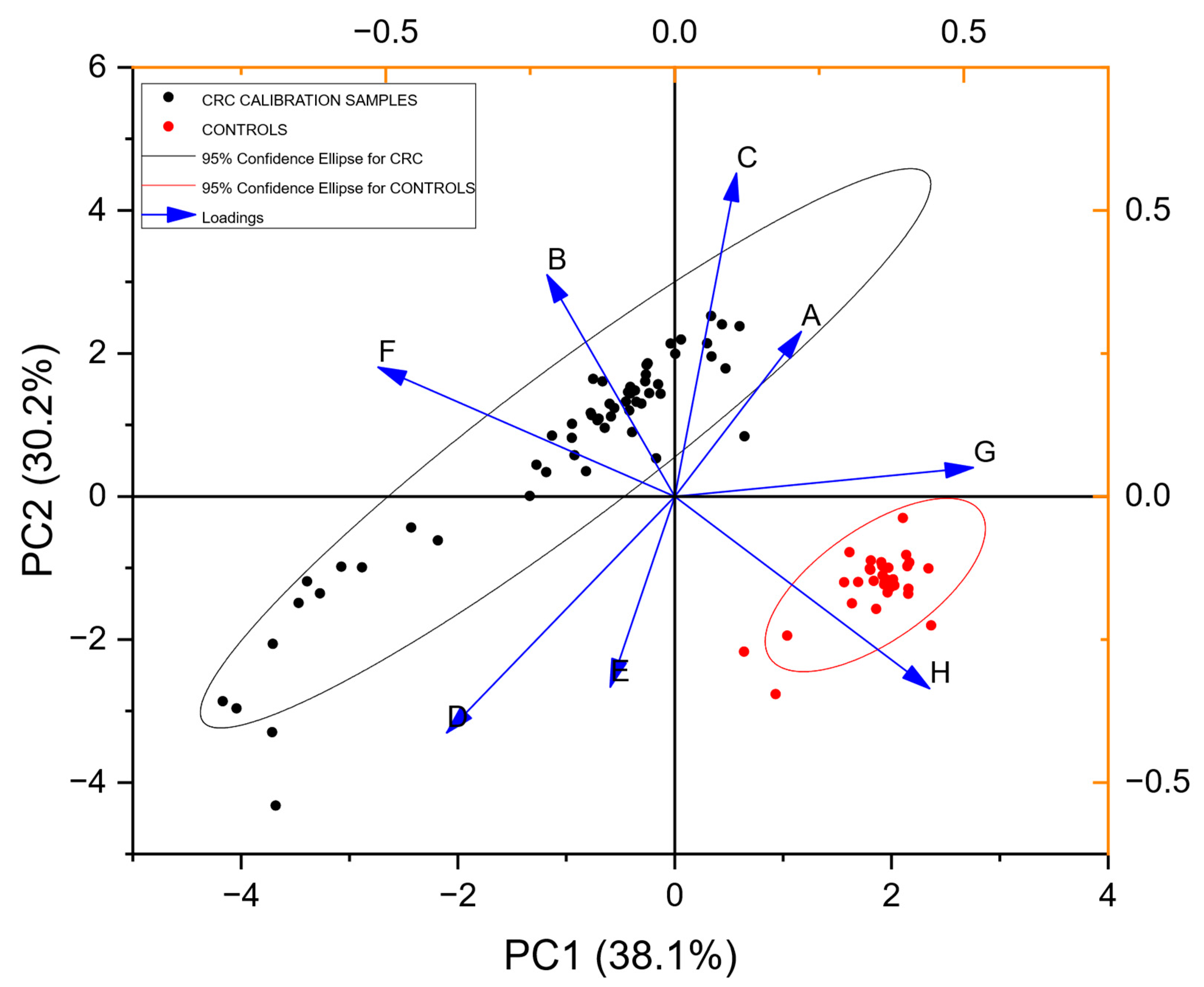
3.4. Defining the Control/Comparator Population
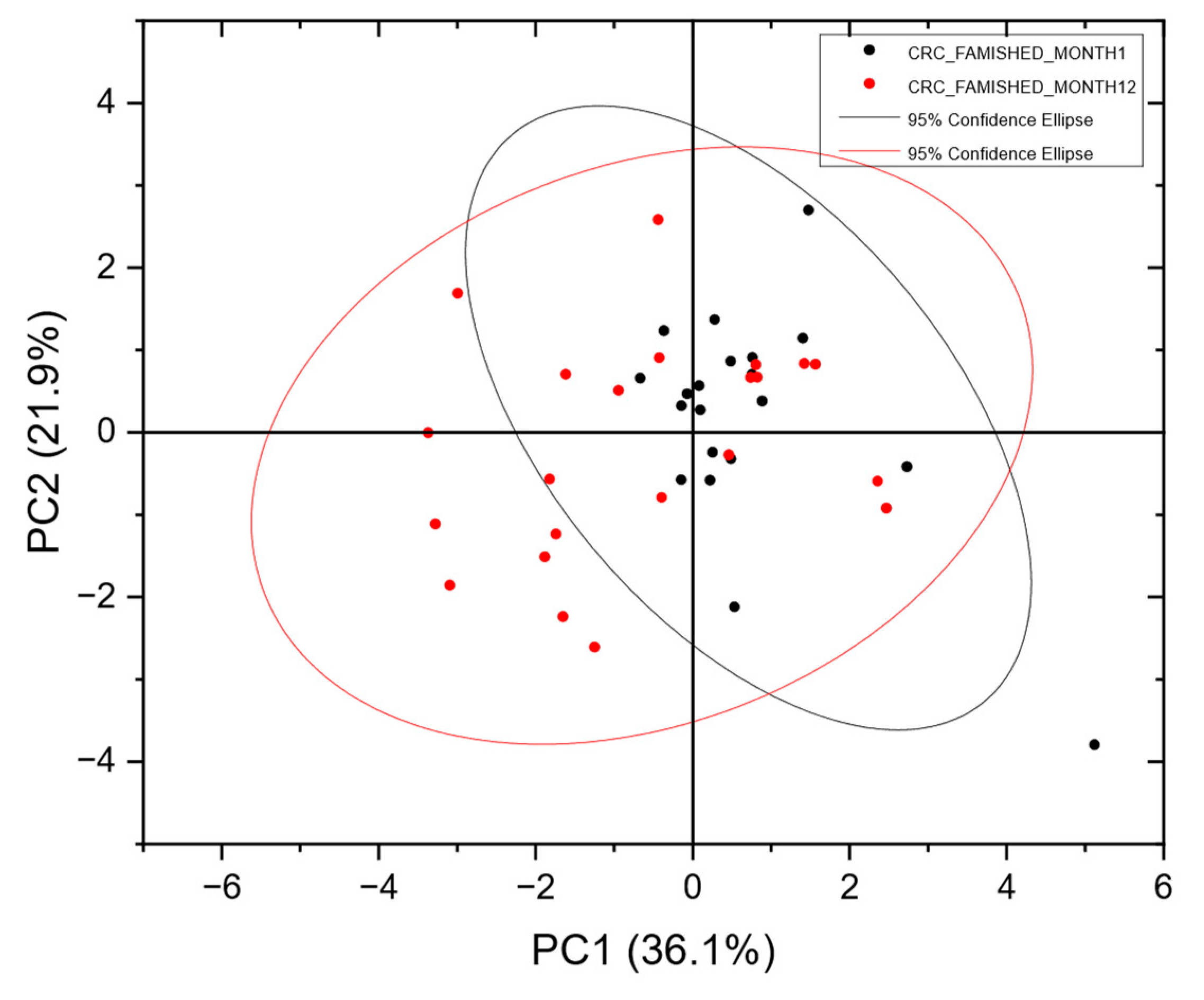
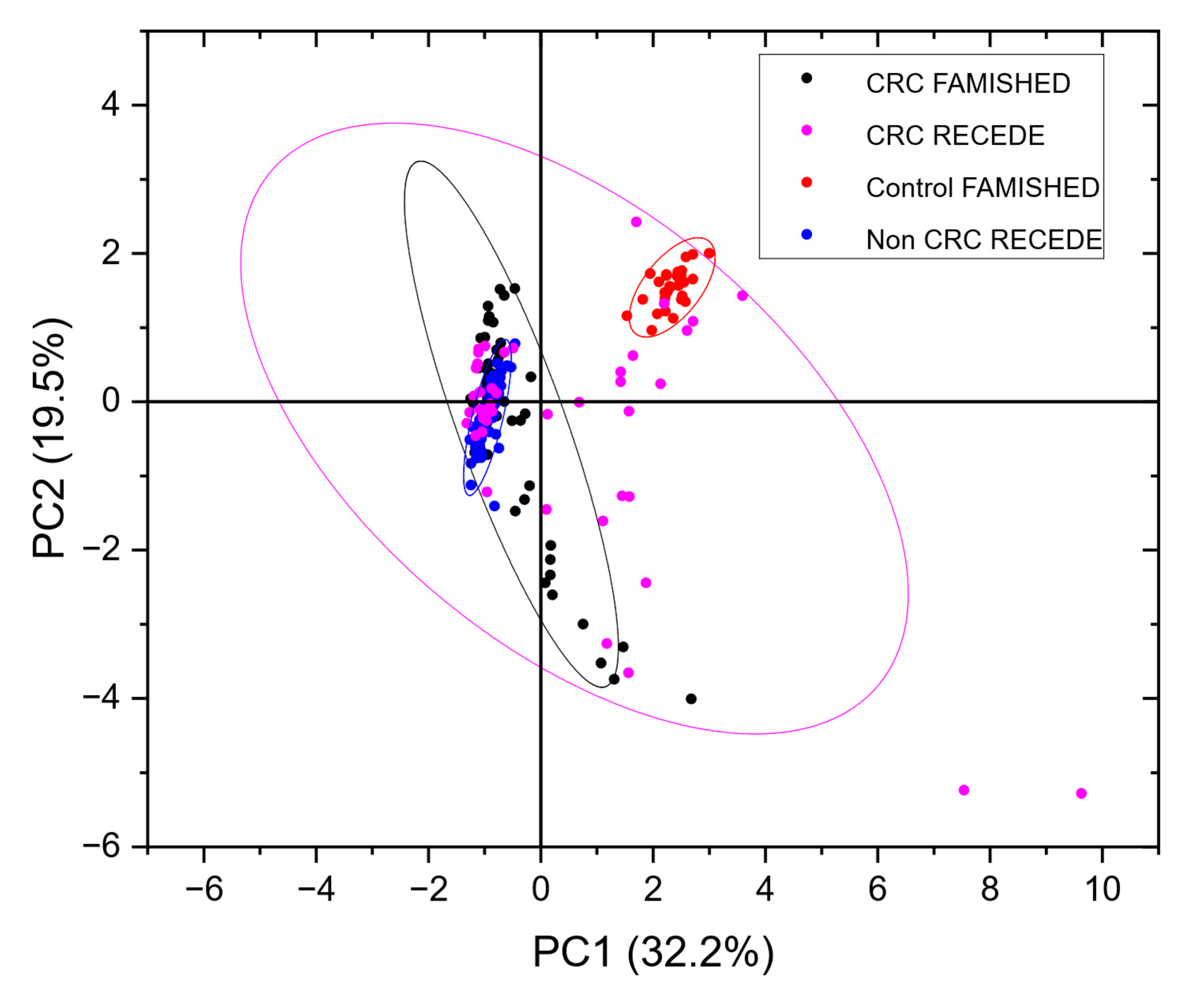
3.5. Resolving Unbalanced Datasets
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | False-Negative Rate | False-Positive Rate | True-Positive Rate | True-Negative Rate |
|---|---|---|---|---|
| Fraction | Fraction | Fraction | Fraction | |
| CRC | 0.03 | 0.22 | 0.78 | 0.97 |
| NEGATIVE | 0.22 | 0.03 | 0.97 | 0.78 |
| CRC | ||||
| Sensitivity (se) | 0.44 | |||
| Specificity (sp) | 0.99 | |||
| Positive predictive value (PPV) | 0.78 | |||
| Negative predictive value (NPV) | 0.97 | |||
| Positive likelihood ratio (PLR) | 80.24 | |||
| Negative likelihood ratio (NLR) | 0.56 | |||
Tab Exposure Calibration

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432. [Google Scholar] [CrossRef]
- Fraser, C.G. Faecal Immunochemical Tests (FIT) in the Assessment of Patients Presenting with Lower Bowel Symptoms: Concepts and Challenges. Surgeon 2018, 16, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Ching, J.Y.L.; Chan, V.C.W.; Lam, T.Y.T.; Luk, A.K.C.; Ng, S.S.M.; Sung, J.J.Y. Factors Associated with False-Positive and False-Negative Fecal Immunochemical Test Results for Colorectal Cancer Screening. Gastrointest. Endosc. 2015, 81, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Katsoula, A.; Paschos, P.; Haidich, A.B.; Tsapas, A.; Giouleme, O. Diagnostic Accuracy of Fecal Immunochemical Test in Patients at Increased Risk for Colorectal Cancer Ameta-Analysis. JAMA Intern. Med. 2017, 177, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of Fecal Immunochemical Tests for Colorectal Cancer. Ann. Intern. Med. 2014, 160, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Fedeli, U.; Schievano, E.; Bovo, E.; Guzzinati, S.; Baracco, S.; Fedato, C.; Saugo, M.; Dei Tos, A.P. Impact on Colorectal Cancer Mortality of Screening Programmes Based on the Faecal Immunochemical Test. Gut 2015, 64, 784–790. [Google Scholar] [CrossRef]
- Widlak, M.M.; Neal, M.; Daulton, E.; Thomas, C.L.; Tomkins, C.; Singh, B.; Harmston, C.; Wicaksono, A.; Evans, C.; Smith, S.; et al. Risk Stratification of Symptomatic Patients Suspected of Colorectal Cancer Using Faecal and Urinary Markers. Color. Dis. 2018, 20, O335–O342. [Google Scholar] [CrossRef] [PubMed]
- Widlak, M.M.; Thomas, C.L.; Thomas, M.G.; Tomkins, C.; Smith, S.; O’Connell, N.; Wurie, S.; Burns, L.; Harmston, C.; Evans, C.; et al. Diagnostic Accuracy of Faecal Biomarkers in Detecting Colorectal Cancer and Adenoma in Symptomatic Patients. Aliment. Pharmacol. Ther. 2017, 45, 354–363. [Google Scholar] [CrossRef]
- Ibañez-Sanz, G.; Garcia, M.; Milà, N.; Rodríguez-Moranta, F.; Binefa, G.; Gómez-Matas, J.; Benito, L.; Padrol, I.; Barenys, M.; Moreno, V. False-Negative Rate Cannot Be Reduced by Lowering the Haemoglobin Concentration Cut-off in Colorectal Cancer Screening Using Faecal Immunochemical Test. Eur. J. Cancer Prev. 2017, 26, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The Human Volatilome: Volatile Organic Compounds (VOCs) in Exhaled Breath, Skin Emanations, Urine, Feces and Saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Chandrapalan, S.; Arasaradnam, R.P. Urine as a Biological Modality for Colorectal Cancer Detection. Expert Rev. Mol. Diagn. 2020, 20, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- van Liere, E.L.S.A.; van Dijk, L.J.; Bosch, S.; Vermeulen, L.; Heymans, M.W.; Burchell, G.L.; de Meij, T.G.J.; Ramsoekh, D.; de Boer, N.K.H. Urinary Volatile Organic Compounds for Colorectal Cancer Screening: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2023, 186, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Non-Invasive Detection and Staging of Colorectal Cancer Using a Portable Electronic Nose. Sensors 2021, 21, 5440. [Google Scholar] [CrossRef]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile Organic Compounds as New Biomarkers for Colorectal Cancer: A Review. Color. Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef]
- Chandrapalan, S.; Bosch, S.; Cubiella, J.; Guardiola, J.; Kimani, P.; Mulder, C.; Persaud, K.; de Meij, T.G.J.; Altomare, D.F.; Brenner, H.; et al. Systematic Review with Meta-Analysis: Volatile Organic Compound Analysis to Improve Faecal Immunochemical Testing in the Detection of Colorectal Cancer. Aliment. Pharmacol. Ther. 2021, 54, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Feil, C.; Staib, F.; Berger, M.R.; Stein, T.; Schmidtmann, I.; Forster, A.; Schimanski, C.C. Sniffer Dogs Can Identify Lung Cancer Patients from Breath and Urine Samples. BMC Cancer 2021, 21, 917. [Google Scholar] [CrossRef]
- Costantini, M.; Filianoti, A.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Ferriero, M.; Mastroianni, R.; Misuraca, L.; Tuderti, G.; Ciliberto, G.; et al. Human Urinary Volatilome Analysis in Renal Cancer by Electronic Nose. Biosensors 2023, 13, 427. [Google Scholar] [CrossRef]
- Goertzen, A.; Kidane, B.; Ahmed, N.; Aliani, M. Potential Urinary Volatile Organic Compounds as Screening Markers in Cancer—A Review. Front. Oncol. 2024, 14, 1448760. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Meng, S.; Arao, Y.; Saito, Y.; Inoue, K.; Alshammari, A.H.; Hatakeyama, H.; di Luccio, E.; Vecchione, A.; Hirotsu, T.; et al. Non-Invasive Detection of Tumors by Volatile Organic Compounds in Urine. Biomedicines 2025, 13, 109. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Quraishi, N.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. A Novel Tool for Noninvasive Diagnosis and Tracking of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and Application of a New Electronic Nose Instrument for the Detection of Colorectal Cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Mcfarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of Colorectal Cancer (CRC) by Urinary Volatile Organic Compound Analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef]
- Covington, J.A.; Van Der Schee, M.P.; Edge, A.S.L.; Boyle, B.; Savage, R.S.; Arasaradnam, R.P. The Application of FAIMS Gas Analysis in Medical Diagnostics. Analyst 2015, 140, 6775–6781. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; Marco, S.; Persaud, K.C.; Schiffman, S.S.; Nagle, H.T. Artificial Olfaction in the 21 St Century. IEEE Sens. J. 2021, 21, 12969–12990. [Google Scholar] [CrossRef]
- Murdocca, M.; Torino, F.; Pucci, S.; Costantini, M.; Capuano, R.; Greggi, C.; Polidoro, C.; Somma, G.; Pasqualetti, V.; Mougang, Y.K.; et al. Urine Lox-1 and Volatilome as Promising Tools towards the Early Detection of Renal Cancer. Cancers 2021, 13, 4213. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, M.H.M.C.; Al-Difaie, Z.; Brandts, L.; Peeters, A.; Van Grinsven, B.; Bouvy, N.D. Diagnostic Performance of Electronic Noses in Cancer Diagnoses Using Exhaled Breath: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, E2219372. [Google Scholar] [CrossRef]
- Gasparri, R.; Sedda, G.; Spaggiari, L. The Electronic Nose’s Emerging Role in Respiratory Medicine. Sensors 2018, 18, 3029. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Developing Electronic-Nose Technologies for Clinical Practice. J. Med. Surg. Pathol. 2018, 3, 168–170. [Google Scholar] [CrossRef]
- Wilson, A.D. Future Applications of Electronic-Nose Technologies in Healthcare and Biomedicine; Wide Spectra of Quality Control; InTech Publishing: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef][Green Version]
- Lai, S.Y.; Deffenderfer, O.F.; Hanson, W.; Phillips, M.P.; Thaler, E.R. Identification of Upper Respiratory Bacterial Pathogens with the Electronic Nose. Laryngoscope 2002, 112, 975–979. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [PubMed]
- Bannaga, A.S.; Kvasnik, F.; Persaud, K.; Asaradanam, R.P. Differentiating Cancer Types Using a Urine Test for Volatile Organic Compounds. J. Breath Res. 2020, 15, 017102. [Google Scholar] [CrossRef] [PubMed]
- Bannaga, A.S.; Tyagi, H.; Daulton, E.; Covington, J.A.; Arasaradnam, R.P. Exploratory Study Using Urinary Volatile Organic Compounds for the Detection of Hepatocellular Carcinoma. Molecules 2021, 26, 2447. [Google Scholar] [CrossRef]
- Amos, G.C.A.; Sergaki, C.; Logan, A.; Iriarte, R.; Bannaga, A.; Chandrapalan, S.; Wellington, E.M.H.; Rijpkema, S.; Arasaradnam, R.P. Exploring How Microbiome Signatures Change across Inflammatory Bowel Disease Conditions and Disease Locations. Sci. Rep. 2021, 11, 18699. [Google Scholar] [CrossRef]
- NG12 Suspected Cancer: Recognition and Referral; Full Guideline; National Institute for Health and Care Excellence: London, UK, 2015.
- Pawliszyn, J. Solid Phase Microextraction. Adv. Exp. Med. Biol. 2001, 488, 73–87. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Ma, R.; Zhuo, J.; Zeng, Y.; Wu, P.; Chu, J. A Novel High Accuracy Fast Gas Detection Algorithm Based on Multi-Task Learning. Measurement 2024, 228, 114383. [Google Scholar] [CrossRef]
- Jollife, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.; Perera, A.; Montoliu, I.; Chaudry, A.; Persaud, K.; Marco, S. Drift Compensation of Gas Sensor Array Data by Orthogonal Signal Correction. Chemom. Intell. Lab. Syst. 2010, 100, 28–35. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; He, X.; Yan, R.; Li, Z.; Jiang, Y.; Li, X. Improved Deep Bidirectional Recurrent Neural Network for Learning the Cross-Sensitivity Rules of Gas Sensor Array. Sens. Actuators B Chem. 2024, 401, 134996. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, C.; Niu, G.; Zhuang, Y.; Wang, F. Gas Sensor Array with Pattern Recognition Algorithms for Highly Sensitive and Selective Discrimination of Trimethylamine. Adv. Intell. Syst. 2022, 4, 2200169. [Google Scholar] [CrossRef]
- Evans, P.; Persaud, K.C.; McNeish, A.S.; Sneath, R.W.; Hobson, N.; Magan, N. Evaluation of a Radial Basis Function Neural Network for the Determination of Wheat Quality from Electronic Nose Data. Sens. Actuators B Chem. 2000, 69, 348–358. [Google Scholar] [CrossRef]
- Bridle, J.S. Probabilistic Interpretation of Feedforward Classification Network Outputs, with Relationships to Statistical Pattern Recognition. In Neurocomputing; Soulie, F.F., Herault, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 227–236. [Google Scholar]



| Population | Total Patient Urine Samples | Mean Age (Years) | Male | Female | Mean Height (cm) | Mean Weight (kg) | Mean BMI | Alcohol Users | Smokers | Current Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| RECEDE (total) | 1681 | 61.0 (sd 13.0) | 870 (51.82%) | 1101 (65.5%) | 168.6 (sd 17.3) | 79.6 (sd 18.2) | 28.1 (sd 6.2) | 966 (57.5%) | 1092 (55.4%) | 1209 (71.9%) |
| RECEDE NEGATIVE controls | 763 | 60.4 (sd 12.6) | 314 | 456 | 168 (sd 10) | 80.9 (sd 50.3) | 28.5 (sd 14.7) | 437 (56.7%) | 301 (39.4%) | 554 (72%) |
| FAMISHED controls | 22 | 36.1 | 8 (36.4%) | 14 (63.6%) | N/A | N/A | 23.2 | 12 (54.5%) | 4 (18.2%) | N/A |
| FAMISHED CRC | 53 | 67.3 | 33 (62.3%) | 20 (37.7%) | N/A | N/A | 27.2 | 30 (56.6%) | 6 (11.3%) | N/A |
| Disease Characteristics | % Prevalence |
|---|---|
| Colorectal cancer (CRC) | 4.2 |
| Crohn’s disease (IBD) | 1.3 |
| Ulcerative colitis (IBD) | 3.4 |
| Polyps (low risk) | 52.8 |
| Polyps > 10 mm or high-grade dysplasia < 10 mm, sessile serrated polyps—classed as high-risk polyps/adenomas (HRA) | 22.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arasaradnam, R.P.; Krishnamoorthy, A.; Hull, M.A.; Wheatstone, P.; Kvasnik, F.; Persaud, K.C. The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer. Sensors 2025, 25, 599. https://doi.org/10.3390/s25030599
Arasaradnam RP, Krishnamoorthy A, Hull MA, Wheatstone P, Kvasnik F, Persaud KC. The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer. Sensors. 2025; 25(3):599. https://doi.org/10.3390/s25030599
Chicago/Turabian StyleArasaradnam, Ramesh P., Ashwin Krishnamoorthy, Mark A. Hull, Peter Wheatstone, Frank Kvasnik, and Krishna C. Persaud. 2025. "The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer" Sensors 25, no. 3: 599. https://doi.org/10.3390/s25030599
APA StyleArasaradnam, R. P., Krishnamoorthy, A., Hull, M. A., Wheatstone, P., Kvasnik, F., & Persaud, K. C. (2025). The Development and Optimisation of a Urinary Volatile Organic Compound Analytical Platform Using Gas Sensor Arrays for the Detection of Colorectal Cancer. Sensors, 25(3), 599. https://doi.org/10.3390/s25030599







