Low-Temperature Stress-Induced Changes in Cucumber Plants—A Near-Infrared Spectroscopy and Aquaphotomics Approach for Investigation
Abstract
1. Introduction
2. Materials and Methods
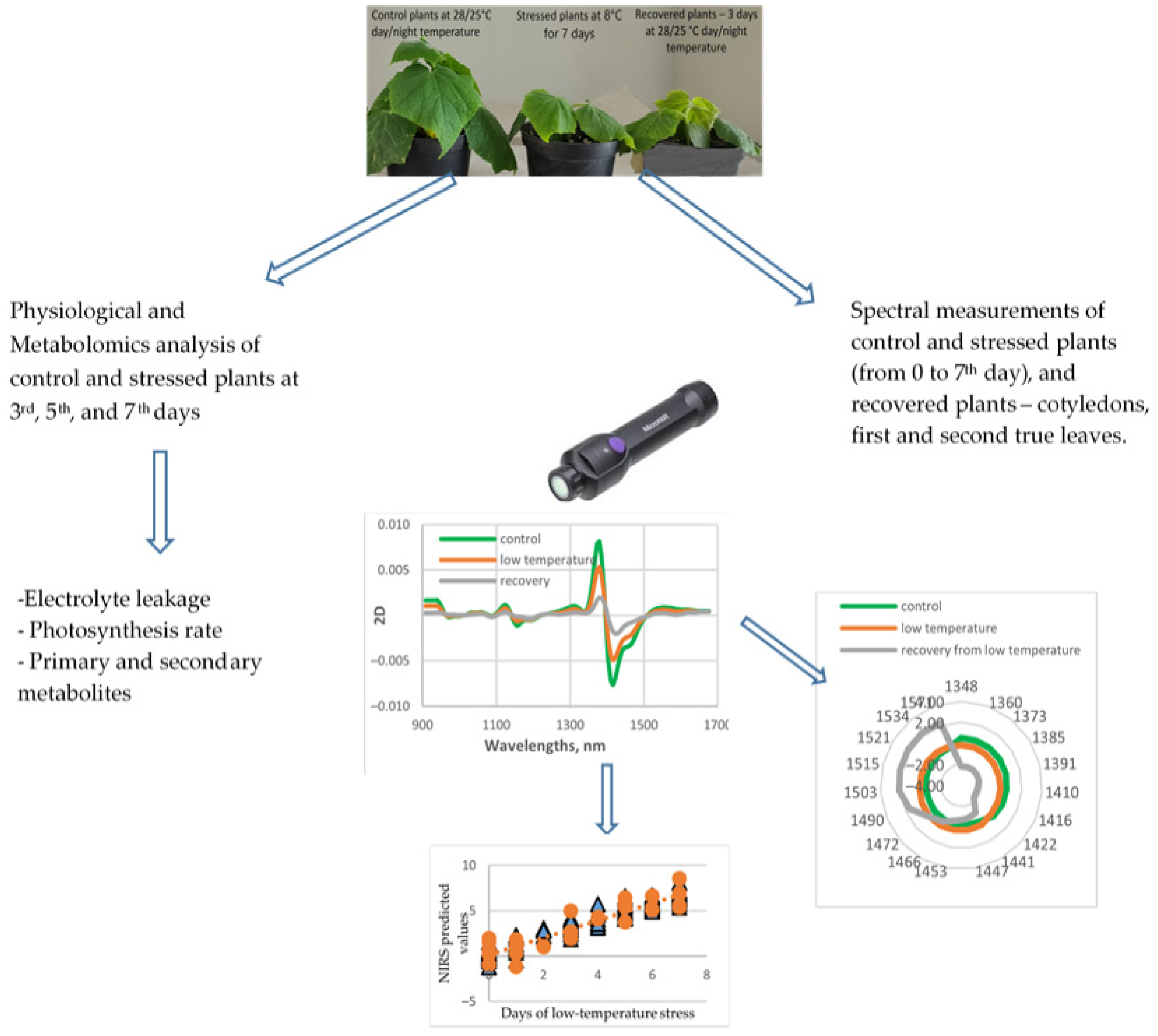
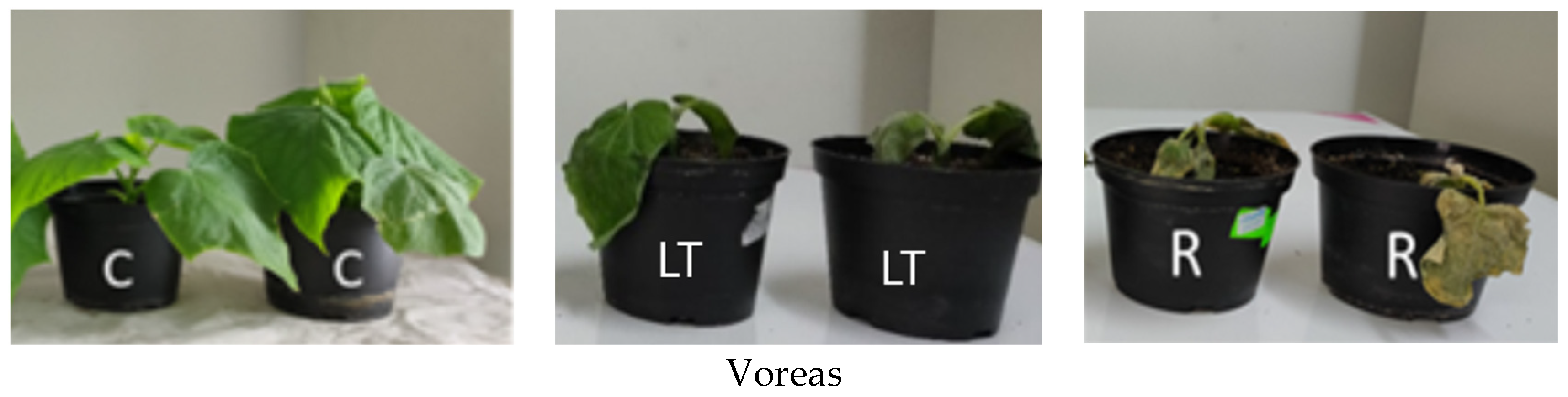
2.1. Plant Materials and Experimental Conditions
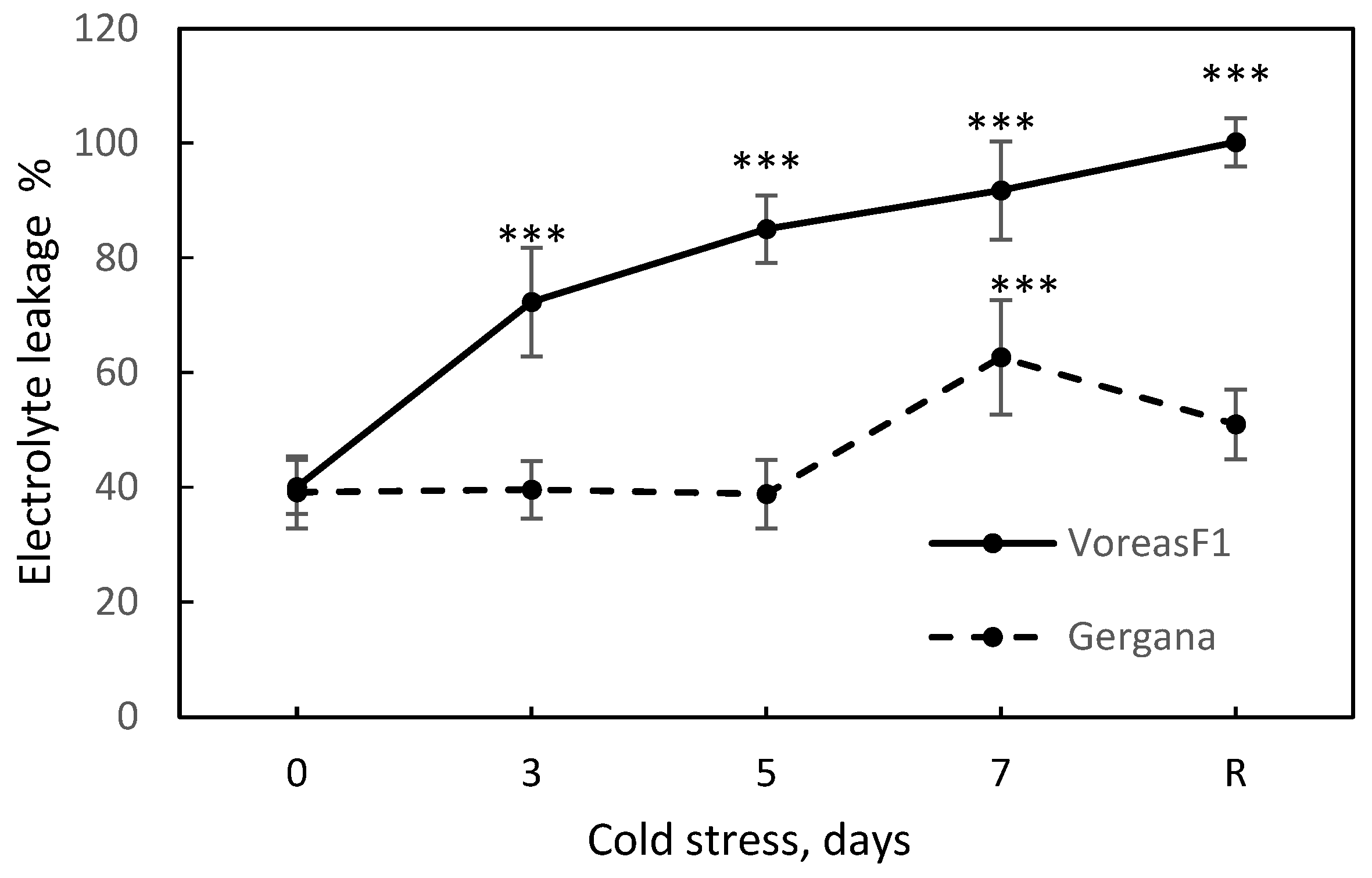
2.2. Electrolyte Leakage
2.3. Measurement of Photosynthesis Rate
2.4. GC-MS Analysis of Primary and Secondary Metabolites
2.5. Statistical Analysis
2.6. Spectral Measurement
2.7. Spectral Data Processing
2.8. Aquaphotomics Analysis
3. Results and Discussion
3.1. Effect of Chilling Stress on Electrolyte Leakage and Photosynthetic Activity
3.2. Metabolomics Changes During Chilling Stress
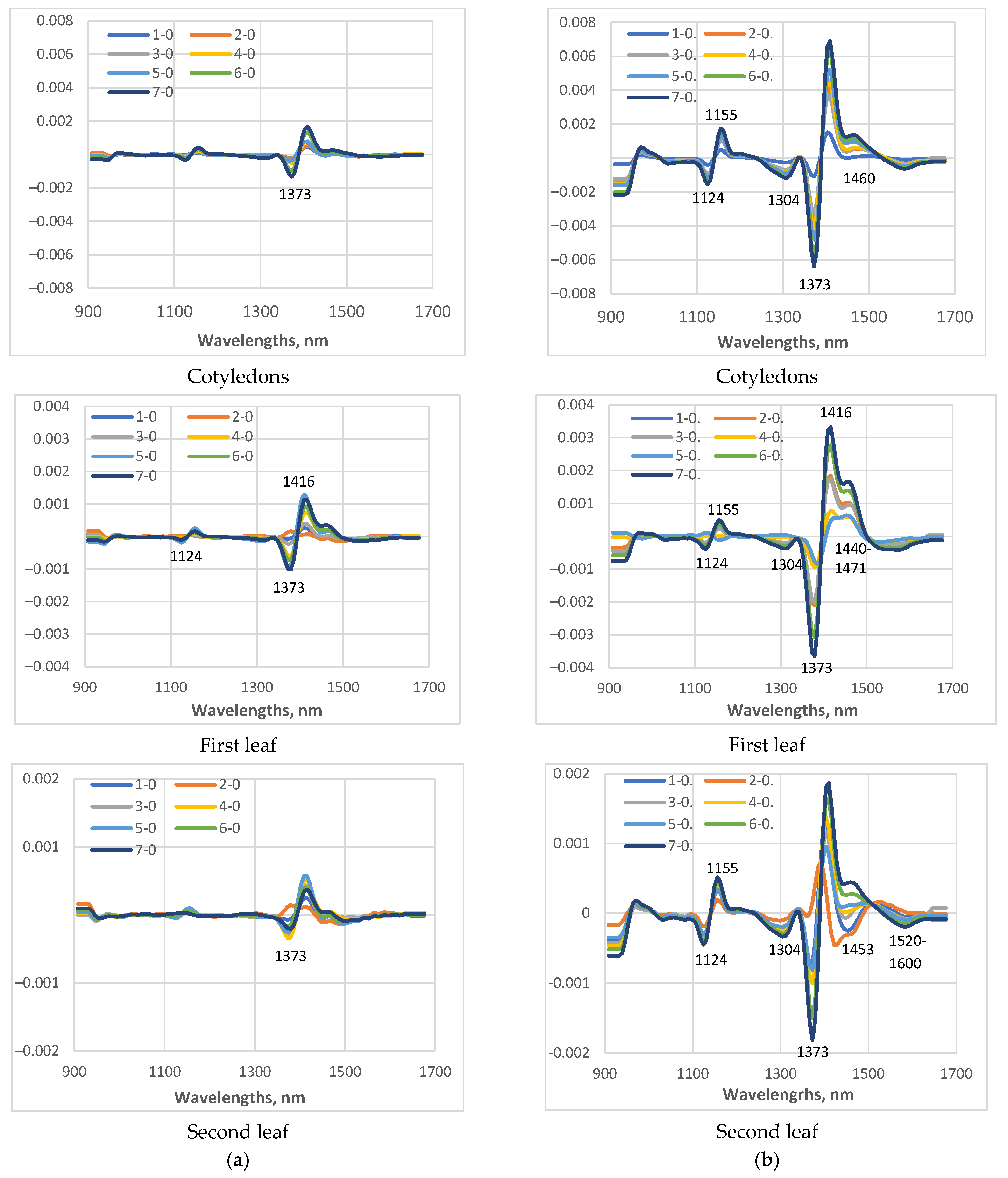
3.3. Near-Infrared Spectra of the Cucumber Plant During Cold Stress
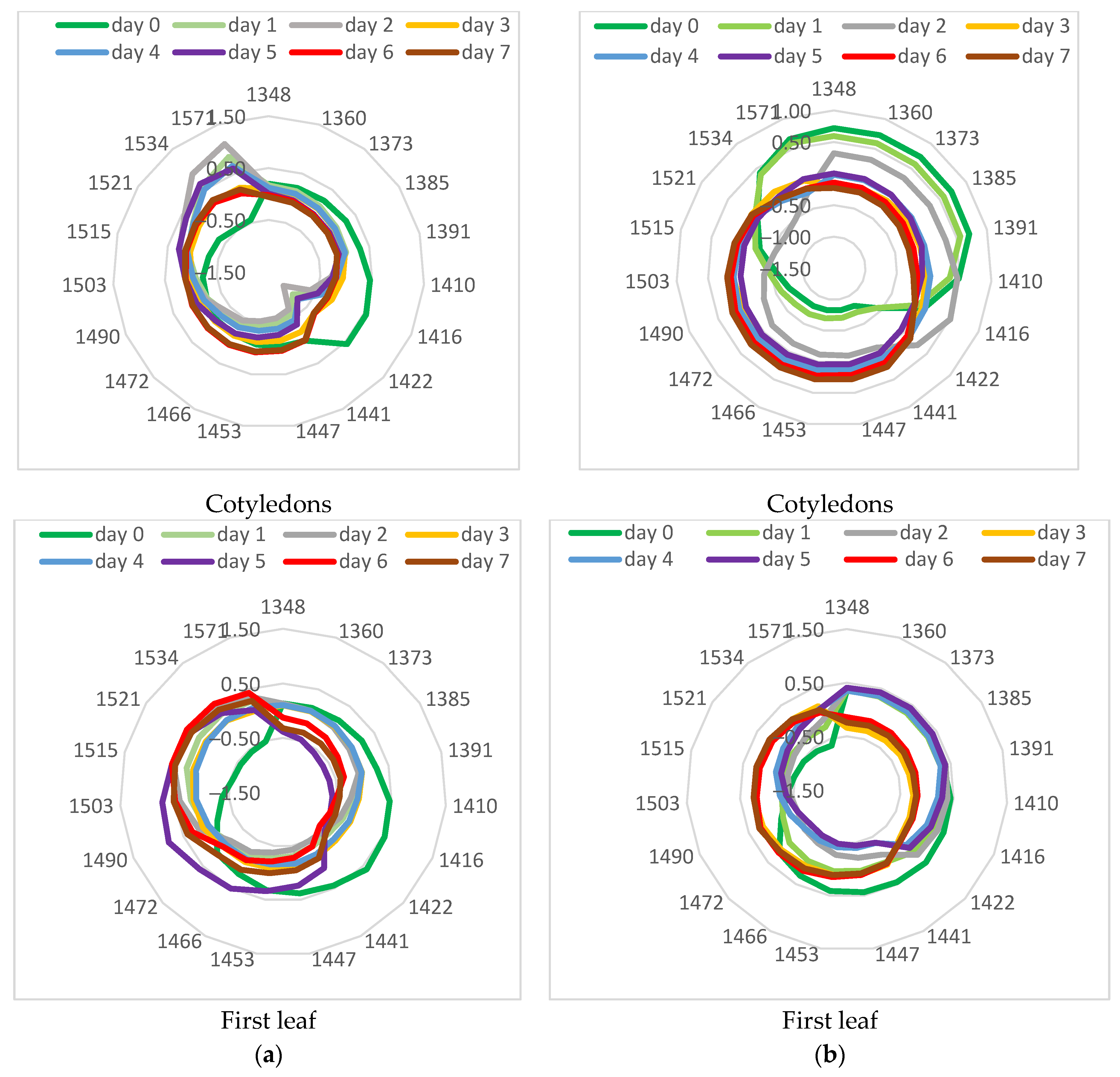
3.4. Aquagram Analysis
3.5. PLS Models for the Determination of Days of Cold Stress
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIR | Near-infrared |
| LT | Low temperature |
| Pn | Net photosynthesis rate |
| E | Transpiration rate |
| EL | Electrolyte leakage |
| C | Stomatal conductance |
| PLS | Partial Least Squares regression |
| Rcal | Multiple correlation coefficients between reference values and NIR predicted values calibration |
| SEC | Standard error of calibration |
| Rcv | Multiple correlation coefficients between reference values and NIR predicted values for cross-validation |
| SECV | Standard error of cross-validation |
| SEP | Standard error of prediction |
References
- Smiti, K.; Mina, U.; Verma, M.; Arya, L. Impact of climate extremes and other key abiotic stresses on cucurbits: A systematic review. Vegetos 2025, 1–21. [Google Scholar] [CrossRef]
- FAOSTAT. Statistics Database of Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#compare (accessed on 21 October 2025).
- Sheng, Y.; Pan, Y.; Li, Y.; Yang, L.; Weng, Y. Quantitative trait loci for fruit size and flowering time-related traits under domestication and diversifying selection in cucumber (Cucumis sativus). Plant Breed. 2019, 139, 176–191. [Google Scholar] [CrossRef]
- Olechowska, E.; Słomnicka, R.; Kaźmińska, K.; Olczak-Woltman, H.; Bartoszewski, G. The genetic basis of cold tolerance in cucumber (Cucumis sativus L.)—The latest developments and perspectives. J. Appl. Genet. 2022, 63, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Sarwar, R.; Zhang, W.; Geng, R.; Zhu, K.-M.; Tan, X.-L. Research progress on the physiological response and molecular mechanism of cold response in plants. Front. Plant Sci. 2024, 15, 1334913. [Google Scholar] [CrossRef]
- Saltveit, M.E., Jr.; Morris, L.L. Overview on Chilling Injury of Horticultural Crops. In Chilling Injury of Horticultural Crops; Wang, C.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 3–16. [Google Scholar]
- Lukatkin, A.S.; Brazaityte, A.; Bobinas, Č.; Duchovskis, P. Chilling injury in chilling-sensitive plants: A review. Žemdirbystė-Agriculture 2012, 99, 111–124. [Google Scholar]
- Zhang, Y.; He, H.; Song, M.; Chen, A.; Chen, M.; Lin, W.; Yang, J.; Luo, D.; Ye, J.; Xu, F. Advances in Physiological and Molecular Mechanisms of Cucumber Response to Low-Temperature Stress. Horticulturae 2025, 11, 1268. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is essential for cucumber survival under cold stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Luo, J.; Wang, C. Quantifying Chilling Injury on the Photosynthesis System of Strawberries: Insights from Photosynthetic Fluorescence Characteristics and Hyperspectral Inversion. Plants 2023, 12, 3138. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Wang, X.; Meng, H.; Ghani, M.I.; Ali, M.; Ding, Y.; Li, X.; Cheng, Z. Effect of low temperature and high humidity stress on physiology of cucumber at different leaf stages. Plant Biol. 2021, 23, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [PubMed]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Kacira, M.; Bartzanas, T.; Kittas, C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Zubler, A.V.; Yoon, J.-Y. Proximal Methods for Plant Stress Detection Using Optical Sensors and Machine Learning. Biosensors 2020, 10, 193. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Jamlos, M.F.; Omar, A.F.; Jamlos, M.A.; Mamat, R.; Muncan, J.; Tsenkova, R. Review—Plant nutritional status analysis employing the visible and near-infrared spectroscopy spectral sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123273. [Google Scholar] [CrossRef]
- Krishnamoorthi, S.; Koh, S.S.; Ang, M.C.; Teo, M.J.T.; Jie, R.A.; Dinish, U.S.; Strano, M.S.; Urano, D. Advancements in Plant Diagnostic and Sensing Technologies. Adv. Sens. Res. 2025, 4, e00045. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Park, S.H.; Lee, M.-A.; Chung, Y.B.; Yang, J.H.; Cho, J.-S.; Min, S.G. Chemometrics and neural networks for estimating the chilling injury severity of kimchi cabbage (Brassica rapa L. ssp. pekinensis) based on hyperspectral images. LWT 2024, 207, 116601. [Google Scholar] [CrossRef]
- Pipatsart, N.; Meenune, M.; Hoonlor, A.; Niamsiri, N.; Punyasuk, N.; Mairhofer, S.; Lertsiri, S. Application of short-wave infrared hyperspectral imaging combined with machine learning on chilling injury detection in fresh coriander. Food Control. 2025, 172, 111176. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Dynamic Spectroscopy of Aqueous and Biological Systems Describes Peculiarities of Water. J. Near Infrared Spectrosc. 2009, 17, 303–313. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of Aquaphotomics and Its Chemometrics Approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef]
- Muncan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [PubMed]
- Jinendra, B.; Tamaki, K.; Kuroki, S.; Vassileva, M.; Yoshida, S.; Tsenkova, R. Near infrared spectroscopy and aquaphotomics: Novel approach for rapid in vivo diagnosis of virus infected soybean. Biochem. Biophys. Res. Commun. 2010, 397, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Jinendra, B.M.S.; Kuroki, S.; Tsenkova, R. Aquaphotomics Research of Cold Stress in Soybean Cultivars with Different Stress Tolerance Ability: Early Detection of Cold Stress Response. Molecules 2022, 27, 744. [Google Scholar] [CrossRef]
- Moyankova, D.; Stoykova, P.; Veleva, P.; Christov, N.K.; Petrova, A.; Atanassova, S. An Aquaphotomics Approach for Investigation of Water-Stress-Induced Changes in Maize Plants. Sensors 2023, 23, 9678. [Google Scholar] [CrossRef]
- Vitalis, F.; Muncan, J.; Anantawittayanon, S.; Kovacs, Z.; Tsenkova, R. Aquaphotomics Monitoring of Lettuce Freshness during Cold Storage. Foods 2023, 12, 258. [Google Scholar] [CrossRef]
- Muncan, J.; Anantawittayanon, S.; Furuta, T.; Kaneko, T.; Tsenkova, R. Aquaphotomics monitoring of strawberry fruit during cold storage—A comparison of two cooling systems. Front. Nutr. 2022, 9, 1058173. [Google Scholar] [CrossRef]
- Dong, X.; Bi, H.; Wu, G.; Ai, X. Drought-induced chilling tolerance in cucumber involves membrane stabilisation improved by antioxidant system. Int. J. Plant Prod. 2013, 7, 67–80. [Google Scholar] [CrossRef]
- Zaharieva, A.; Rusanov, K.; Rusanova, M.; Paunov, M.; Yordanova, Z.; Mantovska, D.; Tsacheva, I.; Petrova, D.; Mishev, K.; Dobrev, P.I.; et al. Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.). Metabolites 2023, 13, 1099. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Hu, J.; Mao, W. Advances in Cold Stress Response Mechanisms of Cucurbits. Horticulturae 2025, 11, 1032. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Dolma, T.; Phuntsog, N.; Katiyar, A.K.; Chaurasia, O.P.; Stobdan, T. Influence of Production Environments on Phenolic Contents in Cucumber Fruit. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2025, 95, 297–300. [Google Scholar] [CrossRef]
- Angmo, D.; Sharma, S.P.; Kalia, A.; Brar, N.S.; Bhardwaj, V. Effect of Cold Stress on Field Performance, Chlorophyll Fluorescence, Electrolyte Leakage and Leaf Gas Exchange Parameters of Potato (Solanum tuberosum L.) Genotypes. Potato Res. 2023, 66, 641–661. [Google Scholar] [CrossRef]
- Wang, M.; An, H.; Cai, W.; Shao, X. Wavelet Transform Makes Water an Outstanding Near-Infrared Spectroscopic Probe. Chemosensors 2023, 11, 37. [Google Scholar] [CrossRef]
- Persson, F.; Söderhjelm, P.; Halle, B. How proteins modify water dynamics. J. Chem. Phys. 2018, 148, 215103. [Google Scholar] [CrossRef]
- Chatani, E.; Tsuchisaka, Y.; Masuda, Y.; Tsenkova, R. Water Molecular System Dynamics Associated with Amyloidogenic Nucleation as Revealed by Real Time Near Infrared Spectroscopy and Aquaphotomics. PLoS ONE 2014, 9, e101997. [Google Scholar] [CrossRef]
- Tsenkova, R.N.; Iordanova, I.K.; Toyoda, K.; Brown, D.R. Prion protein fate governed by metal binding. Biochem. Biophys. Res. Commun. 2004, 325, 1005–1012. [Google Scholar] [CrossRef]
- Ma, L.; Cui, X.; Cai, W.; Shao, X. Understanding the function of water during the gelation of globular proteins by temperature-dependent near infrared spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 20132–20140. [Google Scholar] [CrossRef]
- Uchida, N.; Yoshimura, N.; Takayanagi, M. Variation of the Near-Infrared Spectrum of Water from Dissolved Salts. J. Solut. Chem. 2015, 44, 2167–2178. [Google Scholar] [CrossRef]
- Tsenkova, R.; Kovacs, Z.; Kubota, Y. Aquaphotomics: Near infrared spectroscopy and water states in biological systems. In Membrane Hydration; DiSalvo, E.A., Ed.; Springer: Cham, Switzerland, 2015; pp. 189–211. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; Taylor & Francis: London, UK, 2007. [Google Scholar]
- Marinoni, L.; Buccheri, M.; Bianchi, G.; Cattaneo, T.M.P. Aquaphotomic, E-Nose and Electrolyte Leakage to Monitor Quality Changes during the Storage of Ready-to-Eat Rocket. Molecules 2022, 27, 2252. [Google Scholar] [CrossRef]
- Moll, V.; Beć, K.B.; Grabska, J.; Huck, C.W. Investigation of Water Interaction with Polymer Matrices by Near-Infrared (NIR) Spectroscopy. Molecules 2022, 27, 5882. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A Review of Band Assignments in near Infrared Spectra of Wood and Wood Components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Kuroki, S.; Tsenkova, R.; Moyankova, D.; Muncan, J.; Morita, H.; Atanassova, S.; Djilianov, D. Water molecular structure underpins extreme desiccation tolerance of the resurrection plant Haberlea rhodopensis. Sci. Rep. 2019, 9, 3049, Correction in Sci. Rep. 2020, 10, 9061. [Google Scholar] [CrossRef]







| Gergana | Voreas F1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | Control | 3 Days | 5 Days | 7 Days | Control | 3 Days | 5 Days | 7 Days |
| Sugars | ||||||||
| D-Fructose sum | 0 | −1.7 * | −2.2 * | −0.2 | 0 | −1.7 ** | −1.1 * | −2.7 ** |
| D-Glucose | 0 | −2.5 *** | −2.6 *** | −0.8 | 0 | −2.8 * | −2.9 * | −3.4 * |
| Sucrose | 0 | −2.1 * | −3.8 * | −2.2 * | 0 | −3.3 ** | −0.5 * | −6.7 ** |
| Myo-Inositol | 0 | −0.2 | −0.1 | 0.1 | 0 | 0.2 | 0.3 | 0.1 |
| Alcohols | ||||||||
| Glycerol | 0 | 0.2 | 0.2 | −0.5 * | 0 | 0.5 | 0.6 | −0.1 |
| Galactinol | 0 | −0.9 ** | −1.0 * | −2.5 ** | 0 | −1.5 ** | −1.6 ** | −2.2 *** |
| Organic acids | ||||||||
| Lactic acid | 0 | 0.0 | 0.0 | −0.1 | 0 | 0.1 | 0.0 | −0.2 |
| Glycolic acid | 0 | −0.3 | −0.2 | −0.3 | 0 | −0.6 ** | −0.5 ** | −0.9 *** |
| Succinic acid | 0 | −0.1 | −0.8 *** | −0.8 *** | 0 | −0.6 * | −0.3 | −0.8 * |
| Glyceric acid | 0 | −2.1 *** | −1.9 *** | −2.4 *** | 0 | −0.7 * | −0.6 | −1.4 *** |
| Fumaric acid | 0 | −0.1 | −0.5 | −0.9 ** | 0 | −1.6 * | −1.2 | −2.0 * |
| Malic acid | 0 | −1.6 ** | −2.7 ** | −2.9 ** | 0 | −2.1 ** | −2.9 ** | −3.2 *** |
| Phenolic acids | ||||||||
| Cinnamic acid | 0 | 0.1 | −0.8 * | 1.1 ** | 0 | −0.8 * | −1.9 ** | −1.5 * |
| Ferulic acid | 0 | 0.1 | 0.4 | 0.2 | 0 | −1.2 * | −1.6 * | n.d. |
| Fatty acids | ||||||||
| Palmitic acid | 0 | −0.2 | 0.2 | 0.2 | 0 | 0.5 | 0.8 ** | 0.1 |
| Stearic acid | 0 | 0.4 | 0.2 | −0.2 | 0 | 1.1 ** | 1.4 ** | 0.5 * |
| 1-Monopalmitin | 0 | −0.6 | −0.5 | −3.7 * | 0 | −0.2 | −0.8 | −1.7 |
| Glycerol Monostearate | 0 | 0.4 * | 0.4 | −1.1 | 0 | −0.2 | −0.4 | −4.0 * |
| Leaves | Calibration Set | Validation Set | |||||
|---|---|---|---|---|---|---|---|
| PLS Factors | SECV | Rcv | SEC | Rcal | SEP | Rval | |
| Cotyledons | 5 | 0.67 | 0.96 | 0.63 | 0.97 | 1.04 | 0.91 |
| First leaf | 2 | 0.81 | 0.94 | 0.80 | 0.95 | 1.03 | 0.91 |
| Second leaf | 3 | 1.04 | 0.91 | 0.99 | 0.92 | 1.03 | 0.91 |
| Leaves | Calibration Set | Validation Set | |||||
|---|---|---|---|---|---|---|---|
| PLS Factors | SECV | Rcv | SEC | Rcal | SEP | Rval | |
| Cotyledons | 2 | 1.14 | 0.84 | 1.07 | 0.87 | 1.14 | 0.86 |
| First leaf | 2 | 0.98 | 0.89 | 0.96 | 0.90 | 1.04 | 0.85 |
| Second leaf | 7 | 1.03 | 0.89 | 0.89 | 0.92 | 0.97 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyankova, D.; Stoykova, P.; Veleva, P.; Christov, N.K.; Petrova, A.; Rusanov, K.; Atanassova, S. Low-Temperature Stress-Induced Changes in Cucumber Plants—A Near-Infrared Spectroscopy and Aquaphotomics Approach for Investigation. Sensors 2025, 25, 7602. https://doi.org/10.3390/s25247602
Moyankova D, Stoykova P, Veleva P, Christov NK, Petrova A, Rusanov K, Atanassova S. Low-Temperature Stress-Induced Changes in Cucumber Plants—A Near-Infrared Spectroscopy and Aquaphotomics Approach for Investigation. Sensors. 2025; 25(24):7602. https://doi.org/10.3390/s25247602
Chicago/Turabian StyleMoyankova, Daniela, Petya Stoykova, Petya Veleva, Nikolai K. Christov, Antoniya Petrova, Krasimir Rusanov, and Stefka Atanassova. 2025. "Low-Temperature Stress-Induced Changes in Cucumber Plants—A Near-Infrared Spectroscopy and Aquaphotomics Approach for Investigation" Sensors 25, no. 24: 7602. https://doi.org/10.3390/s25247602
APA StyleMoyankova, D., Stoykova, P., Veleva, P., Christov, N. K., Petrova, A., Rusanov, K., & Atanassova, S. (2025). Low-Temperature Stress-Induced Changes in Cucumber Plants—A Near-Infrared Spectroscopy and Aquaphotomics Approach for Investigation. Sensors, 25(24), 7602. https://doi.org/10.3390/s25247602








