Simultaneous Measurement of Contractile Force and Ca2+ Concentration Distribution in Human iPS Cell-Derived Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
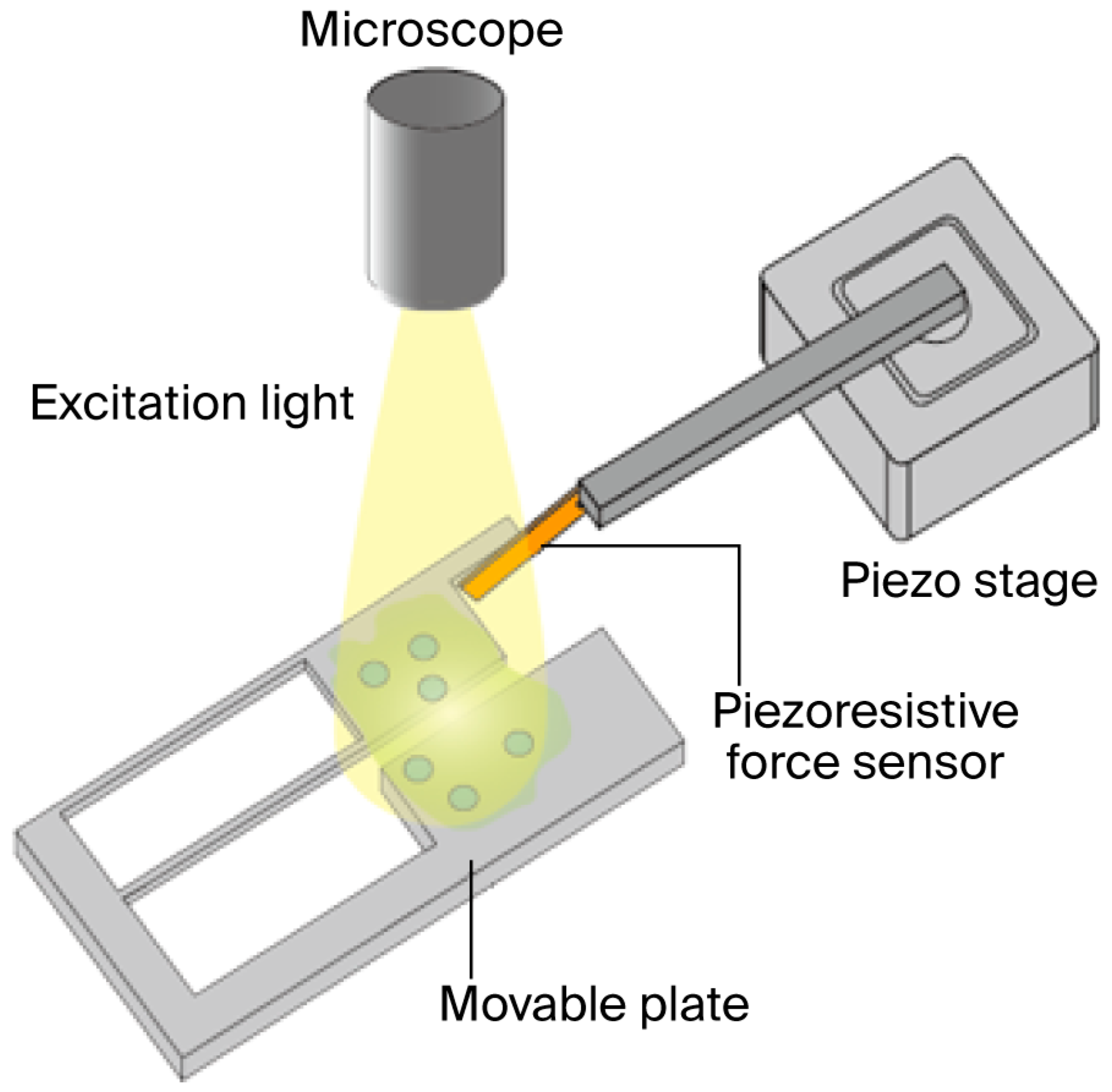
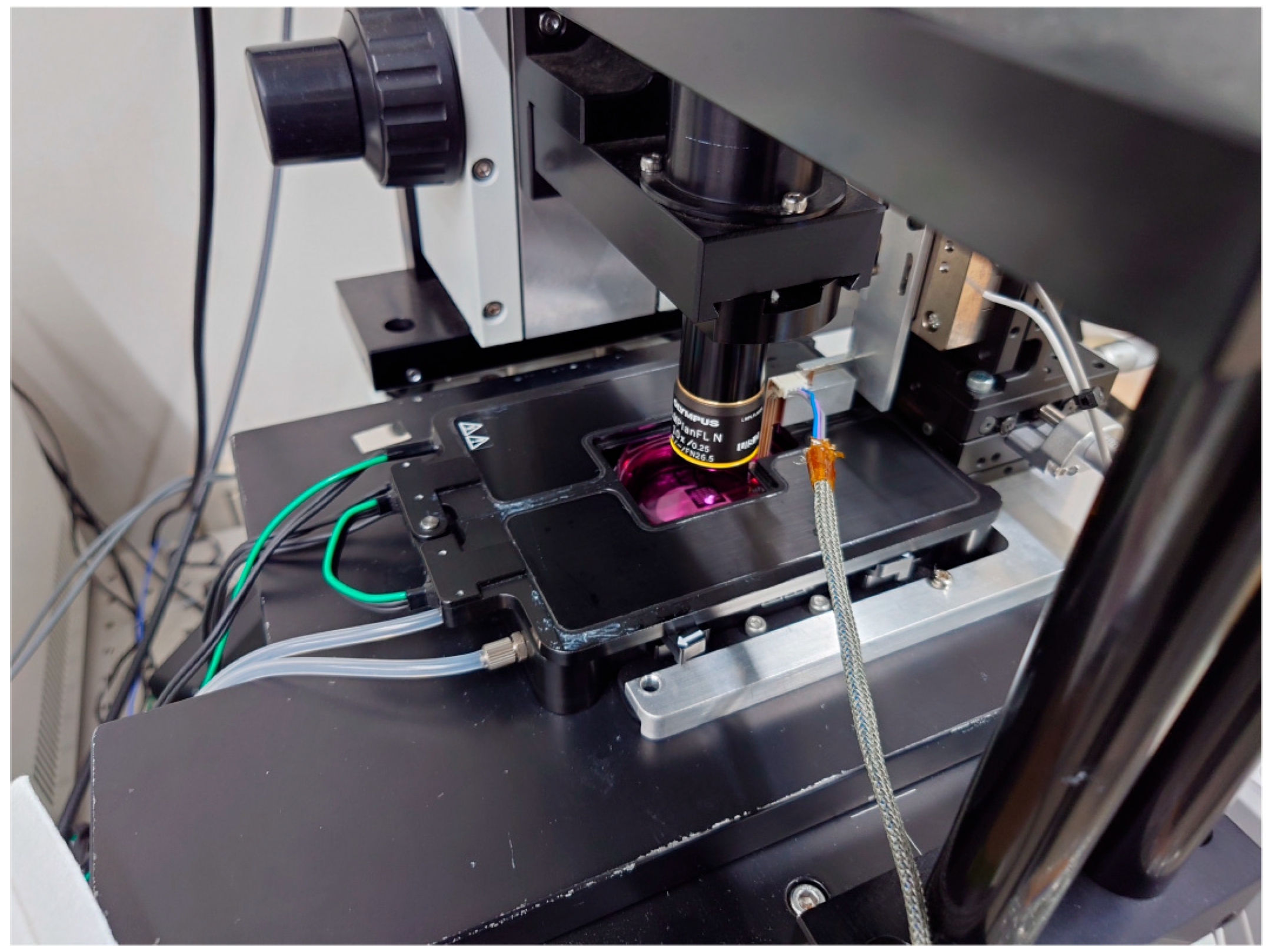
2.1. Measurement System
2.2. Movable Plate
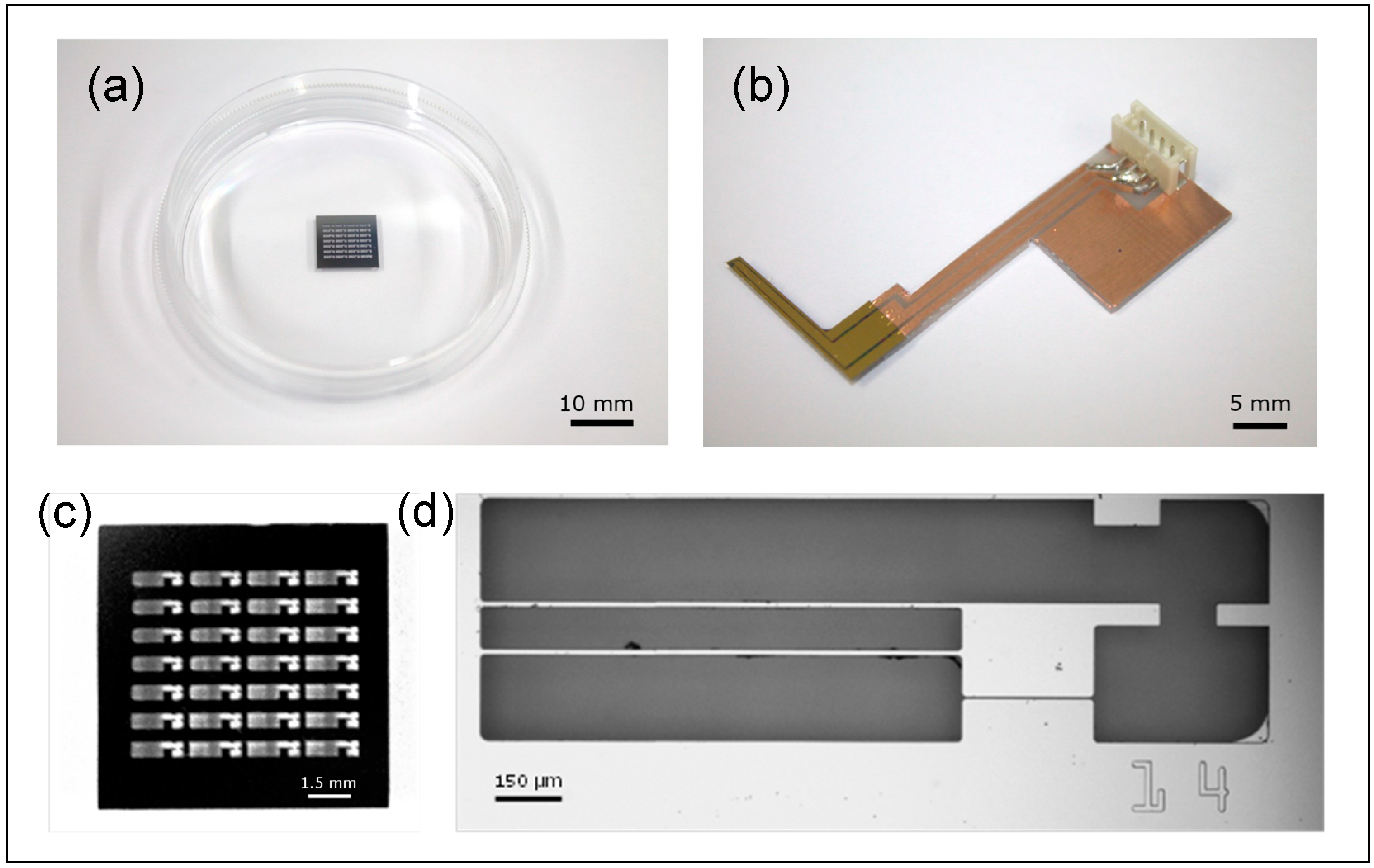
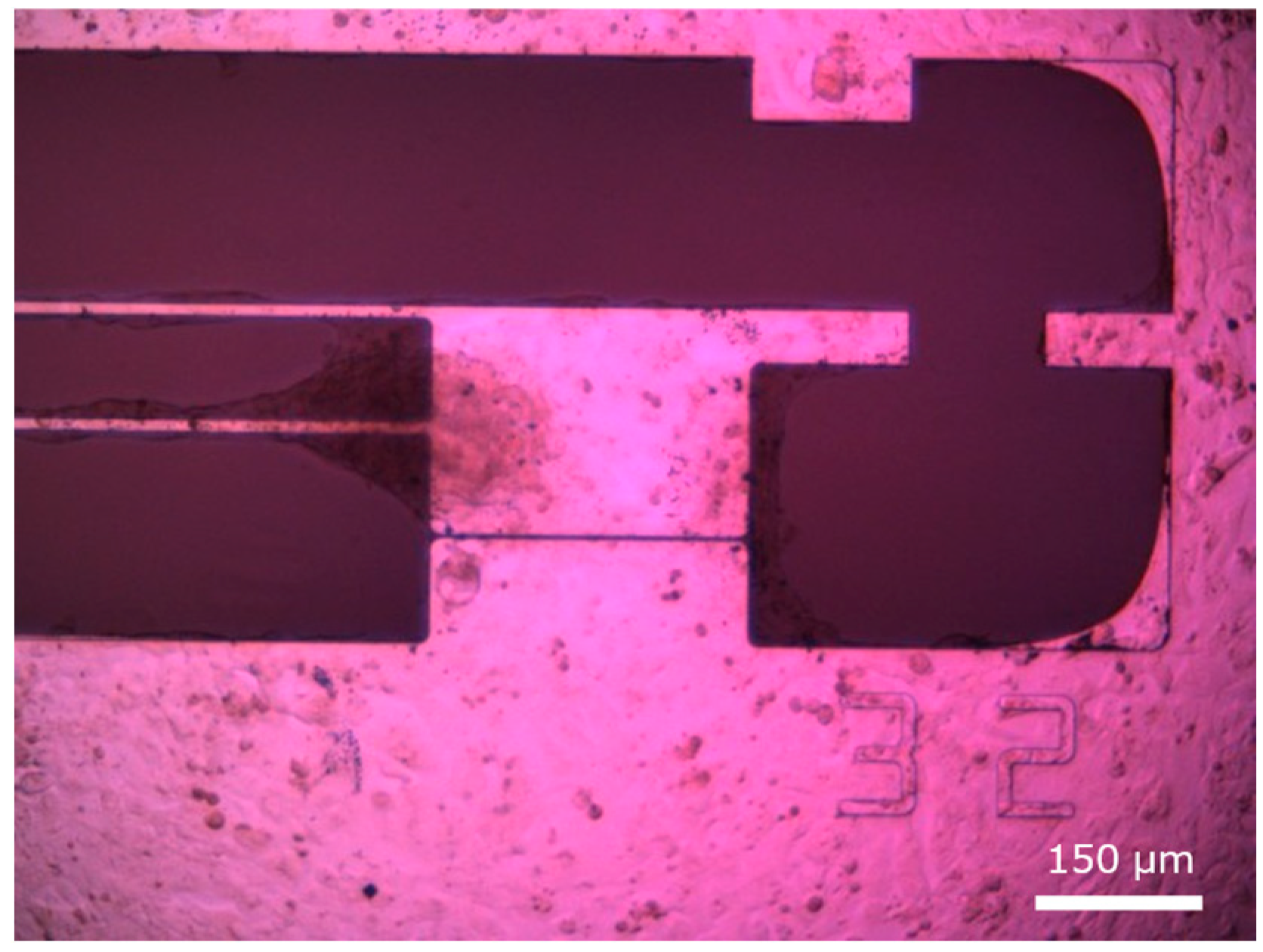
2.3. MEMS Force Sensor
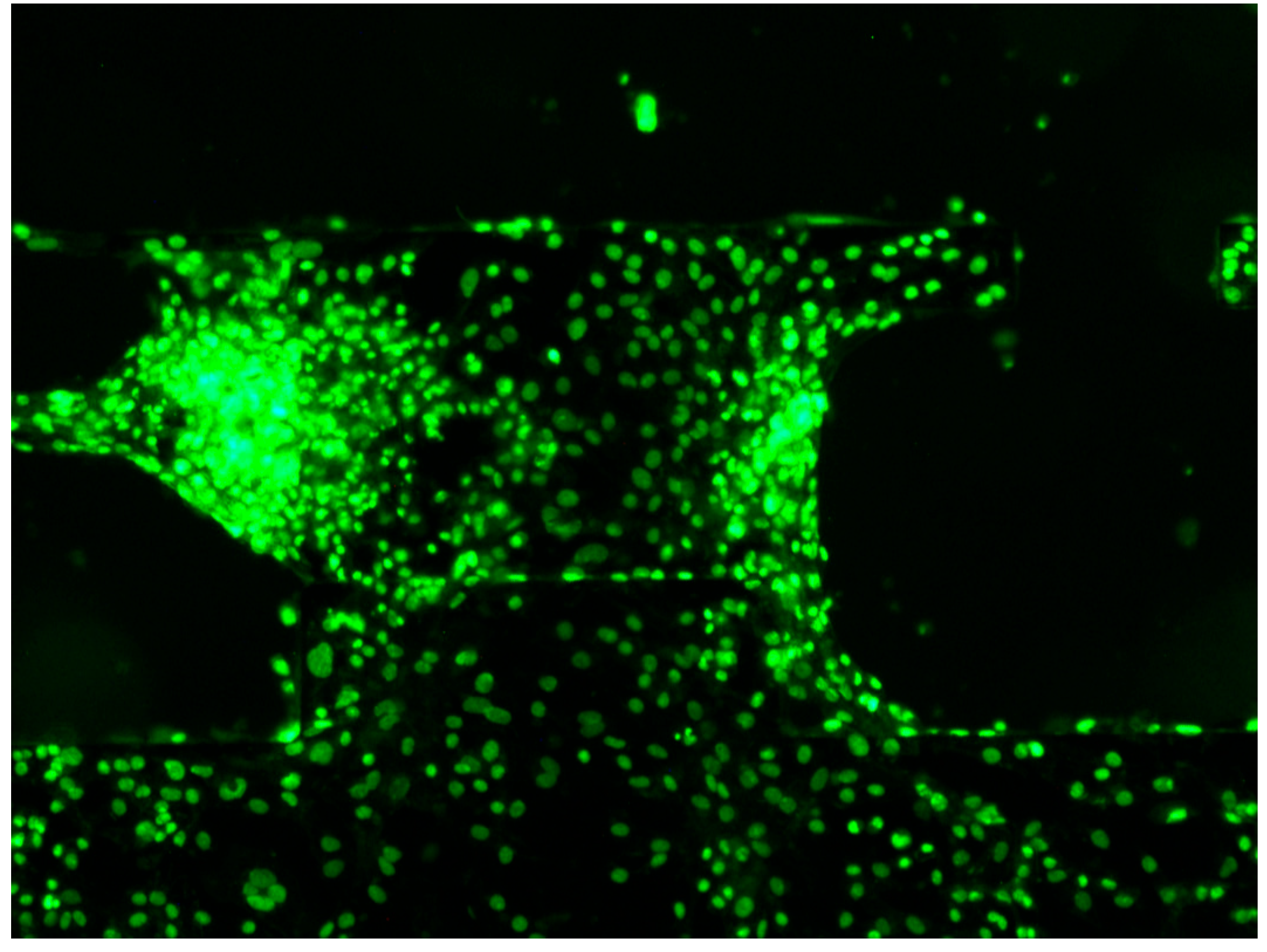
2.4. hiPSC-CM Seeding and Culture
2.5. Visualization of Ca2+ Concentration
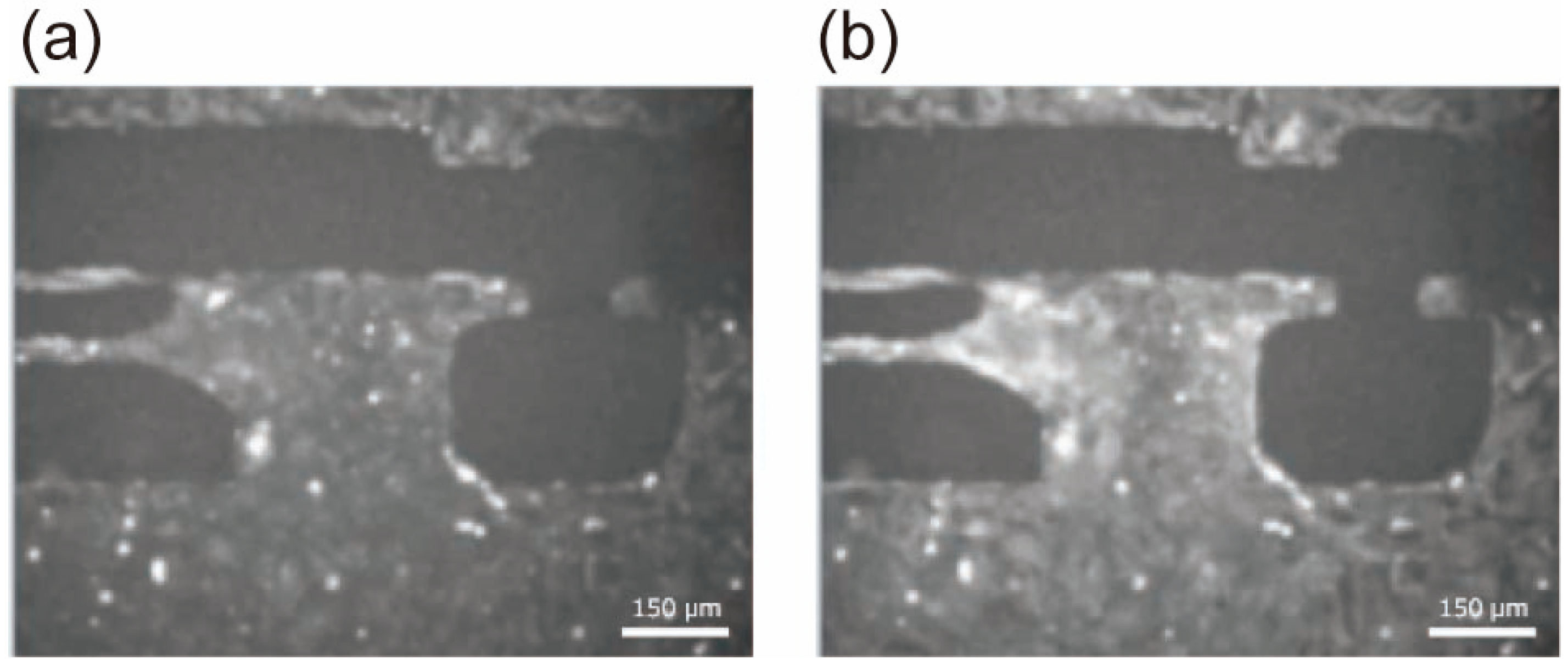
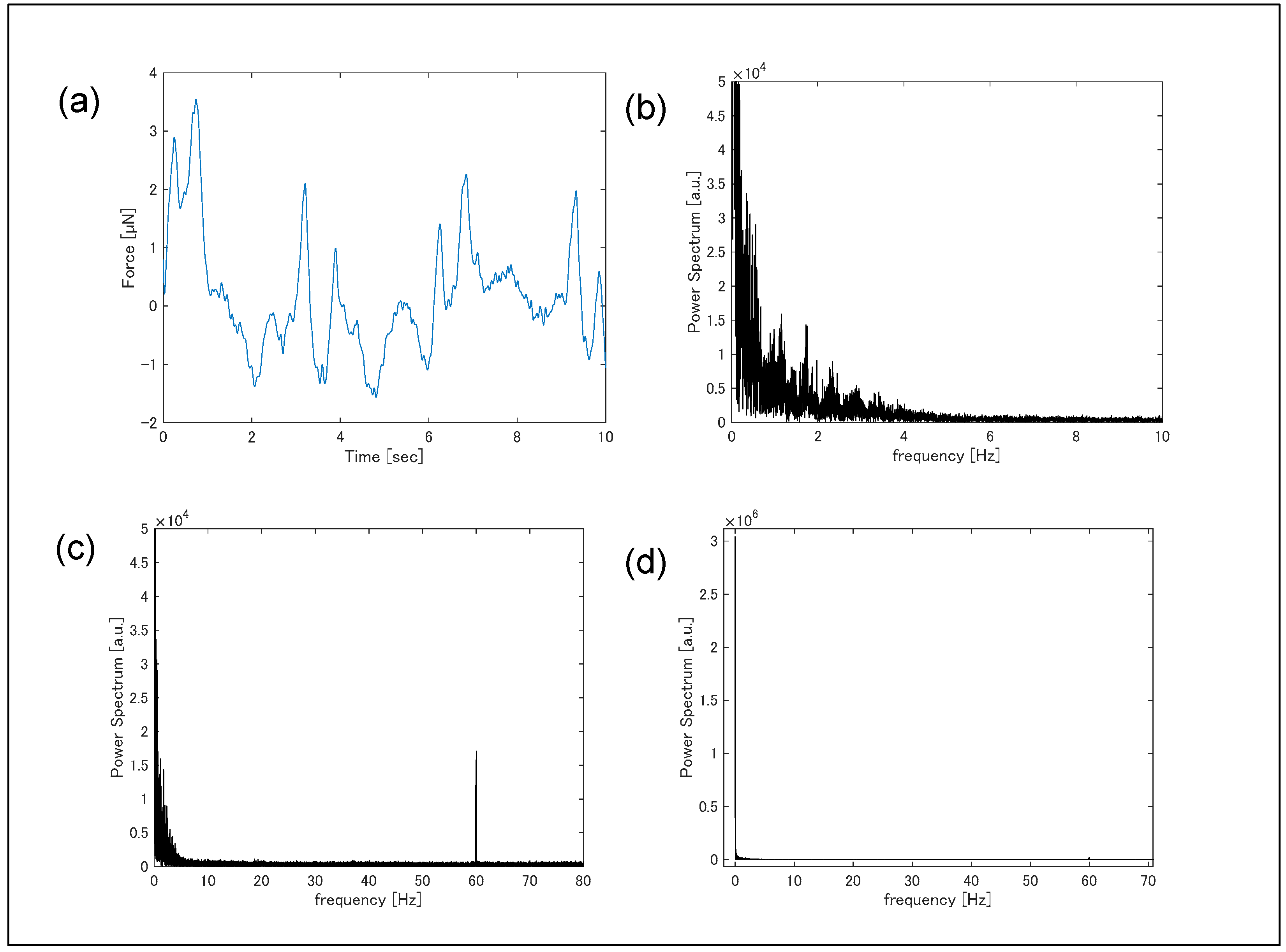
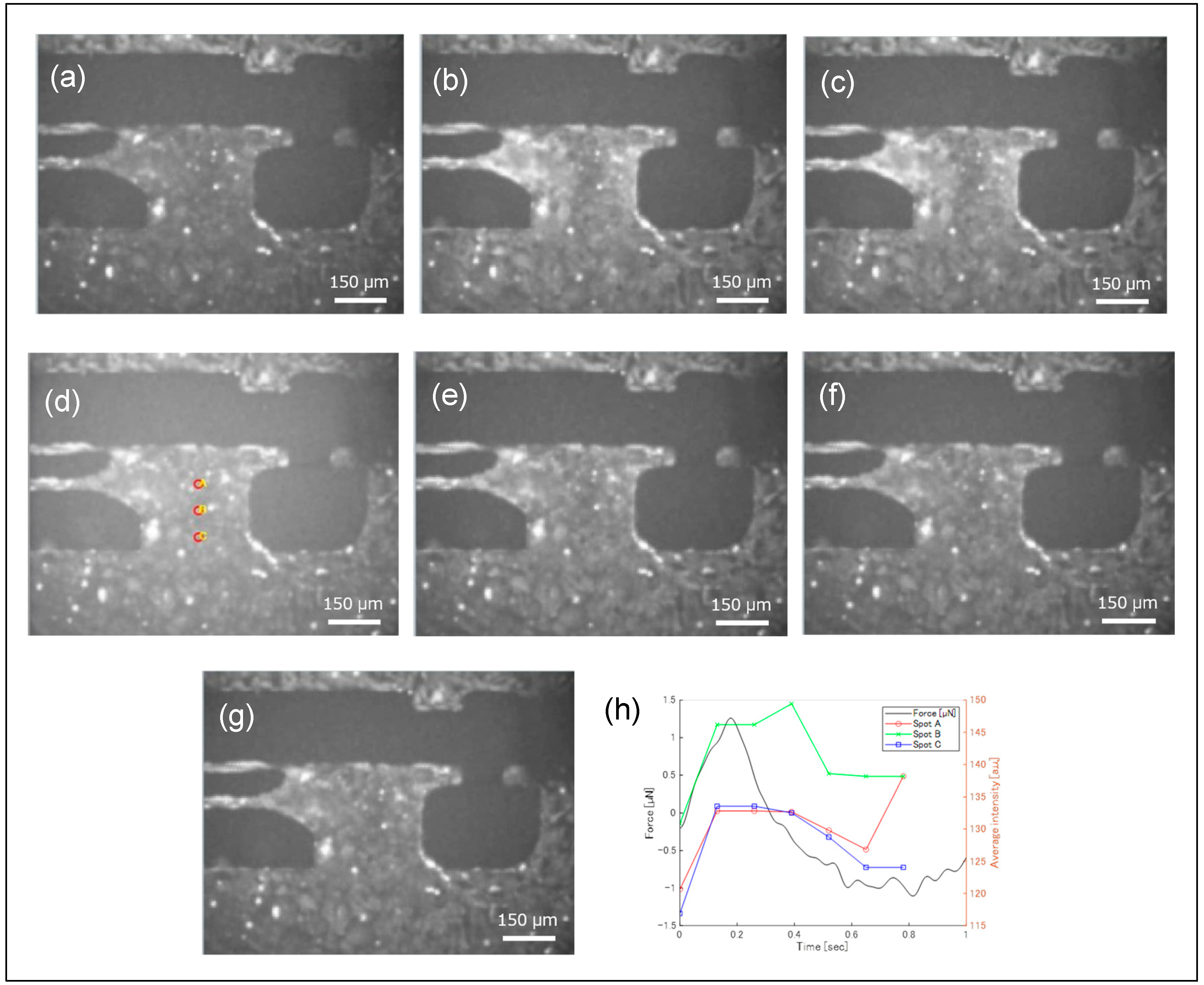
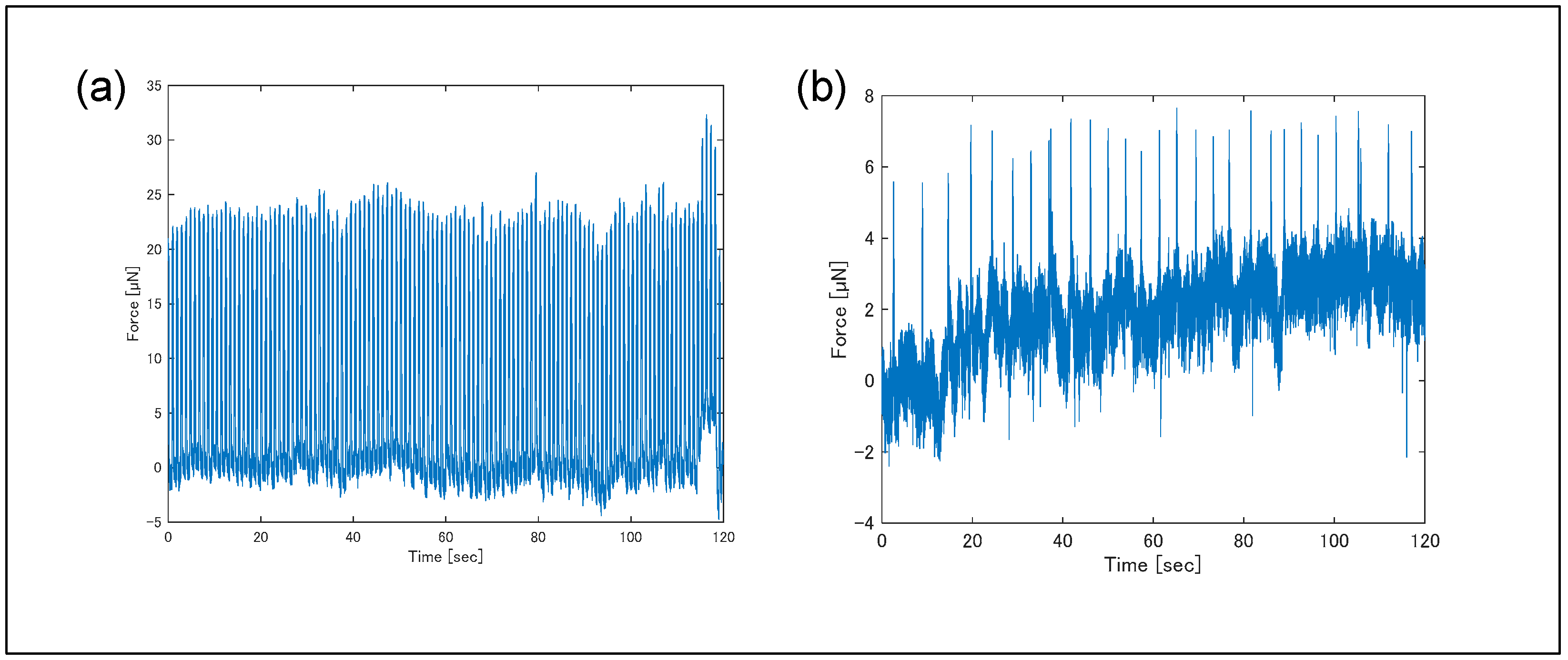
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvakumar, D.; Reyes, L.; Chong, J.J.H. Cardiac Cell Therapy with Pluripotent Stem Cell-Derived Cardiomyocytes: What Has Been Done and What Remains to Do? Curr. Cardiol. Rep. 2022, 24, 445. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, D.; Clayton, Z.E.; Prowse, A.; Dingwall, S.; Kim, S.K.; Reyes, L.; George, J.; Shah, H.; Chen, S.; Leung, H.H.L.; et al. Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Nat. Cardiovasc. Res. 2024, 3, 145. [Google Scholar] [CrossRef]
- Tian, Y.; Lucena-Cacace, A.; Tani, K.; Elvandari, A.P.; Osorio, R.S.A.; Narita, M.; Matsumura, Y.; Paixao, I.C.; Miyoshi, Y.; Inagaki, A.; et al. Generation of mature epicardium derived from human-induced pluripotent stem cells via inhibition of mTOR signaling. Nat. Commun. 2025, 16, 5902. [Google Scholar] [CrossRef]
- Abadi, P.P.S.; Garbern, J.C.; Behzadi, S.; Hill, M.J.; Tresback, J.S.; Heydari, T.; Ejtehadi, M.R.; Ahmed, N.; Copley, E.; Aghaverdi, H.; et al. Engineering of Mature Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Using Substrates with Multiscale Topography. Adv. Funct. Mater. 2018, 28, 170378. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef]
- Wu, P.; Deng, G.; Sai, X.; Guo, H.; Huang, H.; Zhu, P. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. 2021, 41, BSR20200833. [Google Scholar] [CrossRef]
- Liu, J.; Sun, N.; Bruce, M.A.; Wu, J.C.; Butte, M.J. Atomic Force Mechanobiology of Pluripotent Stem Cell-Derived Cardiomyocytes. PLoS ONE 2012, 7, e37559. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, W.; Shao, Y.; Ma, S.; Borg, T.K.; Gao, B.Z. Pulse splitter-based nonlinear microscopy for live-cardiomyocyte imaging. Proc. SPIE 2014, 8948, 89482X. [Google Scholar]
- Hall, M.S.; Long, R.; Feng, X.; Huang, Y.; Hui, C.-Y.; Wu, M. Towards Single Cell Traction Force Microscopy within 3D Collagen Matrices. Exp. Cell Res. 2013, 319, 2396. [Google Scholar] [CrossRef]
- Hazeltine, L.B.; Simmons, C.S.; Salick, M.R.; Lian, X.; Badur, M.G.; Han, W.; Delgado, S.M.; Wakatsuki, T.; Crone, W.C.; Pruitt, B.L.; et al. Effects of Substrate Mechanics on Contractility of Cardiomyocytes Generated from Human Pluripotent Stem Cells. Int. J. Cell Biol. 2012, 2012, 508294. [Google Scholar] [CrossRef] [PubMed]
- Beussman, K.M.; Rodriguez, M.L.; Leonard, A.; Taparia, N.; Thompson, C.R.; Sniadecki, N.J. Micropost arrays for measuring stem cell-derived cardiomyocyte contractility. Methods 2016, 94, 43. [Google Scholar] [CrossRef]
- Pardon, G.; Roest, A.S.V.; Chirikian, O.; Birnbaum, F.; Lewis, H.; Castillo, E.A.; Wilson, R.; Denisin, A.K.; Blair, C.A.; Holbrook, C.; et al. Tracking single hiPSC-derived cardiomyocyte contractile function using CONTRAX an efficient pipeline for traction force measurement. Nat. Commun. 2024, 15, 5427. [Google Scholar] [CrossRef]
- Mandel, Y.; Weissman, A.; Schick, R.; Barad, L.; Novak, A.; Meiry, G.; Goldberg, S.; Lorber, A.; Rosen, M.R.; Itskovitz-Eldor, J.; et al. Human Embryonic and Induced Pluripotent Stem Cell–Derived Cardiomyocytes Exhibit Beat Rate Variability and Power-Law Behavior. Circulation 2012, 125, 883. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, K.; Takahashi, H.; Hirayama-Shoji, K.; Nguyen, T.-V.; Tsukagoshi, T.; Shimoyama, I. A MEMS-based measurement system for evaluating the force-length relationship of human induced pluripotent stem cell-derived cardiomyocytes adhered on a substrate. J. Micromech. Microeng. 2019, 29, 55003. [Google Scholar] [CrossRef]
- Matsudaira, K.; Takahashi, H.; Hirayama-Shoji, K.; Tsukagoshi, T.; Nguyen, T.-V.; Shimoyama, I. Isometric contraction force measurement of hiPSC-CMs on a movable plate with a feedback-controlled MEMS cantilever probe. Meas. Sci. Tech. 2021, 32, 115118. [Google Scholar] [CrossRef]
- Ikegami, R.; Tsukagoshi, T.; Matsudaira, K.; Shoji, K.H.; Takahashi, H.; Nguyen, T.-V.; Tamamoto, T.; Noda, K.; Koyanagi, K.; Oshima, T.; et al. Temperature Dependence of the Beating Frequency of hiPSC-CMs Using a MEMS Force Sensor. Sensors 2023, 23, 3370. [Google Scholar] [CrossRef] [PubMed]
- Chaui-Berlinck, J.G.; Monteiro, L.H.A. Frank-Starling mechanism and short term adjustment of cardiac flow. J. Exp. Biol. 2017, 220, 4391. [Google Scholar] [CrossRef]
- Han, J.-C.; Pham, T.; Taberner, A.J.; Loiselle, D.S.; Tran, K. Solving a century-old conundrum underlying cardiac force-length relations. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H781. [Google Scholar] [CrossRef]
- Louch, W.E.; Koivumäki, J.T.; Tavi, P. Calcium signalling in developing cardiomyocytes: Implications for model systems and disease. J. Physiol. 2015, 593, 1047. [Google Scholar] [CrossRef]
- Fukutani, A.; Westerblad, H.; Jardemark, K.; Bruton, J. Ca2+ and force during dynamic contractions in mouse intact skeletal muscle fibers. Sci. Rep. 2024, 14, 689. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.; Strano, A.; Arun, S.; Gamm, O.; Poetsch, M.S.; Hasse, M.; Steiner, R.-P.; Fischer, K.; Pöche, J.; et al. Comprehensive promotion of iPSC-CM maturation by integrating metabolic medium with nanopatterning and electrostimulation. Nat. Commun. 2025, 16, 2785. [Google Scholar] [CrossRef]
- Jung, P.; Seibertz, F.; Fakuade, F.E.; Ignatyeva, N.; Sampathkumar, S.; Ritter, M.; Li, H.; Mason, F.E.; Ebert, A.; Voigt, N. Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy. Basic Res. Cardiol. 2022, 117, 5. [Google Scholar] [CrossRef] [PubMed]
- Ernst, P.; Bidwell, P.A.; Dora, M.; Thomas, D.D.; Kamdar, F. Cardiac calcium regulation in human induced pluripotent stem cell cardiomyocytes: Implications for disease modeling and maturation. Front. Cell Dev. Biol. 2023, 10, 3389. [Google Scholar] [CrossRef]
- Stavrov, V.; Tomerov, E.; Stavreva, G.; Hardalov, C.; Shulev, A. Lateral Displacement MEMS Sensor. Procedia Eng. 2010, 5, 649. [Google Scholar] [CrossRef]
- Takahashi, H.; Jung, U.G.; Kan, T.; Tsukagoshi, T.; Matsumoto, K.; Shimoyama, I. Rigid two-axis MEMS force plate for measuring cellular traction force. J. Micromech. Microeng. 2016, 26, 105006. [Google Scholar] [CrossRef]
- Behrens, I.; Doering, L.; Peiner, E. Piezoresistive cantilever as portable micro force calibration standard. J. Micromech. Microeng. 2003, 13, S171. [Google Scholar] [CrossRef]
- Gel, M.; Shimoyama, I. Force sensing submicrometer thick cantilevers with ultra-thin piezoresistors sy rapid thermal diffusion. J. Micromech. Microeng. 2004, 14, 423. [Google Scholar] [CrossRef]
- Takahashi, H.; Dung, N.M.; Matsumoto, K.; Shimoyama, I. Differential pressure sensor using a piezoresistive cantilever. J. Micromech. Microeng. 2012, 22, 055015. [Google Scholar] [CrossRef]
- Noda, K. Far Infrared Pas Sensor Using Silicon Piezoresistive Cantilever for Continuous Non-Invasive Blood Glucose Measurement. In Proceedings of the IEEE 35th International Conference on Micro Mechanical Systems (MEMS), Tokyo, Japan, 9–13 January 2022; pp. 9–13. [Google Scholar]
- Takahashi, H.; Takei, Y.; Noda, K.; Matsumoto, K.; Shimoyama, I. Triaxial piezoresistive force sensor probe with high sensitivity and stiffness using 3D notch structure. J. Micromech. Microeng. 2023, 33, 125005. [Google Scholar] [CrossRef]
- Robinson, P.; Sparrow, A.J.; Psaras, Y.; Steeples, V.; Simon, J.N.; Broyles, C.N.; Chang, Y.-F.; Brook, F.A.; Wang, Y.-J.; Blease, A.; et al. Comparing the effects of chemical Ca2+ dyes and R-GECO on contractility and Ca2+ transients in adult and human iPSC cardiomy-ocytes. J Mol Cell Cardiol. 2023, 180, 44–57. [Google Scholar] [CrossRef]
- Dou, W.; Malhi, M.; Zhao, Q.; Wang, L.; Huang, Z.; Law, J.; Liu, N.; Simmons, C.A.; Maynes, J.T.; Sun, Y. Microengineered platforms for characterizing the contractile function of in vitro cardiac models. Microsyst Nanoeng 2022, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.-Q.; Wu, S.-S.; Cheng, Y.-K.; Wu, Y.; Li, Y.-M. Comprehensive review of indicators and tech-niques for optical mapping of intracellular calcium ions. Cereb. Cortex 2024, 34, bhae346. [Google Scholar] [CrossRef]
- Rubart, M.; Wang, E.; Dunn, K.W.; Field, L.J. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am. J. Physiol. Cell Physiol. 2003, 284, C1654–C1668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinani, R.; Manders, E.; Helmes, M.; Wang, L.; Knollmann, B.; Kuster, D.W.D.; van der Velden, J. Real-Time Measurements of Calcium and Contractility Parameters in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Vis. Exp. 2023, 195, e65326. [Google Scholar] [CrossRef] [PubMed]
| Temporal Resolution (Frame Rate) | Image Resolution | ||
|---|---|---|---|
| Typical | Highest | ||
| Optical Mapping /High Speed Camera | 500–1000 fps | 4000 fps | 0.1–1 mm |
| Confocal Laser Scanning Microscopy | 10–50 fps | kHz order (theoretically) | H: 0.2–0.5 µm V: 0.5–1 µm |
| Two-photon Microscopy | 10–100 fps | kHz order | H: 0.3–0.6 µm V: 1–2 µm |
| Piezoresistive Force Sensor (this work) | 2000 S/sec | 1 MS/sec | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikegami, R.; Tsukagoshi, T.; Matsudaira, K.; Takahashi, H.; Nguyen, T.-V.; Noda, K.; Koyanagi, K.; Shimoyama, I. Simultaneous Measurement of Contractile Force and Ca2+ Concentration Distribution in Human iPS Cell-Derived Cardiomyocytes. Sensors 2025, 25, 7478. https://doi.org/10.3390/s25247478
Ikegami R, Tsukagoshi T, Matsudaira K, Takahashi H, Nguyen T-V, Noda K, Koyanagi K, Shimoyama I. Simultaneous Measurement of Contractile Force and Ca2+ Concentration Distribution in Human iPS Cell-Derived Cardiomyocytes. Sensors. 2025; 25(24):7478. https://doi.org/10.3390/s25247478
Chicago/Turabian StyleIkegami, Ryota, Takuya Tsukagoshi, Kenei Matsudaira, Hidetoshi Takahashi, Thanh-Vinh Nguyen, Kentaro Noda, Ken’ichi Koyanagi, and Isao Shimoyama. 2025. "Simultaneous Measurement of Contractile Force and Ca2+ Concentration Distribution in Human iPS Cell-Derived Cardiomyocytes" Sensors 25, no. 24: 7478. https://doi.org/10.3390/s25247478
APA StyleIkegami, R., Tsukagoshi, T., Matsudaira, K., Takahashi, H., Nguyen, T.-V., Noda, K., Koyanagi, K., & Shimoyama, I. (2025). Simultaneous Measurement of Contractile Force and Ca2+ Concentration Distribution in Human iPS Cell-Derived Cardiomyocytes. Sensors, 25(24), 7478. https://doi.org/10.3390/s25247478





